Abstract
Background
Diabetes mellitus (DM) is a major public health problem which prevalence is constantly raising, particularly in low- and middle-income countries. Both diabetes mellitus types (DMT1 and DMT2) are associated with high risk of developing chronic complications, such as retinopathy, nephropathy, neuropathy, endothelial dysfunction, and atherosclerosis.
Methods
This is a review of available articles concerning HDL subfractions profile in diabetes mellitus and the related cardiovascular risk. In this review, HDL dysfunction in diabetes, the impact of HDL alterations on the risk diabetes development as well as the association between disturbed HDL particle in DM and cardiovascular risk is discussed.
Results
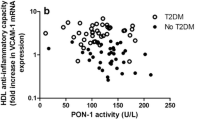
Changes in the amount of circulation lipids, including triglycerides and LDL cholesterol as well as the HDL are frequent also in the course of DMT1 and DMT2. In normal state HDL exerts various antiatherogenic properties, including reverse cholesterol transport, antioxidative and anti-inflammatory capacities. However, it has been suggested that in pathological state HDL becomes “dysfunctional” which means that relative composition of lipids and proteins in HDL, as well as enzymatic activities associated to HDL, such as paraoxonase 1 (PON1) and lipoprotein-associated phospholipase 11 (Lp-PLA2) are altered. HDL properties are compromised in patients with diabetes mellitus (DM), due to oxidative modification and glycation of the HDL protein as well as the transformation of the HDL proteome into a proinflammatory protein. Numerous studies confirm that the ability of HDL to suppress inflammatory signals is significantly reduced in this group of patients. However, the exact underlying mechanisms remains to be unravelled in vivo.
Conclusions
The understanding of pathological mechanisms underlying HDL dysfunction may enable the development of therapies targeted at specific subpopulations and focusing at the diminishing of cardiovascular risk.
Similar content being viewed by others
Background
Diabetes mellitus (DM) is a major public health problem which prevalence is constantly raising, particularly in low- and middle-income countries [1]. In 2012, total burden of deaths worldwide from high blood glucose was estimated to amount to 3.7 million. 1.5 million deaths were associated with the presence of diabetes, while additional 2.2 million deaths was the result of DM-related increased risks of cardiovascular and other diseases [1]. In 2014, 422 million people in the world suffered from diabetes [1]. Both diabetes mellitus types (DMT1 and DMT2) are associated with high risk of developing chronic complications, such as retinopathy, nephropathy, neuropathy, endothelial dysfunction, and atherosclerosis [2]. According to studies, adult patients with type 1 diabetes (DMT1) poses a less atherogenic fasting lipid profile than people without diabetes, however, the incidence of cardiovascular diseases (CAD) is paradoxically high in this group of patients [3,4,5]. Even diabetic women were shown to develop CAD earlier than non-diabetic women and they have CAD rates approaching those of men with DMT1 [6, 7]. Changes in the amount of circulating lipids, including the increase in triglycerides and LDL cholesterol as well as the decrease in HDL are frequent also in the course of DMT2 [8]. However, it has been found that dyslipidaemia may precede DM by several years [9].
Numerous studies have shown that HDL cholesterol is strongly and inversely associated with the occurrence of cardiovascular events [10,11,12,13,14]. HDL cholesterol participates in the efflux of cholesterol efflux from peripheral cells as well as in reverse cholesterol transport from these cells to the liver [8]. According to studies, HDL has antioxidative and anti-inflammatory properties. It reduces LDL oxidation [15], inhibits oxidized LDL-induced MCP-1 (monocyte chemoattractant protein 1) production and monocyte transmigration in a co-culture of human aortic endothelial cells and human aortic smooth muscle cells [16, 17] and blunts inflammatory response of endothelial cells to TNF-α (tumour necrosis factor-1) and IL-1 (interleukin 1) stimuli [18]. Finally, it has been demonstrated to exert anti-thrombotic and anti-apoptotic effects [19]. It has been shown that its biological activity may change in various pathophysiological states. In the past it was believed that high level of HDL protects against the occurrence of cardiovascular disease, however new evidence suggest that in some pathological conditions cholesterol HDL may lose its protective properties and become pro-atherogenic. The results of studies confirm that high levels of HDL cholesterol may not be always beneficial. Systematic review and meta-regression analysis of randomized controlled trials testing lipid modifying interventions provided evidence that increasing circulating HDL-C did not reduce coronary heart disease morbidity or mortality [20]. Also The Initiating Dialysis Early and Late (IDEAL) study, in which the efficacy of high to moderate dose statin regimen for the secondary prevention of cardiovascular disease was compared, and The European Prospective Investigation of Cancer, Norfolk (EPIC-Norfolk) [21] demonstrated that highly elevated HDL-C concentrations did not protect against cardiovascular disease.
HDL becomes “dysfunctional” inter alia in type 2 diabetes [5], which may mean that also in pathological state relative composition of lipids and proteins in HDL, as well as enzymatic activities associated to HDL, such as paraoxonase 1 (PON1) and lipoprotein-associated phospholipase 11 (Lp-PLA2), are altered [22]. It has been suggested that plasma HDL cholesterol is not homogeneous, it comprises different particles varying in size, density, apolipoprotein composition, and lipid content. The hypothesis of dysfunctional HDL-C in relation to its activity and reverse cholesterol transport, has been put forward in type 1 diabetes [23]. Generally increased HDL-C does not translate into lower cardiovascular risk in DMT1 patients, but rather an inverse association was observed. However, the exact mechanisms of such relationship remain not fully elucidated. It has been suggested that different HDL subfractions relate to coronary artery disease (CAD) incidence in a different manner.
In this review, HDL dysfunction in diabetes, the impact of HDL alterations on the risk diabetes development as well as the association between disturbed HDL particle in DM and cardiovascular risk is discussed.
