Abstract
Background
Hepatitis virus B (HBV) has infected millions of people worldwide. Notably, such infections can be associated with hepatic complications. Levels of apolipoprotein M (apoM), a component of high-density lipoprotein (HDL), are known to be significantly elevated in patients with chronic hepatitis B (CHB). The aim of this study was to investigate the relationship between HBV DNA load in serum and serum apoM levels in patients with CHB.
Methods
A total of 73 HBeAg-negative CHB patients, 50 HBeAg-positive CHB patients, and 79 non-CHB controls were included in the study cohort. The age and body mass index (BMI) of the study participants were matched. Serum levels of apoM and the HBV antigens HBsAg and HBeAg were measured by enzyme-linked immunosorbent assay (ELISA) analysis. Serum levels of alanine aminotransferase (ALT), aspartate transaminase (AST), cholesterol, and triglycerides (TG) were assessed using an automatic biochemical analyzer. Serum HBV DNA levels were quantified by real-time PCR analysis. Data were analyzed by Spearman’s rank correlation coefficient, Pearson correlation coefficient, and multivariate linear regression model (continuous variables), or Student’s t-test (mean differences).
Results
Both the HBeAg-negative CHB and HBeAg-positive CHB patient groups exhibited elevated serum levels of apoM. Moreover, serum apoM levels were positively correlated with serum HBV DNA levels in HBeAg-negative CHB patients (r = 0.394, p < 0.001). Conversely, there was no significant relationship between apoM and HBV DNA levels in the HBeAg-positive CHB group (r = 0.197, p = 0.170). The median log copies/mL value for HBV DNA (4.00) was considered the cutoff point for the HBeAg-negative CHB group. Notably, a significant number of patients with HBV DNA levels above the cutoff point also had higher serum apoM levels (63.38 ± 29.84 vs. 41.41 ± 21.84; p = 0.001).
Conclusions
Our findings reveal that the correlation between serum apoM levels and viral loads may depend on HBeAg status, as serum apoM levels were positively correlated with HBV DNA levels in HBeAg-negative CHB patients. These results suggest that HBeAg may play a role in apoM-related lipid metabolism and anti-inflammatory functions in hepatitis B patients. Thus, our findings may facilitate the clinical management of HBV infection.
Similar content being viewed by others
Background
Hepatitis B virus (HBV) infection causes chronic liver disease in >350 million people worldwide and this disease is positively correlated with incidences of liver cirrhosis and hepatocellular carcinoma. Chronic liver diseases can interfere with hepatic metabolism of lipoproteins and apolipoproteins. Moreover, the ongoing replication of HBV in chronic hepatitis B (CHB) induces oxidative stress and is associated with inflammation of the liver [1–3]. Factors that govern viral replication and determine infection outcome remain unclear, but it is known that host factors play a major role in these processes [4].
Apolipoprotein M (apoM) is a 26-kD apolipoprotein that is primarily associated with high-density lipoproteins (HDL); however, a small proportion of this protein also interacts with triglyceride-rich lipoprotein (TGRLP) and low-density lipoprotein (LDL) in the serum. apoM is a member of the lipocalin protein superfamily and is exclusively expressed in hepatocytes and kidney tubular cells [5, 6]. This protein plays a variety of biological functions, e.g., anti-oxidative function [7], anti-inflammatory function [8], promoting pre-β HDL formation [9], and increasing cholesterol efflux from foam cells [10].
Previous studies reported that chronic hepatitis patients exhibit higher serum apoM levels than healthy control subjects [11, 12]. Similarly, HepG2 cells transfected with an infectious HBV clone were found to express significantly higher levels of apoM mRNA and protein than uninfected HepG2 cells in vitro. Moreover, apoM suppressed HBV replication in HepG2 cells [13]. However, the association between apoM expression and HBV replication rate in CHB patients is poorly understood. Therefore, the aim of the present study was to investigate the association between apoM and HBV replication rate to facilitate the management of anti-viral treatment in clinical practice.
