Abstract
Background
There is limited longitudinal evidence supporting the association between the hypertriglyceridemic-waist (HTGW) phenotype and hyperuricemia. This study aimed to examine the longitudinal relationship between hyperuricemia and the HTGW phenotype among males and females.
Methods
A total of 5562 hyperuricemia-free participants aged 45 or over from the China Health and Retirement Longitudinal Study (mean age: 59.0) were followed for 4 years. The HTGW phenotype was defined as having elevated triglyceride levels and enlarged waist circumference (cutoffs for males: 2.0 mmol/L and 90 cm; females: 1.5 mmol/L and 85 cm). Hyperuricemia was determined by uric acid cutoffs (males: 7 mg/dl; females: 6 mg/dl. Multivariate logistic regression models were used to assess the association between the HTGW phenotype and hyperuricemia. The joint effect of the HTGW phenotype and sex on hyperuricemia was quantified, and the multiplicative interaction was assessed.
Results
During the four-year follow-up, 549 (9.9%) incident hyperuricemia cases were ascertained. Compared with those with normal levels of triglycerides and waist circumference, participants with the HTGW phenotype had the highest risk of hyperuricemia (OR: 2.67; 95% CI: 1.95 to 3.66), followed by an OR of 1.96 (95% CI: 1.40 to 2.74) for only higher triglyceride levels and 1.39 (95% CI: 1.03 to 1.86) for only greater waist circumference. The association between HTGW and hyperuricemia was more prominent among females (OR = 2.36; 95% CI: 1.77 to 3.15) than males (OR = 1.29; 95% CI: 0.82 to 2.04), with evidence of a multiplicative interaction (P = 0.006).
Conclusions
Middle-aged and older females with the HTGW phenotype may at the highest risk of hyperuricemia. Future hyperuricemia prevention interventions should be primarily targeted for females with the HTGW phenotype.
Similar content being viewed by others
Introduction
Hyperuricemia is a metabolic disease caused by excessive production of uric acid or reduced renal excretion and is usually defined as a condition where the level of uric acid exceeds the normal range [1, 2]. Previous studies have revealed that hyperuricemia may increase the risk of several diseases, such as hypertension, diabetes, and kidney disease [3, 4], and lead to gout and nephrolithiasis [2]. However, the prevalence of hyperuricemia has been increasing, e.g., in China, from 8.5% in 2001 to 18.4% in 2017, with the incidence increasing with age [5, 6]. This implies an urgent need to identify people at risk of hyperuricemia.
Previous studies reported that elevated triglycerides and enlarged waist circumference were associated with a higher risk of hyperuricemia [7, 8]. The hypertriglyceridemic-waist (HTGW) phenotype (i.e., coexistence of elevated triglyceride levels and enlarged waist circumference) was first introduced in 2000 and has been confirmed as a measure of increased visceral adiposity and a predictor of chronic kidney disease [9, 10]. A previous study determined the potential mechanism among them, i.e., insulin resistance induced by visceral obesity subsequently reduces the excretion of uric acid from the renal system, resulting in an increased risk of hyperuricemia [11]. To our knowledge, only one study including participants at high risk of cardiovascular disease examined the cross-sectional association of the HTGW phenotype with hyperuricemia, leaving the longitudinal association for the general Chinese population uninvestigated [12].
It is worth noting that sex differences in relation to metabolic syndrome components are common, especially among adults 45 years and older [13]. For example, previous studies have shown a higher prevalence of high triglycerides and high waist circumference among females than males [14]. A cross-sectional study from China observed a higher likelihood among females than males for developing hyperuricemia with higher triglycerides [15]. Recent studies have shown that the correlation between the HTGW phenotype and diabetes and kidney disease might be stronger among females [16, 17]. This aforementioned evidence emphasizes the importance of sex in the association between HTGW and the incidence of hyperuricemia.
In this study, we used 5562 participants from the China Health and Retirement Longitudinal Study (CHARLS) to examine the prospective relationship between the HTGW phenotype and hyperuricemia among middle-aged and older adults. An HTGW phenotype-sex interaction was also investigated.