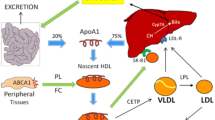
Types of HDL particles obtained using various methods of separation
Numerous studies indicated that HDL particles are highly heterogeneous in size, shape, density and properties. Abundance of different methodologies used to analyse HDL subclasses resulted in the generation of numerous classifications with do not relate with each other. It is generally believed that at the beginning of its formation, HDL is a discoidal and lipid poor [24]. Then, Apo A-I acquires cholesterol and phospholipids via its interaction with the ATP-binding cassette 10 (ABCA1) leading to the formation of pre-β1 HDL particles [24]. These particles gradually accumulate more and more cholesterol. Following the esterification by the enzyme lecithin-cholesterol acyltransferase (LCAT) cholesterol is transferred to the core of HDL particle, forming larger, spherical, α-mobility HDL particles, which may undergo clearance by the hepatic scavenger receptor [24]. Cholesteryl esters can be transferred to VLDL/LDL for catabolism via cholesteryl ester transfer protein (CETP) enzyme.
HDL can be divided into various subclasses, according to its density, size, electrophoretic mobility and apolipoprotein cargo. The use of ultracentrifugation allows for the separation of HDL2 (1.063–1.125 g/mL) and HDL3 (1.125–1.21 g/mL), while gradient gel electrophoresis allows to receive HDL2b (9.7–12.0 nm), HDL2a (8.8–9.7 nm), HDL3a (8.2–8.8 nm), HDL3b (7.8–8.2 nm) and HDL3c (7.2–7.8 nm) or HDL1-HDL10 (Lipoprint) [25]. Also. the obtaining of large HDL, medium HDL, small HDL, spherical, discoidal HDL and many others is possible using various methods.
Proteins, which constitute important part of HDL particles are responsible for their structure and function. Among the most important components of HDL cholesterol there are apolipoproteins (e.g. ApoA-I, ApoA-II, ApoE, ApoJ), enzymes (e.g. LCAT, PON1; LpPLA2’ PAF-AH, GSPx-3), lipid transfer proteins (e.g. PLTP, CETP), acute-phase response proteins (SAA1, SAA4, alpha-2-HS-glycoprotein), complement components and proteinase inhibitors (alpha-1-antitrypsin) and many other [25].
HDL levels and diabetes risk
Recent studies have indicated that cholesterol HDL may directly alter glucose metabolism [26, 27]. Indeed, HDL cholesterol promotes pancreatic β-cell insulin secretion and modifies glucose uptake in skeletal muscle as shown in different experimental and human settings [27,28,29,30]. Therefore, low levels of HDL cholesterol has been suggested to be associated with higher risk of type 2 diabetes in epidemiological studies [31, 32]. Moreover, plasma HDL level increase has been proposed as a therapeutic measure to reduce the risk of type 2 diabetes [33,34,35]. However, the results of genetic studies evaluating the relationship between HDL cholesterol levels and glycaemic control and risk of type 2 diabetes are conflicting [36,37,38]. Some studies demonstrated the relationship between HDL particles and lower risk of type 2 diabetes [22, 39, 40]. Hwang et al. [10] found an inverse association between total cholesterol and HDL2 and future development of type 2 DM and this relationship was independent of well-established risk factors for type 2 diabetes. However, they failed to find any correlation between HDL3 cholesterol and future diabetes risk. Also Tabara et al. [41] suggested that high-density lipoprotein (HDL) may exert an antidiabetes function. In their study HDL2 cholesterol levels were inversely associated with HOMA-IR (β = −0.169, p < 0.001) and type 2 diabetes (OR = 0.96, p = 0.001 Opposite relationship was observed in case of HDL3-C and HOMA-IR (β = 0.054, p < 0.001) and type 2 diabetes (OR = 1.04, p = 0.181). In turn, a longitudinal analysis demonstrated inverse relationship between HDL2-C and the exacerbation of insulin resistance (β = −0.163, p < 0.001) and the inverse risk of type 2 diabetes incidence (odds ratio = 0.98, p = 0.006) [41].
It has been proposed that the deletion within ABCA1 may be associated with cholesterol accumulation within cell membrane of beta cells and further results in the hampering of the exocytosis of insulin from secretory granules, and inhibition of insulin secretion [42, 43]. The mutation R230C in ABCA-1, which is associated with reduced cholesterol efflux capacities, was demonstrated to be more frequent in young persons with DMT2 [44]. Animal studies provided the explanation of this phenomenon. It seems that cholesterol accumulation in islet β-cells is the responsible for the pathology.
Moreover, beneficial, apoptosis-inhibiting effects of HDLs on beta cells have also been demonstrated [45]. Fryirs et al. [46] revealed that the incubation of Min6 cells and primary islets with HDLs isolated from human plasma or a constituent of discoidal reconstituted HDLs (rHDLs) or apolipoprotein (apo) A-I or apoA-II enhanced insulin secretion up to 5-fold in a calcium-dependent as well as time and concentration dependent manner [46]. The observation that intravenous reconstituted HDL (rHDL) reduced plasma glucose in patients with type 2 diabetes mellitus by increasing plasma insulin and stimulating AMP-activated protein kinase in skeletal muscle further supports the view that HDLs have the capacity to improve diabetic control and probably postpone the development of new diabetes via several mechanisms [27]. According to Han et al. [30] study, apo A-I was able to stimulate the phosphorylation of the key metabolic regulatory enzyme AMPK and increased glucose uptake in C2C12 myocytes. In turn, Rapizzi et al. [47] reported that HDL-associated sphingolipid S1P could enhance glucose uptake in skeletal muscle through transactivation of the insulin receptor. HDL was reported to reverse the deleterious effects of oxidized LDL on insulin secretion [48, 49]. Recently, it has been shown that HDL can reciprocally increase adiponectin expression in a PI3K-dependent way, which offers a novel indirect way of glucose homeostasis regulation [50]. The treatment with the apo A-I mimetic peptide L-4F increased serum adiponectin levels and decreased IL-1β and IL-6 levels in obese mice and this was accompanied by increased the presence of insulin-sensitive adipocytes [51], improved insulin sensitivity and improved glucose tolerance [52]. Van Linthout et al. [53] reported a decrease in cardiac glycogen content following apo A-I gene transfer in an experimental model of diabetic cardiopathy. They suggested that this effect may be associated with Akt-glycogen synthase kinase (GSK)-3β dependent pathway.