Methods
Subjects
CHB patients were recruited at the Second Xiangya Hospital (Changsha, Hunan, PR China) between June 2015 and June 2016. From the 200 individuals screened, 73 HBsAg(+) HBeAg(−) CHB patients and 50 HBsAg(+) HBeAg(+) CHB patients were enrolled in this study. The mean age of the patients in the HBsAg(+) HBeAg(−) group was 44 ± 13 years, and the group was comprised of 62 males and 11 females. Meanwhile, the mean age of the patients in the HBsAg(+) HBeAg(+) group was 40 ± 12 years, and the group was comprised of 43 males and 7 females (Table 1). CHB was diagnosed based on serum HBsAg positivity for at least 6 months. The exclusion criteria for CHB were as follows: (1) positive for hepatitis C virus, hepatitis D virus, and human immunodeficiency virus antibodies; (2) cirrhosis and hepatocellular carcinoma; (3) autoimmune hepatitis and alcoholic liver disease; (4) diabetes; (5) immunological disease; (6) cancer; (7) renal disease; (8) heart disease; and (9) antiviral therapy treatment. Lastly, the control group was comprised of 79 age- and sex-matched HBsAg(−) volunteers who underwent physical examination in our hospital. All subjects provided signed Informed Consent Forms and the study protocol was approved by the Second Xiangya Hospital Investigational Review Board.
Blood sampling
Following overnight fasting (12 h) and at least 20 min of rest, blood samples were collected from each subject. After centrifugation at 1200 × g for 5 min, sera were collected, aliquoted, and stored at −80 °C until use.
Enzyme-linked immunosorbent assay (ELISA) for apoM determinations
Serum apoM levels were measured by sandwich ELISA (Yuan Tai Bio Inc., Changsha, Hunan, People’s Republic of China) analysis. Absorbance was measured at 450 nm, with a background reading at 620 nm, using an ELX-800 absorbance reader (BioTek Instruments, Inc., Winooski, VT, USA). The concentration of apoM in each sample were determined using a standard curve.
Virological assessment
Serum levels of HBsAg and HBeAg were measured via ELISA analysis using HBV S antigen and HBV E antigen diagnostic kits (Ke Hua Bio Inc., Shanghai, PR China), respectively. HBV DNA was extracted from 200 μL aliquots of serum using a Hepatitis B Viral DNA Quantitative Fluorescence Diagnostic Kit (Sheng Xiang Bio Inc., Changsha, Hunan, PR China), and quantified by real-time polymerase chain reaction (PCR) using an ABI 7000 real-time detection system (Applied Biosystems, Foster City, CA, USA).
Lipoprotein and liver biochemical assays
Levels of serum triglyceride (TG), total cholesterol (THOL), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), apolipoprotein A (apoA), apolipoprotein B (apoB), lipoprotein a (Lpa), alanine aminotransferase (ALT), aspartate transaminase (AST), and total bile acid (TBA) were determined using an ARCHITECTc8000 System (Abbott Laboratories, Irving, TX, USA).
Statistical analyses
Continuous data are expressed as means ± standard deviations. Relationships between continuous variables were tested by Spearman’s rank correlation coefficient, Pearson correlation coefficient, and multivariate linear regression model analyses. Mean differences were compared by Student’s t-test. Statistical analyses were performed using SPSS 20.0 (SPSS Statistics, Inc., Chicago, IL, USA) or GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) software. Two-tailed p-values < 0.05 were considered statistically significant.
Results
Demographic and clinical characteristics of the study subjects
Of the 200 CHB patients screened for participation in this study, 73 HBeAg-negative and 50 HBeAg-positive CHB patients were identified and assessed according to the criteria delineated in the Methods section. Additionally, of the 100 control subjects screened for participation, 11 failed the eligibility criteria and 10 refused to participate. As such, 79 control subjects were evaluated in this study (Fig. 1). The demographic and biochemical characteristics of the subjects are summarized in Table 1. Notably, there were no differences between the control, HBeAg-negative, and HBeAg-positive CHB groups in regard to age (44 ± 12, 44 ± 13, and 40 ± 12), gender (87, 85, and 86% male), and BMI (22.2 ± 2.2, 22.3 ± 2.1, and 22.1 ± 2.1), respectively. Conversely, the patients in the HBeAg-negative and HBeAg-positive groups exhibited significantly higher (p < 0.05) mean serum apoM levels (52.42 ± 28.09 and 58.32 ± 27.06 mg/L, respectively) than those in the control group (13.95 ± 7.11 mg/L). Meanwhile, there was no significant difference between the HBeAg-negative and HBeAg-positive groups in average apoM serum levels or HBV DNA levels (4.28 ± 2.07 and 5.61 ± 1.97 log copies/mL, respectively).