Method
Data and sample
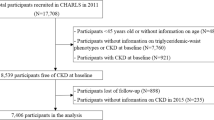
The data were obtained from the China Health and Retirement Longitudinal Study (CHARLS), a nationally representative cohort survey consisting of community residents aged 45 years or older. Initial samples were recruited from 2011 by multistage probability sampling and followed up every 2 years. Questionnaire surveys and physical measurements are conducted at every follow-up, and blood sample collection is performed once every two follow-up cycles [18, 19]. In the current study, we used three waves of data from CHARLS (2011, 2013, and 2015). As shown in fig. 1, after excluding those who 1) had hyperuricemia or kidney disease or were undergoing chemotherapy for malignancies at baseline (n = 1732); 2) had missing information on triglycerides (n = 10), uric acid (n = 3), waist circumference (n = 1524) and both triglycerides and uric acid (n = 5567); 3) were lost or refused to follow-up (n = 2926); and 4) had no information on uric acid in 2015 (n = 13), 5562 participants remained in the analytical sample.
Exposure and outcome
Fasting venous blood samples were collected from participants and tested at the Clinical Laboratory of Capital Medical University in 2011 and 2015 [19]. Triglycerides were measured using an enzymatic color metric test, with an elevated triglyceride level defined as ≥ 1.5 mmol/L for females or ≥ 2.0 mmol/L for males. Waist circumference was measured by trained assessors using soft measuring tape, and enlarged waist circumference was defined as ≥ 85 cm in females or ≥ 90 cm in males [9, 10]. We divided participants into the following four triglyceride-waist phenotypes: 1) NTNW, normal triglyceride levels and normal waist circumference; 2) NTGW, normal triglyceride levels and enlarged waist circumference; 3) HTNW, elevated triglyceride levels and normal waist circumference; and 4) HTGW, elevated triglyceride levels and enlarged waist circumference [10]. Serum uric acid was determined by the Uric Acid Plus method [19]. Hyperuricemia was defined as a serum uric acid concentration ≥ 7 mg/dl in males and ≥ 6 mg/dl in females [1]. To focus on participants with elevated triglyceride levels and enlarged waist circumference and to facilitate the interpretation of the interaction effect between the HTGW phenotype and sex on hyperuricemia, we combined ‘NTNW’, ‘NTGW’ and ‘HTNW’ as ‘non-HTGW’ in the analyses concerning interaction.
Covariates
Covariates were collected at baseline mainly through standardized questionnaires and anthropometric measurements. Maximum years of schooling (educational level: less than or equal to 6 years vs. more than 6 years), marital status (married vs. nonmarried, i.e., divorced/widowed/single), residential location (rural vs. urban), smoking (current smokers vs. current nonsmokers), alcohol consumption (occasional drinkers, i.e., less than or equal to 3 times per week vs. habitual drinkers, i.e., more than 3 times per week) were dichotomized. Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2) and categorized as underweight (< 18.5 kg/m2), normal weight (18.5–23.9 kg/m2), overweight (24–27.9 kg/m2) and obese (≥ 28 kg/m2), according to the revised Asia-Pacific BMI criteria by the World Health Organization [20]. Health status referred to self-reported history of doctor diagnosed diseases (e.g., diabetes, hypertension, and hyperlipidemia) or treatments of these diseases. People who responded affirmatively to one or more diseases were categorized as unhealthy or otherwise healthy.
Statistical analyses
To test the differences in characteristics between participants with different hyperuricemia statuses, chi-square (χ2) and one-way ANOVA were used for categorical variables and continuous variables, respectively. We also compared the characteristics of those with and without information on triglycerides and waist circumference. Multivariate logistic regression models were performed to detect the associations between the triglyceride-waist phenotypes and hyperuricemia after adjusting for age, sex, education, marital status, residential location, smoking, alcohol consumption, BMI, and health status. Furthermore, the joint effect of the HTGW phenotype and sex on hyperuricemia was quantified, and the two-way multiplicative interaction was examined.
Multiple imputation by chained equations was performed for missing data on triglycerides and waist circumference, and then we repeated the analyses and compared the results with those conducted on the observed data.