However, the recent Haase et al. [28] study demonstrated that lifelong low levels of HDL cholesterol due to genetic variation in HDL cholesterol-related genes were not associated with increased risk of type 2 diabetes in the general population. They also suggested that low levels of HDL cholesterol per se do not cause type 2 diabetes and but they may be explained by reverse causation, due to a state of prediabetes prior to the diabetes diagnosis. The results of large Schou et al. [38] study also failed to find any association between loss-of-function mutations in ABCA1 and ABCG1 and risk of type 2 diabetes in 40,000 individuals. No relationship between HDL cholesterol related genes and type 2 diabetes has also been reported in recent genome-wide association studies and meta-analyses [54, 55]. Contrary to the aforementioned results, smaller studies of genetic variation in ABCA1 (ATP-binding cassette transporter 1) demonstrated that R230C variant was associated with increased risk of type 2 diabetes [56], while loss-of-function mutations in ABCA1 were proposed to correlate with impaired β-cell function, but not with development of type 2 diabetes [57]. Also, Mackey et al. [58] study demonstrated that decreases in large HDL particles adjusted for confounders were significantly associated with the incidence of diabetes.
Influence of DM on HDL composition and level
The direct impact of insulin resistance on lipid metabolism in type 2 diabetes DMT2 is quite well-known, while in DM type 1 the mechanisms related to insulin deficiency and dyslipidaemia remain poorly understood and controversial. According to studies, diabetes generally promotes not only quantitative changes in the amount of circulating lipids – particularly an increase in triglycerides and LDL as well as a reduction in HDL but also qualitative and kinetic in nature [59,60,61]. Decreased plasma concentration, triacylglycerol enrichment, reduced phospholipids, ApoE and ApoM, glycation and increased HDL catabolism are the main changes occurring in diabetes [19]. Altered HDL composition in patients with diabetes results in diminished ability to promote reverse cholesterol transport. Impaired cholesterol efflux from adipose and hepatic cells is mainly related to increased triglyceride and decreased cholesterol content in HDL [62].
Miettinen et al. [63] demonstrated increased markers of cholesterol absorption and decreased markers of cholesterol synthesis in patients with DM type 1 in comparison to control subjects, suggesting that the occurrence of high cholesterol absorption and low cholesterol synthesis in this group of patients with type 1 diabetes. The relationship between gender and lipid levels in patients with DMT1 was analysed by Maahs et al. [64]. They observed that male type 1 diabetic subjects showed higher content of large and lower content of small HDL-C particles than non-diabetic subjects, while DMT1 women had smaller amount of large and higher amount of small dense LDL lipoproteins and reduced LDL size [64]. However, it remains unclear whether the aforementioned lipid abnormalities are due to impaired lipid metabolism associated with DMT1 rather than with glucose control, gender, insulin resistance, and non-regular lifestyle of these patients, or by all these factors in combination. The study of 127 patients with DMT1 demonstrated higher levels of total HDL-C and the lowest density HDL subfraction, apolipoprotein A-I, LPL activity, and adiponectin levels in comparison to control subjects (P < .05) [65]. Moreover, Calderon et al. found a relationship between adiponectin and LPL activity and total HDL and its lowest density subfraction.
DM type 2 is associated with dyslipidaemia which involves abnormalities in all types of lipoproteins [19, 66, 67]. According to studies, concentrations of HDL cholesterol are diminished in patients with diabetes mellitus type 2 [68, 69]. Moreover, the predominance of small dense HDL particles which undergo rapid catabolism has been also reported [8, 68, 69].
Hypertriglyceridemia which appears in the course of T2 diabetes mellitus is associated with insulin resistance, hyperglycaemia and hyperinsulinemia. Insulin resistance was shown to increase free fatty acids availability, while hyperinsulinemia and hyperglycaemia promote triglyceride synthesis via the activation of carbohydrate-responsive element-binding protein (ChREBP) [65] and sterol regulatory element-binding transcription factor 1 (SREBF1c) [66], respectively and the consequent increase in cholesteryl ester transfer protein (CETP) activation. Enhanced CETP activity is associated with the enrichment of HDL particles with triglycerides, which is responsible for increased HDL catabolism. Hepatic lipase (HL), which expression and activity is augmented in the presence of hyperglycaemia and insulin resistance, metabolizes triglyceride-rich HDL leading at first to the formation of small HDL particles and then to their accelerated clearance [70, 71]. Therefore, the amount of circulating smaller HDL particles (HDL3) is increased, while the number of large HDL particles (HDL2) is diminished [72]. Also, the content of cholesteryl esters is diminished in HDL particles of DM patients [73]. The alteration of HDL particle lipid composition results in Apo A-I destabilization and its shedding form HDL during lipolysis [19, 74]. The decrease in HDL cholesterol levels may be also associated with the lowering of plasma adiponectin levels in patients with insulin resistance and type 2 diabetes. Vergès et al. [75] demonstrated a negative correlation between HDL-ApoA-I catabolism rate and plasma levels of adiponectin, which was independent of abdominal obesity, insulin sensitivity, age, and sex and plasma lipids. Their finding suggests a direct impact of adiponectin on HDL metabolism, however, the exact mechanism has not been unraveled. Moreover, in patients with type 2 DM decreased plasma concentrations of campesterol and increased levels of lathosterol were observed which mirrors reduced cholesterol absorption and enhanced cholesterol synthesis [76, 77]. Alteration in HDL function and structure are also associated with glycation and oxidation of HDL-associated proteins, changes in gene expression and activity of HDL-metabolizing enzymes. Prolonged inflammation present in DM promotes changes in HDL proteome. All these changes results in the loss of HDL of its normal function and in its “transformation” into a proatherogenic particle. In vitro studies have revealed that HDL glycation is accompanied by the oxidation of HDL lipids and results in diminished cholesterol efflux and reduced HDL affinity binding to fibroblasts. [8, 78,79,80,81]. Hyperglycaemia and glycation contribute to disturbed cholesterol efflux, reduced expression of ABCA-1 [82] and scavenger receptor class B type I (SR-BI) [83] and lower HDL antioxidative capacity. Moreover, glycation has been shown to decrease paraoxonase 1 (PON1) activity and to inactivate LCAT. In vitro glycation of HDL was also shown to hamper HDL ability to suppress TNF-α and IL-1β production by lipopolysaccharide-stimulated macrophages [84] as well as to reduce monocyte adhesion to human aortic endothelial cells induced by oxidized LDL [85, 86]. Nobécourt et al. [87] demonstrated that non-enzymatic glycation impaired the anti-inflammatory properties of apolipoprotein A-I and therefore it exerted deleterious effects on HDL key functions. The presence of advanced glycation end products (AGEs) induces changes in the conformation and surface charge of HDL apolipoproteins and decreases the activity of HDL-bound enzymes, including lecithin-cholesterol acyltransferase and paraoxonase-1 [86,87,88]. However, due to the fact that AGE levels in HDL after in vitro glycation were 5- to 150-fold higher than after in vivo glycation in T2D subjects [86, 87, 89] it is difficult to confirm that such glycation of HDL exerts a direct effect on its anti-inflammatory effects in DMT2.