Serum liver biochemical profiles of HBeAg-negative CHB patients, HBeAg-positive CHB patients, and normal subjects
As shown in Fig. 2, the average serum ALT and AST levels of the HBeAg-negative CHB patients were 99.73 ± 4.50 IU/L and 99.74 ± 2.95 IU/L, respectively, which were significantly higher than those of the normal subjects (18.25 ± 1.56 IU/L and 20.11 ± 1.25 IU/L, respectively; p < 0.01). Notably, however, the ALT and AST levels of the patients in the HBeAg-positive group (216.77 ± 4.17 IU/L and 171.25 ± 2.8 IU/L; p < 0.01) were even higher than those observed in the HBeAg-negative group.
Serum liver biochemical profiles of chronic hepatitis B (CHB) patients. Serum concentrations of liver biochemical markers in CHB patients, grouped according to HBeAg status, and healthy control subjects. *p < 0.05, **p < 0.01, compared to the control group. #p < 0.05, between two groups indicated by the horizontal line
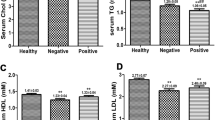
Routine serum lipid levels in HBeAg-negative CHB patients, HBeAg-positive CHB patients, and normal subjects
HBeAg-positive CHB patients exhibited significantly higher (p < 0.01 for each) serum Trig levels (2.74 ± 0.62 mmol/L) than those in the HBeAg-negative (1.72 ± 0.50 mmol/L) and control (1.1 ± 0.41 mmol/L) groups (Fig. 3). Moreover, the serum Trig levels of the HBeAg-negative group were also significantly higher than those of the control (p < 0.01). Meanwhile, serum HDL-C levels were higher in both the HBeAg-negative and HBeAg-positive CHB patients than in control subjects; however, there was no difference in serum HDL-C levels between the HBeAg-negative and HBeAg-positive CHB groups. Likewise, there were no differences in THOL and LDL-C levels between the HBeAg-positive CHB patients and control subjects. Conversely, the HBeAg-negative CHB patients exhibited elevated levels of THOL and LDL-C, compared to those in the other two groups (Fig. 3).
Chronic hepatitis B (CHB) patients exhibit elevated serum triglyceride levels and decreased serum levels of total cholesterol (THOL), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Serum concentrations of THOL, HDL-C, and LDL-C in CHB patients, grouped according to HBeAg status, and healthy control subjects. *p < 0.05, **p < 0.01, compared to the control subjects. #p < 0.05, between the two groups indicated by the horizontal line
Serum apolipoprotein levels in HBeAg-negative CHB patients, HBeAg-positive CHB patients, and normal subjects
While serum apoM levels were higher in both the HBeAg-negative and HBeAg-positive CHB patients than control subjects, these patients exhibited lower serum levels of apoA and Lpa than the controls (Fig. 4). In addition, there were no differences in apoA and Lpa levels between the two CHB groups. Conversely, HBeAg-positive patients exhibited higher serum apoB levels than both the HBeAg-negative patients and control subjects.
Chronic hepatitis B (CHB) patients exhibit elevated serum apoM and apoB levels and decreased serum apoA and lipoprotein a (Lpa) levels. Serum concentrations of apolipoproteins in CHB patients, grouped according to HBeAg status, and healthy control subjects. *p < 0.05, **p < 0.01, compared to the control group. #p < 0.05, between two groups indicated by the horizontal line
Serum apoM and HBV DNA load in HBeAg-negative and HBeAg-positive CHB patients
As summarized in Table 2, there was no significant difference in the serum levels of apoM between the HBeAg-negative and HBeAg-positive groups (52.42 ± 28.09 vs. 58.32 ± 27.06 mg/L; p = 0.248); however, the HBeAg-positive CHB patients exhibited significantly higher HBV DNA loads than those in the HBeAg-negative group (4.28 ± 2.07 vs. 5.61 ± 1.97 log copies/mL; p < 0.01).
Correlation analysis of serum apoM and HBV DNA levels in HBeAg-negative and HBeAg-positive CHB patients
Notably, we detected a positive association between serum apoM and serum HBV DNA levels in the HBeAg-negative CHB group (r = 0.394; p < 0.01), but not in the HBeAg-positive CHB patients group (r = 0.197; p = 0.170) (Fig. 5).