To test the reliability in the classification of the HTGW phenotype, we conducted two sensitivity analyses: 1) adjusting the treatment of dyslipidemia as a confounder; 2) people with treatment of dyslipidemia were excluded, and then the main analysis was repeated.
All analyses were performed using Stata 16.0 (Stata Corp, College Station, TX, USA). Odds ratios (ORs) and 95% confidence intervals (CIs) were used to describe the associations.
Ethics review
All interviewees were required to sign the informed consent form, and the data collection of CHARLS was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052–11015).
Results
Demographic characteristics
Table 1 shows the baseline characteristics of participants classified on the basis of their waist circumference and triglyceride levels. Of the 5562 participants at baseline, 3061 (55.0%) were females, with a mean age of 59 years, and 964 (17.3%) participants had the HTGW phenotype. Compared to the participants with normal waist circumference and triglyceride levels, participants with the HTGW phenotype were more likely to be younger, females, current nonsmokers, occasional drinkers, obese, unhealthy, and live in urban areas. Compared with those without missing information on triglycerides and waist circumference, participants with missing information tended to have higher education levels, live in urban areas, and be younger, males, and healthy (Table S1).
Triglyceride-waist phenotypes and hyperuricemia
After the four-year follow-up, 549 (9.9%) incident hyperuricemia cases were ascertained. In the fully adjusted model, participants with the NTGW (OR: 1.39; 95% CI: 1.03 to 1.86), HTNW (OR: 1.96; 95% CI: 1.40 to 2.74), and HTGW (OR: 2.67; 95% CI: 1.95 to 3.66) phenotypes had significantly higher hyperuricemia incidence than those with the NTNW phenotype (Table 2). Moreover, the risk of hyperuricemia was obviously higher in participants with the HTGW phenotype (OR: 2.00; 95% CI: 1.58 to 2.54) than in those with the non-HTGW phenotype after adjusting for full covariates (Table 3). Similar results were found in the analyses where uric acid level was treated as a continuous variable (Table 3).
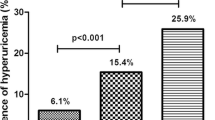
In the sex-stratified analysis, we found that the association of the HTGW phenotype and hyperuricemia was statistically significant in females (OR: 2.36; 95% CI: 1.77 to 3.15; P < 0.001) but not in males (OR: 1.29; 95% CI: 0.82 to 2.04; P = 0.269) (Table 3). Notably, a significant multiplicative interaction between sex and the triglyceride-waist phenotype for the risk of hyperuricemia (P two-way multiplicative = 0.006) was observed, which suggests that females with the HTGW phenotype had a 1.41-fold (95% CI: 1.05 to 1.91) higher risk of hyperuricemia than males with non-HTGW conditions (Fig. 2) (Table S2).
The interaction between the HTGW phenotype and female sex on the risk of hyperuricemia. Notes: Non-HTGW includes 3 phenotypes: NTNW, normal triglyceride levels and normal waist circumference; NTGW, normal triglyceride levels and enlarged waist circumference; HTNW, elevated triglyceride levels and normal waist circumference. HTGW, elevated triglyceride levels and enlarged waist circumference; OR, odds ratio; CI, confidence interval. The model was adjusted for age, education, marital status, residential location, smoking, alcohol consumption, body mass index, and health status.
Sensitive analysis
After imputation of missing data on triglycerides and waist circumference, all results remained almost unchanged (Table S3). In addition, similar results were obtained regardless of adjusting the treatment of dyslipidemia or excluding people with treatment of dyslipidemia (Tables S4 and S5).
Discussion
In this national longitudinal cohort study, we found that both elevated triglyceride levels and enlarged waist circumference (i.e., the HTGW phenotype) were associated with a higher risk of hyperuricemia among middle-aged and older adults. In addition, female sex and the HTGW phenotype interact in their relationship with hyperuricemia, suggesting that the HTGW phenotype was associated with a much higher odds of hyperuricemia in females but not in males.