Apart from glycation, also oxidative modifications of HDL are associated with disturbed HDL function. Negative correlation between HDL oxidation and ABCA1-dependent cholesterol efflux was observed by Zeng et al. [90] who suggested that apolipoprotein A-I was a selective target for myeloperoxidase-catalysed oxidation and its functional impairment was frequent in subjects with cardiovascular disease. Other studies have indicated that oxidative modifications of Apo-AI affect two amino acids (Tyr-166 and Met-148) which are placed within lecithin–cholesterol acyltransferase binding site thus preventing LCAT binding and abolishing its activity [91, 92]. Also paraoxonase 1 is sensitive to oxidation and it becomes inactive following HDL oxidation [93]. Diabetes is associated with the presence of prolonged inflammation. According to studies, inflammation changes HDL proteome converting it from an antiatherogenic particle to a raft of immunological proteins [8]. During acute phase response, Apo A-I content was observed to be diminished due to its replacement by acute phase protein - serum amyloid A [94]. Moreover, the activity of HDL antioxidant enzymes, including PON1, PAF-AH and LCAT, is also reduced during an acute phase response [94]. It has been recently hypothesized that the relative distribution of Lp-PLA2 between LDL and HDL determines whether it exerts pro- or anti-inflammatory effects - Lp-PLA2 in HDL is anti-inflammatory while Lp-PLA2 associated to apoB-containing lipoproteins is pro-inflammatory [95].
The presence of chronic inflammatory state promotes the transformation of HDL into proinflammatory particle which no longer mitigates inflammatory response stimulated by oxidized LDL, but it aggravates the situation [96]. Moreover, Chiba et al. suggested that HDL proteasome change by the inflammation results in HDL ability to bind components of the extracellular matrix, such as vascular proteoglycans. Also Dullaart et al. [97] demonstrated reduced PON-1 activity, HDL cholesterol and apoA-I in T2DM (all p < 0.05). Despite the lack of HDL particle concentration change, their distribution was found to be different. Large & medium HDL particles, and HDL particle size were decreased, whereas small HDL particles were increased in T2DM (all p < 0.05). It is noteworthy that many of the aforementioned lipids-related alterations are present before the onset of diabetes mellitus as a result of insulin-resistant metabolic syndrome [19].
HDL subfractions and cardiovascular risk in DM patients
Numerous large epidemiologic studies and clinical trials indicate that the mortality of CAD reasons is much higher in patients with DMT2, even after the adjustment for age, ethnicity or the presence of risk factor [98, 99]. Haffner et al. [100] found that diabetic persons with no history of myocardial infarction (MI) had equivalent rates of CAD mortality to non-diabetic individuals with a MI history. Moreover, diffuse, severe atherosclerotic lesions were observed in patients with diabetes mellitus. Potential mechanisms responsible for these worse outcomes in patients with T2D have not been fully elucidated. However, it was suggested that strict glycaemic control alone can only slightly improve diabetes-related cardiovascular events [101], Also dyslipidaemia was proposed as a causal factor of diabetic atherosclerosis as well as clinical outcomes. Also, individuals with type 1 diabetes show a considerably increased risk for cardiovascular disease in comparison to the general population [102]. Costacou et al. [24] demonstrated a smooth linear inverse association between HDL-C and CAD incidence in men. However, in DMT1 women an apparent increase in risk is observed below an HDL-C of 50 mg/dL as well as above 80 mg/dL. Moreover, their findings revealed that HDL3 subfraction was mainly associated with CAD risk. Asztalos and Schaefer [103] demonstrated deficiencies in the α1 and pre-α1–3 HDL subspecies and increase in the α3 HDL subspecies among individuals with CAD in comparison to normal controls. Their results may suggest a disturbance in the progressive increase of HDL particle size in those with CAD.
Some cross-sectional and prospective studies have suggested that HDL2 may be more protective than HDL3 [104,105,106]. Gordon et al. [107] revealed that young patients with T2DM exhibited decreased phospholipid content in fractions containing large HDL particles, which inversely correlated with pulse wave velocity (PWV) (P < 0.001). However, no relationship was observed between HDL-C and PWV. They also reported changes in 7 out of 45 identified proteins in the T2D group, including apolipoprotein (apo) A-II, apoE, and paraoxonase-1 (p < 0.05). Diminished ApoE content in large HDL particles may have an atherogenic effect, due to the fact that large, ApoE-rich HDL usually prevents LDL binding to proteoglycans in the vessel wall [107]. The content of ApoM which mediates the enrichment of HDL in sphingosine-1-phosphate (which promotes arterial vasodilation by stimulating endothelial nitric oxide formation [108]) was also reduced in their study. Gordon et al. [107] recommend the analysis of HDL composition, rather than HDL-C level as an useful tool in the evaluation of cardiovascular risk in this population. In patients with DMT2, reduced antioxidative properties of HDL due to the presence of hyperglycaemia and triacylglycerol enrichment has been reported [109]. In comparison to normolipidemic, non-diabetic controls, in diabetic patients specific antioxidative activity of small dense HDL3b and 3c particles was reduced up to 47% (on a particle mass or particle number basis). Moreover, plasma 8-isoprostanes were found to be considerably elevated (2.9-fold) in diabetic patients and they negatively correlated with specific antioxidative activity of HDL3 subfractions [109]. Perségol et al. [110] demonstrated the inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Results of their study suggest that HDL are less atheroprotective in type 2 diabetic patients than in control subjects. In turn, Sorrentino et al. [111] reported weaker stimulatory effect on endothelial nitric oxide synthesis in patients with type 2 diabetes. However, extended-release niacin therapy improved the capacity of HDL to stimulate endothelial nitric oxide, to decrease superoxide production, and to stimulate endothelial progenitor cell-mediated endothelial repair. Chinese prospective study of patients with stable CAD indicated that high levels of large HDL-C was inversely associated with cardiovascular risk including traditional risk factors, severity of CAD, and future cardiovascular outcomes [112]. Moreover, high large HDL-C negatively and independently correlated with the occurrence of major adverse cardiovascular events (MACEs), after adjustment for multiple confounders. The present study provided potential evidence that HDL subfraction analysis might prove useful in CAD risk assessment. However, in this study only 25% of patients had type 2 diabetes [112]. Pennathur et al. [113] study demonstrated increased levels of HDL modified by products of the myeloperoxidase system in atherosclerotic lesions and in plasma of coronary artery disease patients. Some of these modifications (i.e. chlorination) impair ABCA-1-specific cholesterol efflux [114, 115].