Association between serum apoM and HBV DNA levels in HBeAg-negative CHB patients
Using the median value of 4.28 log copies/mL HBV DNA as a cutoff point, we observed that patients with HBV DNA levels exceeding that value also showed elevated levels of apoM (63.38 ± 29.84 vs. 41.41 ± 21.84 mg/L; p = 0.001), ALT (213.8 ± 3.4 vs. 45.3 ± 3.9 IU/L; p < 0.001) and AST (179.8 ± 2.6 vs. 54.6 ± 2.4 IU/L; p < 0.001), respectively (Table 3). Moreover, multivariate regression analysis, using log HBV DNA as a continuous dependent variable and apoM, HDL-C, LDL-C, CHOL, TG, apoA, apoB, age, sex, BMI, AST, ALT, and TBA as independent variables, detected a statistically significant positive association between log HBV DNA levels and serum apoM levels (p < 0.001; β = 0.029) (Table 4).
Discussion
In this study, we measured the serum levels of apoM and lipids in HBeAg-negative CHB, HBeAg-positive CHB, and control subjects, and screened for associations between serum apoM levels and HBV DNA load. Indeed, the key finding of our study was a significant positive correlation in vivo between serum apoM and HBV DNA levels in HBeAg-negative patients. These results are consistent with and extend previous findings that CHB patients exhibit elevated serum apoM levels [11, 12].
In this study, CHB patients exhibited lower serum levels of HDL-C and apoA than the control subjects. In contrast, these patients also presented with higher serum levels of apoM, which primarily interacts with HDL-C, than the controls. Elevated apoM levels were also reported in cases of chronic inflammatory disease and decreased in acute inflammatory disease [14, 15]. The potential mechanisms underlying the anti-inflammatory activities and immune-modulatory properties of apoM have been previously characterized [16–18], and apoM is known to be widely distributed within the pool of serum lipoproteins under inflammatory conditions [19, 20]. In chronic inflammatory disease, apoM functions as an innate protective factor that is up-regulated to resist inflammatory effects over long periods. Recently studies showed that apoM could limits endothelial and vascular inflammation though sphingosine 1-phosphate-sphingosine 1-phosphate receptor 1 pathway (S1P-SIP1) [21, 22]. Furthermore, Wang et al. [18] showed that apoM contributed to the maintenance of CD4+ T lymphocytes or benefited for modifying T lymphocyte subgroups in murine spleen. Indeed, apoM-sphingosine 1-phosphate (apoM-S1P) was shown to contribute to innate and adaptive immunity by directly interacting with bone marrow lymphocyte progenitors and inhibiting their proliferation via activation of the S1P1/ERK pathway [17]. We proposed that apoM-S1P may play a role in resisting chronic inflammation in CHB patients. What’s more, it may be used as a potential helpful agent in CHB patients.
The pronounced replication of HBV during CHB has been linked to the development of cirrhosis and hepatocellular carcinoma [23, 24]. Notably, this replication also seems to be affected by host factors. Previous studies showed that HBV enhances apoM mRNA and protein expression, and that elevated levels of apoM suppress HBV protein expression and viral replication in HepG2 cells. However, these studies found no correlation between HBV viral load and apoM levels in patients with CHB [13]. Conversely, in this study, we found that higher HBV DNA levels were associated with higher serum apoM concentrations in patients with HBeAg-negative, but not HBeAg-positive, CHB. Moreover, while HBV DNA loads were also higher in HBeAg-positive CHB than in the control subjects, there was no significant difference in serum apoM levels between the HBeAg-positive and HBeAg-negative CHB patients. It is therefore conceivable that high HBeAg titers have an inhibitory effect on the HBV DNA-mediated enhancement of apoM expression. Liver is a major organ for the synthesis of lipoproteins and metabolism of lipids. HBV infection disturbs the synthesis of almost all lipoproteins and enzymes envolving in lipid metabolism [25, 26]. It was reported that HBV down-regulated the expression of apoA through affecting ApoA1 promoter activities [27], probably via promotor hypermethylation [28]. Consistent with these findings, our results found that apoA was lower in CHB patients. Previous study showed that serum leptin was elevated in patients with hepatitis B patients [29], and apoM expression was lower in leptin deficient ob/ob mice. After leptin administration, apoM expression was elevated in the liver and kidney of ob/ob mice [30]. We proposed that HBV may increase apoM level through enhancing leptin expression. Several studies have also reported that HBx promote the expression of LXR [31, 32], and that the LXR agonist TO901317 enhances the expression of apoM in Caco-2 cells [33, 34]. Therefore, HBV may mediate the upregulation of apoM via the RXR/LXR pathway. However, the molecular mechanism underlying these phenomena was not analyzed in the present study. As such, future studies are needed to elucidate the molecular effects of HBeAg on apoM expression.