In our study, the incidence proportion of hyperuricemia was 9.9% after a four-year follow-up, which was comparable with a longitudinal study using the same database (prevalence: 10.12%) [8]. The positive association between the HTGW phenotype and hyperuricemia observed in this study was in line with Shuang Chen et al., who showed a cross-sectional association between the HTGW phenotype and a higher prevalence of hyperuricemia among 11,576 Chinese adults (aged ≥35 years) [12]. Our study extends their work by providing longitudinal evidence.
Furthermore, the present study showed that the association between the HTGW phenotype and hyperuricemia was modified by sex, with females experiencing the highest risk. This is consistent with a prospective study that considered the triglyceride-glucose index (Tyg) (a marker of insulin resistance) as a better index of hyperuricemia in females (OR: 6.08; 95% CI: 4.43 to 8.34) than in males (OR: 2.68; 95% CI: 2.11 to 3.41) [21]. However, Shuang Chen et al. showed that males with the HTGW phenotype (OR: 4.59; 95% CI: 3.53 to 5.98) had a higher risk of hyperuricemia than females (OR: 3.55; 95% CI: 2.60 to 4.86) [12]. One of the possible explanations for this inconsistent finding may be the limitation to the rural population, thus, the causal association between the HTGW phenotype and hyperuricemia in the general population could not be determined [12].
Some possible mechanisms may explain our current findings. First, the HTGW phenotype has been proven to be related to increased visceral fat and insulin resistance [22]. The increase in insulin concentration caused by insulin resistance can enhance the reabsorption of sodium in renal tubules, thereby reducing the clearance rate of uric acid and causing the development of hyperuricemia [23]. In this study, we found that compared with the model2 (without adjusting BMI), the model3 (fully adjusted model) experienced a 23.0% attenuation of the effects from 1.82 (95% CI: 1.36 to 2.42) in model2 to 1.41 (95% CI: 1.05 to 1.91) in model3, this might support aforementioned pathway (Table S2). Second, estrogen is known to promote the excretion of uric acid [24]. This possibly due to the estrogen level in postmenopausal women decreases, which may cause an increase in lipoprotein lipase activity or a decrease in fat decomposition, leading to more severe abdominal fat accumulation [25], and then increased abdominal fat is associated with a series of metabolic abnormalities, such as insulin resistance and dyslipidemia, which may increase the level of uric acid in postmenopausal women [26]. Previous studies might support this speculation that females have higher risk of elevated triglyceride level, enlarged waist circumference, faster growth level of uric acid in comparison to males after aged 45 or 50 [14, 27]. Therefore, possible reasons for the higher risk of hyperuricemia caused by the HTGW phenotype combined with females are that the coexistence of insulin resistance and estrogen deficiency hinders the clearance rate of uric acid.
Our findings have important public health implications. China is experiencing an epidemic of obesity and metabolic diseases due to rapid economic development and lifestyle changes [28]. For example, an epidemiologic study indicated that the prevalence of abdominal obesity increased greatly among Chinese adults (especially those aged 40–80) from 1993 to 2015 [29]. In addition, hypertriglyceridemia, the most common dyslipidemia in the general population, is less frequent with advancing age in males but more frequent in females [30]. Therefore, intervention strategies aimed at reducing hyperlipidemia or abdominal obesity, such as weight loss, changing dietary habits, physical exercise and drug treatment, are essential to reduce the risk of hyperuricemia [31].
Study strengths and limitations
The current study has several strengths, including the use of nationally representative data with a large cohort sample size and objective measures of exposures and outcome indicators. The use of longitudinal design minimize the chance of reverse causation. Nevertheless, some limitations should be considered. First, waist circumference and triglyceride levels were only measured at baseline, which prevented assessment of their impact on hyperuricemia over time, resulting in a potential underestimation of the association. Second, there were substantial missing values (n = 2766) for triglycerides and waist circumference, which may lead to selection bias, as healthy individuals seemed to contribute more to these missing values. However, the results remained similar in the sensitivity analyses when missing values were replaced by imputation. We have also adjusted, e.g., health status into the model to minimize this selection bias. Third, several variables that would have better explained the association between the HTGW phenotype and hyperuricemia, such as diet, genetics, sex hormone levels, and menopausal status of females, were not available in this dataset.