Conclusions
In normal state HDL exerts various antiatherogenic properties, including reverse cholesterol transport, antioxidative and anti-inflammatory capacities. However, these properties are compromised in patients with diabetes mellitus (DM), due to oxidative modification and glycation of the HDL protein as well as the transformation of the HDL proteome into a proinflammatory protein. Numerous studies confirm that the ability of HDL to suppress inflammatory signals is significantly reduced in this group of patients. However, the exact underlying mechanisms remains to be unravelled in vivo. The understanding of pathological mechanisms underlying HDL dysfunction may enable the development of therapies targeted at specific subpopulations and focusing at the diminishing of cardiovascular risk.
Abbreviations
- ABCA1:
-
ATP-binding cassette 1
- AGEs:
-
advanced glycation end products
- ApoE:
-
apolipoprotein E
- ApoM:
-
apolipoprotein M
- CAD:
-
cardiovascular disease
- CETP:
-
cholesteryl ester transfer protein
- ChREBP:
-
carbohydrate-responsive element-binding protein
- DM:
-
diabetes mellitus
- DMT1:
-
diabetes mellitus type 1
- DMT2:
-
diabetes mellitus type 2
- GSPx-3:
-
glutathione peroxidase 3
- HDL:
-
high-density lipoprotein
- HL:
-
hepatic lipase
- IL-1:
-
interleukin 1
- LCAT:
-
lecithin-cholesterol acyltransferase
- LDL:
-
low-density lipoprotein
- Lp-PLA2:
-
lipoprotein-associated phospholipase A2
- MACEs:
-
major adverse cardiovascular events
- MCP-1:
-
monocyte chemoattractant protein 1
- PAF-AH:
-
platelet activating factor-acetylhydrolase
- PLTP:
-
phospholipid transfer protein
- PON1:
-
paraoxonase 1
- PWV:
-
pulse wave velocity
- SAA1:
-
serum amyloid 1
- SAA4:
-
serum amyloid 4
- SR-BI:
-
scavenger receptor class B type I
- SREBF1c:
-
sterol regulatory element-binding transcription factor 1
- TNF-α:
-
tumour necrosis factor-1
References
Global report on diabetes. World Health Organization 2016 http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf.
Bardini G, Rotella CM, Giannini S. Dyslipidemia and Diabetes: Reciprocal impact of impaired lipid metabolism and Beta-cell dysfunction on micro- and macrovascular complications. Rev Diabet stud. Summer-Fall. 2012;9(2–3):82–93.
Cree-Green M, Maahs DM, Ferland A, Hokanson JE, Wang H, Pyle L, Kinney GL, King M, Eckel RH, Nadeau KJ. Lipoprotein subfraction cholesterol distribution is more atherogenic in insulin resistant adolescents with type 1 diabetes. Pediatr Diabetes. 2016;17(4):257–65.
Maahs DM, Maniatis AK, Nadeau K, Wadwa RP, McFann K, Klingensmith GJ. Total cholesterol and high-density lipoprotein levels in pediatric subjects with type 1 diabetes mellitus. J Pediatr. 2005;147(4):544–6.
Lachin JM, Orchard TJ, Nathan DM. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:39–43.
Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46:760–5.
Lloyd CE, Kuller LH, Ellis D, Becker DJ, Wing RR, Orchard TJ. Coronary artery disease in IDDM. Gender differences in risk factors but not risk. Arterioscler Thromb Vasc Biol. 1996;16:720–6.
Farbstein D, Levy AP. HDL dysfunction in diabetes: causes and possible treatments. Expert Rev Cardiovasc Ther. 2012;10(3):353–61. doi: 10.1586/erc.11.182.
Adiels M, Olofsson SO, Taskinen MR, Borén J. Dabetic dyslipidaemia. Curr Opin Lipidol. 2006;17(3):238–46.
Hwang Y-C, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, Boyko EJ. Differential association between HDL subclasses and the development of type 2 diabetes in a prospective study of Japanese Americans. Diabetes Care. 2015;38:2100–5.
Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham study. JAMA. 1986;256:2835–8.
Olsson AG, Schwartz GG, Szarek M, et al. High-density lipoprotein, but not low-density lipoprotein cholesterol levels influence short-term prognosis after acute coronary syndrome: results from the MIRACL trial. Eur Heart J. 2005;26:890–6.
Boekholdt SM, Arsenault BJ, Hovingh GK, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128:1504–12.
Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23). BMJ. 1998;316:823–8.
Modified HDL. Biological and physiopathological consequences. Norata GD, Pirillo a, Catapano AL. Nutr Metab Cardiovasc Dis. 2006 Jul;16(5):371–86.
Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL Becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995 Dec;96(6):2758–67.
Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, Valente AJ, Berliner JA, Drinkwater DC, Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991;88(6):2039–46.