There are several limitations to our present study. First, the number of patients with CHB evaluated herein was relatively small. Further studies with a larger patient cohort are therefore needed to confirm our findings. Another limitation was the imbalanced sex ratio of the cohort; evaluation of an approximately equal number of male and female subjects would provide more conclusive results. Finally, because we did not characterize the HBV genotypes of the infected patients, it is not clear whether the correlation between HBV DNA and apoM levels is dependent on HBV sub-genotypes. Such data would help determine whether apoM could be utilized as a serum marker to facilitate the management of CHB in clinical practice or a potential helpful agent in CHB treatments.
Conclusions
In summary, we detected a correlation between serum apoM levels and viral loads that may depend on HBeAg status. Specifically, we detected a positive correlation between serum apoM and serum HBV DNA levels in HBeAg-negative, but not HBeAg-positive, CHB patients. To enhance the reliability of our results, we adjusted for age, sex, BMI, ALT, AST, TBA, HDL-C, LDL-C, CHOL, TG, apoA, apoB, and LPa when screening for a correlation between apoM and HBV DNA levels. Our results support an association between apoM levels and HBV replication in HBeAg-negative CHB. Since apoM functions as an anti-inflammatory and immune-modulatory factor, these results suggest that HBeAg may play a role in apoM-related lipid metabolism and anti-inflammatory functions in hepatitis B patients. Thus, our findings may facilitate the clinical management of HBV infection.
Abbreviations
- ALT:
-
Alanine aminotransferase
- apoA:
-
Apolipoprotein A
- apoB:
-
Apolipoprotein B
- apoM:
-
Apolipoprotein M
- AST:
-
Aspartate transaminase
- BMI:
-
Body mass index
- CHB:
-
Chronic hepatitis B
- HBV:
-
Hepatitis B virus
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- TBA:
-
Total bile acid
- TG:
-
Triglycerides
- THOL:
-
Total cholesterol
References
Lavanchy D. Hepatitis B, virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107.
Teng YC, Shen ZQ, Kao CH, Tsai TF. Hepatocellular carcinoma mouse models: Hepatitis B virus-associated hepatocarcinogenesis and haploinsufficient tumor suppressor genes. World J Gastroenterol. 2016;22:300–25.
Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–63.
Thursz M. Genetic susceptibility in chronic viral hepatitis. Antiviral Res. 2001;52:113–6.
Zhang X-Y, Dong X, Zheng L, Luo G-H, Liu Y-H, Ekstrom U, et al. Specific tissue expression and cellular localization of human apolipoprotein M as determined by in situ hybridization. Acta Histochem. 2003;105:67–72.
Xu N, Dahlback B. A novel human apolipoprotein (apoM). J Biol Chem. 1999;274:31286–90.
Elsoe S, Ahnstrom J, Christoffersen C, Hoofnagle AN, Plomgaard P, Heinecke JW, et al. Apolipoprotein M binds oxidized phospholipids and increases the antioxidant effect of HDL. Atherosclerosis. 2012;221:91–7.
Huang XS, Zhao SP, Hu M, Luo YP. Apolipoprotein M likely extends its anti-atherogenesis via anti-inflammation. Med Hypotheses. 2007;69:136–40.
Wolfrum C, Poy MN, Stoffel M. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat Med. 2005;11:418–22.
Christoffersen C, Jauhiainen M, Moser M, Porse B, Ehnholm C, Boesl M, et al. Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J Biol Chem. 2008;283:1839–47.
Jiang J, Zhang X, Wu C, Qin X, Luo G, Deng H, et al. Increased plasma apoM levels in the patients suffered from hepatocellular carcinoma and other chronic liver diseases. Lipids Health Dis. 2008;7:25.
Jiang J, Wu C, Luo G, Zheng L, Chen L, Zhang X, et al. Expression of apolipoprotein M in human hepatocellular carcinoma tissues. Acta Histochem. 2011;113:53–7.