Conclusion
In summary, females with the HTGW phenotype were more likely to suffer from hyperuricemia among middle-aged and older adults. Future interventions to prevent hyperuricemia should target females with both enlarged waist circumference and elevated triglyceride levels.
Availability of data and materials
The datasets analyzed in the current study are available in the China Health and Retirement Longitudinal Study repository, http://charls.pku.edu.cn/index/en.html.
Abbreviations
- HTGW:
-
Hypertriglyceridemic waist
- CHARLS:
-
China Health and Retirement Longitudinal Study
- BMI:
-
Body Mass Index
- WHO:
-
World Health Organization
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol. 2014;26(2):186–91. https://doi.org/10.1097/bor.0000000000000028.
Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet (London, England). 2016, 388(10055):2039–2052.https://doi.org/10.1016/s0140-6736(16)00346-9.
Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008;324(1):1–7. https://doi.org/10.1124/jpet.107.129031.
Liu J, Tao L, Zhao Z, Mu Y, Zou D, Zhang J, et al. Two-year changes in hyperuricemia and risk of diabetes: a five-year prospective cohort study. J Diabetes Res. 2018;2018:6905720. https://doi.org/10.1155/2018/6905720.
Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16(7):380–90. https://doi.org/10.1038/s41584-020-0441-1.
Li Y, Shen Z, Zhu B, Zhang H, Zhang X, Ding X. Demographic, regional and temporal trends of hyperuricemia epidemics in mainland China from 2000 to 2019: a systematic review and meta-analysis. Glob Health Action. 2021;14(1):1874652. https://doi.org/10.1080/16549716.2021.1874652.
Zhang Y, Wei F, Chen C, Cai C, Zhang K, Sun N, et al. Higher triglyceride level predicts hyperuricemia: a prospective study of 6-year follow-up. J Clin Lipidology. 2018;12(1):185–92. https://doi.org/10.1016/j.jacl.2017.10.009.
Zhou Z, Li K, Li X, Luan R, Zhou R. Independent and joint associations of body mass index, waist circumference, waist-height ratio and their changes with risks of hyperuricemia in middle-aged and older Chinese individuals: a population-based nationwide cohort study. Nutr Metab. 2021;18(1):62. https://doi.org/10.1186/s12986-021-00590-z.
Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102(2):179–84. https://doi.org/10.1161/01.cir.102.2.179.
Chen R, Sun G, Liu R, Sun A, Cao Y, Zhou X, et al. Hypertriglyceridemic waist phenotype and risk of chronic kidney disease in community-dwelling adults aged 60 years and older in Tianjin, China: a 7-year cohort study. BMC Nephrol. 2021;22(1):182. https://doi.org/10.1186/s12882-021-02339-5.
Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary artery risk development in young adults. Ann Epidemiol. 1998;8(4):250–61. https://doi.org/10.1016/s1047-2797(97)00204-4.
Chen S, Guo X, Dong S, Yu S, Chen Y, Zhang N, et al. Association between the hypertriglyceridemic waist phenotype and hyperuricemia: a cross-sectional study. Clin Rheumatol. 2017;36(5):1111–9. https://doi.org/10.1007/s10067-017-3559-z.
Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. 2017;120:34–42. https://doi.org/10.1016/j.phrs.2017.03.008.
Azimi-Nezhad M, Aminisani N, Ghasemi A, Farimani AR, Khorashadizadeh F, Mirhafez SR, et al. Sex-specific prevalence of metabolic syndrome in older adults: results from the Neyshabur longitudinal study on aging. Iran J Diab Metab Dis. 2022;21(1):263–73. https://doi.org/10.1007/s40200-022-00969-6.
Qi J, Dai X, Zhou B, Su Y, Xie Z, Liu D. Association between lipid profiles and serum urate: a cross-sectional study in southwestern China. Int J Endocrinol. 2021;2021:2741131. https://doi.org/10.1155/2021/2741131.
Zhang M, Gao Y, Chang H, Wang X, Liu D, Zhu Z, et al. Hypertriglyceridemic-waist phenotype predicts diabetes: a cohort study in Chinese urban adults. BMC Public Health. 2012;12:1081. https://doi.org/10.1186/1471-2458-12-1081.