Ashby DT, Rye KA, Clay MA, Vadas MA, Gamble JR, Barter PJ. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18(9):1450–5.
Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–99.
Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, Whitt I. Alves da Silva S, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O’Regan C, Mills EJ, Bucher HC, Montori VM. Guyatt GH Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis BMJ. 2009;338:b92.
van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ES, Tikkanen MJ, Wareham NJ, Faergeman O, Olsson AG, Pedersen TR, Khaw KT, Kastelein JJ. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol. 2008;51:634–42.
Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–8.
Orchard TJ. Dyslipoproteinemia and diabetes. Endocrinol Metab Clin N Am. 1991;19:361–80.
Costacou T1, Evans RW, Orchard TJ. High-density lipoprotein cholesterol in diabetes: is higher always better? J Clin Lipidol. 2011;5(5):387–94.
Kontush A, Lindahl M, Lhomme M, Calabresi L, Chapman MJ, Davidson WS. Structure of HDL: Particle Subclasses and Molecular Components. High Density Lipoproteins. In: Handbook of Experimental Pharmacology, vol. 224: Springer International Publishing AG; 2014. p. 3–51.
Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein: it’s not just about lipid transport anymore. Trends Endocrinol Metab. 2011;22:9–15.
Drew BG, Duffy SJ, Formosa MF, et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–11.
Haase CL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. High-density lipoprotein cholesterol and risk of type 2 diabetes: a Mendelian randomization study. Diabetes. 2015;64:3328–33. https://doi.org/10.2337/db14-1603.
Brunham LR, Kruit JK, Pape TD, Rimmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodriguew B, Johnson JD, Parks JS, Verchere CB, Hayden MR. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13:340–7.
Han R, Lai R, Ding Q, Wang Z, Luo X, Zhang Y, Cui G, He J, Liu W, Chen Y, Apolipoprotein A-I. Stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia. 2007;50:1960–8.
Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, Folsom AR, Chambless LE. For the atherosclerosis risk in communities investigators. Identifying individuals at high risk for diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28:2013–8.
Wilson PF, Meigs JB, Sullivan L, Fox CS, Nathan DM, RB D’A Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham offspring study. Arch Intern Med. 2007;167:1068–74.
Siebel AL, Natoli AK, Yap FY, Carey AL, Reddy-Luthmoodoo M, Sviridow D, Weber CI, Meneses-Lorente G, Maugeais C, Forbes JM, Kingwell BA. Effects of high-density lipoprotein elevation with cholesteryl ester transfer protein inhibition on insulin secretion. Circ Res. 2013;113:167–75.
Fazio S, Linton MF. Killing two birds with one stone, maybe: CETP inhibition increases both high-density lipoprotein levels and insulin secretion. Circ Res. 2013;113:94–6.
Drew BG, Rye KA, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. 2012;8:237–45.
Hirano M, Nakanishi S, Kubota M, Maeda S, Yoneda M, Yamane K, Kira S, Sasaki H, Kohno N. Low high‐density lipoprotein cholesterol level is a significant risk factor for development of type 2 diabetes: Data from the Hawaii–Los Angeles–Hiroshima study. J Diabetes Investig. 2014;5(5):501–6.
Sturek JM, Castle JD, Trace AP, Sturek JM, Castle JD, Trace AP, Page LC, Castle AM, Evans-Molina C, Parks JS, Mirmira RG, Hedrick CC. An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic β-cells. J Clin Invest. 2010;120:2575–89.
Schou J, Tybjærg-Hansen A, Møller HJ, Nordestgaard BG, Frikke-Schmidt RABC. Transporter genes and risk of type 2 diabetes: a study of 40,000 individuals from the general population. Diabetes Care. 2012;35:2600–6.
Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, RB D’A Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham offspring study. Arch Intern Med. 2007;167:1068–74.
Hwang YC, Ahn HY, Park SW, Park CY. Association of HDL-C and apolipoprotein A-I with the risk of type 2 diabetes in subjects with impaired fasting glucose. Eur J Endocrinol. 2014;171:137–42.
Tabara Y, Arai H, Hirao Y, Takahashi Y, Setoh K, Kawaguchi T, Kosugi S, Ito Y, Nakayama T, Matsuda F. Nagahama study group. Different inverse association of large high-density lipoprotein subclasses with exacerbation of insulin resistance and incidence of type 2 diabetes: the Nagahama study. Diabetes Res Clin Pract. 2017;127:123–31.
Barter PJ. The causes and consequences of low levels of high density lipoproteins in patients with diabetes. Diabetes Metab J. 2011;35(2):101–6.
Kruit JK, Kremer PH, Dai L, Tang R, Ruddle P, de Haan W, Brunham LR, Verchere CB, Hayden MR. Cholesterol efflux via ATP-binding cassette transporter A1 (ABCA1) and cholesterol uptake via the LDL receptor influences cholesterol-induced impairment of beta cell function in mice. Diabetologia. 2010;53:1110–9.
Brunham LR, Kruit JK, Hayden MR, Verchere CB. Cholesterol in beta-cell dysfunction: the emerging connection between HDL cholesterol and type 2 diabetes. Curr Diab Rep. 2010;10(1):55–60.
Kruit JK, Brunham LR, Verchere CB, Hayden MRHDL. LDL cholesterol significantly influence beta-cell function in type 2 diabetes mellitus. Curr Opin Lipidol. 2010;21:178–85.
Fryirs MA, Barter PJ, Appavoo M, Tuch BE, Tabet F, Heather AK, Rye KA. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler Thromb Vasc Biol. 2010;30:1642–8.
Rapizzi E, Taddei ML, Fiaschi T, Donati C, Bruni P, Chiarugi P. Sphingosine 1-phosphate increases glucose uptake through transactivation of insulin receptor. Cell Mol Life Sci. 2009;66:3207–18.
Van Linthout S, Spillmann F, Schultheiss HP, Tschöpe C. High-density lipoprotein at the interface of type 2 diabetes mellitus and cardiovascular disorders. Curr Pharm Des. 2010 May;16(13):1504–16.
Abderrahmani A, Niederhauser G, Favre D, Abdelli S, Ferdaoussi M, Yang JY, Regazzi R, Widmann C, Waeber G. Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia. 2007;50(6):1304–14.
Van Linthout S, Foryst-Ludwig A, Spillmann F, Peng J, Feng Y, Meloni M, Van Craeyveld E, Kintscher U, Schultheiss HP, De Geest B, Tschöpe C. Impact of HDL on adipose tissue metabolism and adiponectin expression. Atherosclerosis. 2010;210(2):438–44.
Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, et al. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–304.
Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49:1658–69.
Van Linthout S, Spillmann F, Riad A, Trimpert C, Lievens J, Meloni M, et al. Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation. 2008;117:1563–73.
Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, et al. DIAbetes genetics replication and meta-analysis (DIAGRAM) consortium. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–90.
Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, Rybin D, et al. Genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44:659–69.
Villarreal-Molina MT, Flores-Dorantes MT, Arellano-Campos O, Villalobos-Comparan M, Rodríguez-Cruz M, Miliar-García A, Huertas-Vazquez A, Menjivar M, Romero-Hidalgo S, Wacher NH, Tusie-Luna MT, Cruz M, Aguilar-Salinas CA, Canizales-Quinteros S. The metabolic study group. Association of the ATP-binding cassette transporter 10 R230C variant with early-onset type 2 diabetes in a Mexican population. Diabetes. 2008;57:509–13.
Vergeer M, Brunham LR, Koetsveld J, Kruit JK, Verchere CB, Kastelein JJ, Hayden MR, Stroes ES. Carriers of loss-of-function mutations in ABCA1 display pancreatic β-cell dysfunction. Diabetes Care. 2010;33:869–74.
Mackey RH, Mora S, Bertoni AG, Wassel CL, Carnethon MR, Sibley CT, et al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. 2015;38(4):628–36.
Fonseca MIH, da Silva IT, Ferreira SRG. Impact of menopause and diabetes on atherogenic lipid profile: is it worth to analyse lipoprotein subfractions to assess cardiovascular risk in women? Diabetology & Metabolic Syndrome 2017;9:22.
Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46(6):733–49.
Vergès B. New insight into the pathophysiology of lipid abnormalities in type 2 diabetes. Diabetes Metab. 2005 Nov;31(5):429–39.
Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17(10):594–603.
Miettinen TA, Gylling H, Tuominen J, Simonen P, Koivisto V. Low synthesis and high absorption of cholesterol characterize type 1 diabetes. Diabetes Care. 2004;27(1):53–8.
Maahs DM, Hokanson JE, Wang H, Kinney GL, Snell-Bergeon JK, East A, Bergman BC, Schauer IE, Rewers M, Eckel RH. Lipoprotein subfraction cholesterol distribution is proatherogenic in women with type 1 diabetes and insulin resistance. Diabetes. 2010;59(7):1771–9.
Calderon RM, Diaz S, Szeto A, Llinas JA, Hughes TA, Mendez AJ, Goldberg RB. Elevated lipoprotein lipase activity does not account for the association between adiponectin and HDL in type 1 diabetes. J Clin Endocrinol Metab. 2015;100(7):2581–8.
Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23). BMJ. 1998 Mar 14;316(7134):823–8.
Eliasson B, Cederholm J, Eeg-Olofsson K, Svensson AM, Zethelius B, Gudbjörnsdottir S. National Diabetes Register. Clinical usefulness of different lipid measures for prediction of coronary heart disease in type 2 diabetes: a report from the Swedish National Diabetes Register. Diabetes Care. 2011;34(9):2095–100.
Adiels M, Olofsson SO, Taskinen MR, Borén J. Diabetic dyslipidaemia. Curr Opin Lipidol. 2006;17(3):238–46.
Vergès B, Florentin E, Baillot-Rudoni S, Petit JM, Brindisi MC, Pais de Barros JP, Lagrost L, Gambert P, Duvillard L. Rosuvastatin 20 mg restores normal HDL-apoA-I kinetics in type 2 diabetes. J Lipid Res. 2009;50(6):1209–15.
van Deursen D, Jansen H, Verhoeven AJ. Glucose increases hepatic lipase expression in HepG2 liver cells through upregulation of upstream stimulatory factors 1 and 2. Diabetologia. 2008;51(11):2078–87.
Lewis GF, Murdoch S, Uffelman K, Naples M, Szeto L, Albers A, Adeli K, Brunzell JD. Hepatic lipase mRNA, protein, and plasma enzyme activity is increased in the insulin-resistant, fructose-fed Syrian golden hamster and is partially normalized by the insulin sensitizer rosiglitazone. Diabetes. 2004;53(11):2893–900.
Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003 Feb;52(2):453–62.
Lamarche B, Rashid S, Lewis GFHDL. Metabolism in hypertriglyceridemic states: an overview. Clin Chim Acta. 1999 Aug;286(1–2):145–61.
Sparks DL, Davidson WS, Lund-Katz S, Phillips MC. Effects of the neutral lipid content of high density lipoprotein on apolipoprotein A-I structure and particle stability. J Biol Chem. 1995 10; 270(45):26910–26917.
Vergès B, Petit JM, Duvillard L, Dautin G, Florentin E, Galland F, Gambert P. Adiponectin is an important determinant of apoA-I catabolism. Arterioscler Thromb Vasc Biol. 2006 Jun;26(6):1364–9.
Ooi EM, Ng TW, Chan DC, Watts GF. Plasma markers of cholesterol homeostasis in metabolic syndrome subjects with or without type-2 diabetes. Diabetes Res Clin Pract. 2009;85(3):310–6.
Simonen PP, Gylling HK, Miettinen TA. Diabetes contributes to cholesterol metabolism regardless of obesity. Diabetes Care. 2002;25(9):1511–5.
Hedrick CC, Thorpe SR, MX F, Harper CM, Yoo J, Kim SM, Wong H, Peters AL. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43(3):312–20.
Ferretti G, Bacchetti T, Marchionni C, Caldarelli L, Curatola G. Effect of glycation of high density lipoproteins on their physicochemical properties and on paraoxonase activity. Acta Diabetol. 2001 Dec;38(4):163–9.