Qiu Y, Zhao Q, Wang N, Yu Y, Wang R, Zhang Y, et al. Association of hypertriglyceridemic waist phenotype with renal function impairment: a cross-sectional study in a population of Chinese adults. Nutr Metab. 2020;17:63. https://doi.org/10.1186/s12986-020-00483-7.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. 2014;43(1):61–8.
Zhao YSJYGGJ, Hu P, Hu Y, et al. China health and retirement longitudinal study–2011–2012 national baseline users’ guide. Beijing: National School of Development, Peking University; 2013. p. 2011–56. https://doi.org/10.1093/ije/dys203.
Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
Zhang L, Wan Q, Zhou Y, Xu J, Yan C, Ma Y, et al. Age-related and gender-stratified differences in the association between high triglyceride and risk of hyperuricemia. Lipids Health Dis. 2019;18(1):147. https://doi.org/10.1186/s12944-019-1077-5.
Sam S, Haffner S, Davidson MH, D'Agostino RB Sr, Feinstein S, Kondos G, et al. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care. 2009;32(10):1916–20. https://doi.org/10.2337/dc09-0412.
Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. Jama. 1991;266(21):3008–11. https://doi.org/10.1016/s0140-6736(99)92381-4.
Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet (London, England). 1999;354(9179):650. https://doi.org/10.1001/jama.1991.03470210076036.
Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obesity Related Metabolic Dis. 2000;24(2):226–31. https://doi.org/10.1038/sj.ijo.0801118.
Kissebah AH, Krakower GRJPr. Regional adiposity and morbidity 1994, 74(4):761–811. https://doi.org/10.1152/physrev.1994.74.4.761.
Akizuki S. Serum uric acid levels among thirty-four thousand people in Japan. Ann Rheum Dis. 1982;41(3):272–4. https://doi.org/10.1136/ard.41.3.272.
Li JJ, Liu HH, Li S. Landscape of cardiometabolic risk factors in Chinese population: a narrative review. Cardiovasc Diabetol. 2022;21(1):113. https://doi.org/10.1186/s12933-022-01551-3.
Ma S, Xi B, Yang L, Sun J, Zhao M, Bovet P. Trends in the prevalence of overweight, obesity, and abdominal obesity among Chinese adults between 1993 and 2015. Int J Obes (2005). 2021;45(2):427–37. https://doi.org/10.1038/s41366-020-00698-x.
Jiang B, Zheng Y, Chen Y, Chen Y, Li Q, Zhu C, et al. Age and gender-specific distribution of metabolic syndrome components in East China: role of hypertriglyceridemia in the SPECT-China study. Lipids Health Dis. 2018;17(1):92. https://doi.org/10.1186/s12944-018-0747-z.
Simha V. Management of hypertriglyceridemia. BMJ (Clinical research ed). 2020;371:m3109. https://doi.org/10.1136/bmj.m3109.
Acknowledgments
We are grateful to all China Health and Retirement Longitudinal Study (CHARLS) participants and the research team.
Funding
This work was supported by the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province, China (grant number: 20KJB320035), Hu Xin Foundation (grant number: HX2008), Research Foundation for Talented Scholars, Fujian Medical University (XRCZX2022006) and Young and Middle-aged Teacher Education Research Foundation of Fujian Province (JAT220103).
Author information
Authors and Affiliations
Contributions
The study was designed by Huihui He, Raoping Tu and Tianwei Xu; Suhang Wang, Wenbin Liu, Yueping Li and Guangyu Lu analyzed and interpreted the data; Huihui He was responsible for drafting the manuscript; and the manuscript was reviewed by Raoping Tu and Tianwei Xu. All authors have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board at Peking University approved all CHARLS waves (IRB00001052–11015). Informed consent was obtained from all participants included in the study.
Consent for publication
All authors provide consent for publication of this paper.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, H., Wang, S., Xu, T. et al. Sex-related differences in the hypertriglyceridemic-waist phenotype in association with hyperuricemia: a longitudinal cohort study. Lipids Health Dis 22, 38 (2023). https://doi.org/10.1186/s12944-023-01795-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01795-2