Duell PB, Oram JF, Bierman EL. Non-enzymatic glycosylation of HDL and impaired HDL-receptor-mediated cholesterol efflux. Diabetes. 1991 Mar;40(3):377–84.
Duell PB, Oram JF, Bierman EL. Non-enzymatic glycosylation of HDL resulting in inhibition of high-affinity binding to cultured human fibroblasts. Diabetes. 1990 Oct;39(10):1257–63.
Passarelli M, Tang C, McDonald TO, O'Brien KD, Gerrity RG, Heinecke JW, Oram JF. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54(7):2198–205.
Ohgami N, Nagai R, Miyazaki A, Ikemoto M, Arai H, Horiuchi S, Nakayama H. Scavenger receptor class B type I-mediated reverse cholesterol transport is inhibited by advanced glycation end products. J Biol Chem. 2001;276(16):13348–55.
Liu D, Ji L, Zhang D, et al. Nonenzymatic glycation of high-density lipoprotein impairs its anti-inflammatory effects in innate immunity. Diabetes Metab Res Rev. 2012;28:186–95.
Hedrick CC, Thorpe SR, MX F, et al. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43:312–20.
Lemmers RFH, van Hoek M, Lieverse AG, Verhoeven AJM, Sijbrands EJG, Mulder MT. The anti-inflammatory function of high-density lipoprotein in type II diabetes: A systematic review. J Clin Lipidol. 2017;11(3):712–724.e5.
Nobécourt E, Tabet F, Lambert G, Puranik R, Bao S, Yan L, Davies MJ, Brown BE, Jenkins AJ, Dusting GJ, Bonnet DJ, Curtiss LK, Barter PJ, Rye KA. Nonenzymatic glycation impairs the antiinflammatory properties of apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2010;30(4):766–72.
Mastorikou M, Mackness B, Liu Y, Mackness M. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolize membrane lipid hydroperoxides. Diabet Med. 2008;25:1049–55.
Duell PB, Oram JF, Bierman EL. Nonenzymatic glycosylation of HDL and impaired HDL-receptor-mediated cholesterol efflux. Diabetes. 1991;40:377–84.
Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL, Apolipoprotein A-I. Is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004 Aug;114(4):529–41.
Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM 3rd, Smith JD, Gogonea V, Hazen SL. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14(9):861–8.
Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci U S A. 2008;105(34):12224–9.
Karabina SA, Lehner AN, Frank E, Parthasarathy S, Santanam N. Oxidative inactivation of paraoxonase-implications in diabetes mellitus and atherosclerosis. Biochim Biophys Acta. 2005;1725(2):213–21.
Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M, Anti-inflammatory HDL. Becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96(6):2758–67.
Rosenson RS, Stafforini DM. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase 11. J Lipid Res. 2012;53(9):1767–82.
Navab M, Reddy ST, Van Lenten BJ, Anantharamaiah GM, Fogelman AM. The role of dysfunctional HDL in atherosclerosis. J Lipid Res. 2009; 50 Suppl():S145–149.
Dullaart RP, Otvos JD, James RW. Serum paraoxonase-1 activity is more closely related to HDL particle concentration and large HDL particles than to HDL cholesterol in type 2 diabetic and non-diabetic subjects. Clin Biochem. 2014;47(12):1022–7.
Zhao X, Zhang HW, Zhang Y, Li S, RX X, Sun J, Zhu CG, NQ W, Gao Y, Guo YL, Liu G, Dong Q, Li JJ. Analysis of lipoprotein subfractions in 920 patients with and without type 2 diabetes. Heart Lung Circ. 2017 Mar;26(3):211–8.
Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 1993;16(2):434–44.
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34.
Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–38.
Asztalos BF, Schaefer EJ. High-density lipoprotein subpopulations in pathologic conditions. Am J Cardiol. 2003;91(Suppl):12E–7E.
Morgan J, Carey C, Lincoff A, Capuzzi D. High-density lipoprotein subfractions and risk of coronary artery disease. Curr Atheroscler Rep. 2004;6:359–65.
Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21:305–11.
Pirillo A, Norata GD, Catapano AL. High-density lipoprotein subfractions–what the clinicians need to know. Cardiology. 2013;124:116–25.
Gordon SM, Davidson WS, Urbina EM, Dolan LM, Heink A, Zang H, LJ L, Shah AS. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 2013;62(8):2958–67.
Karuna R, Park R, Othman A, Holleboom AG, Motazacker MM, Sutter I, Kuivenhoven JA, Rohrer L, Matile H, Hornemann T, Stoffel M, Rentsch KM, von Eckardstein A. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 2011 Dec;219(2):855–63.
Nobécourt E, Jacqueminet S, Hansel B, Chantepie S, Grimaldi A, Chapman MJ, Kontush A. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia. 2005;48(3):529–38.
Perségol L, Vergès B, Foissac M, Gambert P, Duvillard L. Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia. 2006 Jun;49(6):1380–6.
Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horváth T, Doerries C, Heinemann M, Flemmer S, Markowski A, Manes C, Bahr MJ, Haller H, von Eckardstein A, Drexler H, Landmesser U. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121(1):110–22.
Li JJ, Zhang Y, Li S, Cui C-J, Zhu C-G, Guo Y-L, N-Q W, R-X X, Liu G, Dong Q, Sun J, Large HDL. Subfraction but not HDL-C is closely linked with risk factors, coronary severity and outcomes in a cohort of nontreated patients with stable coronary artery disease. A prospective observational study. Medicine (Baltimore). 2016 Jan;95(4):e2600.
Pennathur S, Bergt C, Shao B, et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279(41):42977–83.
Bergt C, Pennathur S, Fu X, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101(35):13032–13037. [PMC free article] [PubMed].
Shao B, Bergt C, Fu X, et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280(7):5983–93.
Acknowledgements
none.
Funding
No funding has been received for this study.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
MF, AG-B and AC-R prepared the manuscript, MF performed also the search in order to find appropriate articles, JR revised and corrected the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Femlak, M., Gluba-Brzózka, A., Ciałkowska-Rysz, A. et al. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis 16, 207 (2017). https://doi.org/10.1186/s12944-017-0594-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-017-0594-3