Gu J-G, Zhu C-L, Cheng D-Z, Xie Y, Liu F, Zhou X. Enhanced levels of apolipoprotein M during HBV infection feedback suppresses HBV replication. Lipids Health Dis. 2011;10:154.
Li H, Liu Y, Wang L, Shen T, Du W, Liu Z, et al. High apolipoprotein M serum levels correlate with chronic obstructive pulmonary disease. Lipids Health Dis. 2016;15:59.
Kumaraswamy SB, Linder A, Akesson P, Dahlback B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit Care. 2012;16:R60.
Ma X, Hub YW, Zhaoa ZL, Zheng L, Qiu YR, Huang JL, et al. Anti-inflammatory effects of propofol are mediated by apolipoprotein M in a hepatocyte nuclear factor-1α-dependent manner. Arch Biochem Biophys. 2013;533:1–10.
Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M, et al. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature. 2015;523:342–6.
Wang Z, Luo G, Feng Y, Zheng L, Liu H, Liang Y, et al. Decreased Splenic CD4(+) T-Lymphocytes in Apolipoprotein M Gene Deficient Mice. Biomed Res Int. 2015;2015:293512.
Khovidhunkit W, Memon RA, Feingold KR, Grunfeld C. Infection and inflammation-induced proatherogenic changes of lipoproteins. J Infect Dis. 2000;181:S462–72.
Clifton PM, Mackinnon AM, Barter PJ. Effects of serum amyloid A protein (SAA) on composition, size, and density of high density lipoproteins in subjects with myocardial infarction. J Lipid Res. 1985;26(12):1389–98.
Christensen PM, Liu CH, Swendeman SL, et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 2016;30(6):2351–9.
Galvani S, Sanson M, Blaho VA, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8(389):79.
Alavian SM, Imanieh MH, Imanieh MH. Predictive factors in the incidence of cirrhosis in chronic hepatitis B virus infections. Hepat Mon. 2016;16(5):e34790.
Lyu X, Liu K, Chen Y, Wang Z, Yao J, Cai G, et al. Analysis of risk factors associated with the development of hepatocellular carcinoma in chronic HBV-infected Chinese: a meta-analysis. Int J Environ Res Public Health. 2016;17:604.
Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–91.
Luo L, Pu X, Wang Y, Xu N. Impaired plasma lipid profiles in acute hepatitis. Lipids Health Dis. 2010;9:5.
Weichao J, Lei Z, Qianqian Y. Investigation into the effect of hepatitis B virus on apoliprotein A1 expression and its mechanism. Lipids Health Dis. 2014;13:130.
Yuanyuan W, Junli H, Xiaohong L, et al. The mechanism of apoliprotein A1 down-regulated by Hepatitis B virus. Lipids Health Dis. 2016;15:64.
Tungtrongchitr R, Treeprasertsuk S, Ei NN, Thepouyporn A. Serum leptin concentrations in chronic hepatitis. J Med Assoc Thai. 2006;89(4):490–9.
Xu N, Nilsson-Ehle P, Hurtig M. Both leptin and leptin-receptor are essential for apolipoprotein M expression in vivo. Biochem Biophys Res Commun. 2004;321(4):916–21.
Shieh YS, Chang YS, Hong JR, Chen LJ, Jou LK, Hsu CC, et al. Increase of hepatic fat accumulation by liver specific expression of Hepatitis B virus X protein in zebrafish. Biochim Biophys Acta. 2010;1801(7):721–30.
Na TY, Shin YK, Roh KJ, Kang SA, Hong I, Oh SJ, et al. Liver X receptor mediates hepatitis B virus X protein-induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2009;49:1122–31.
Zhu C, Di D, Zhang X, Luo G, Wang Z, Wei J, et al. TO901317 regulating apolipoprotein M expression mediates via the farnesoid X receptor pathway in Caco-2 cells. Lipids Health Dis. 2011;10:199.
Calayir E, Becker TM, Kratzer A, Ebner B, Panzenböck U, Stefujl J, et al. LXR-agonists regulate ApoM expression differentially in liver and intestine. Curr Pharm Biotechnol. 2008;9:516–21.
Acknowledgements
We thank Ms. Li Hui for her assistance with statistical analyses. We thank Ms. Diaodiao Bian collection patients’ information
Funding
This study was supported by the Science and Technology Innovation Investment Programs of the Development and Reform Commission of Hunan Province [Document no. 658 (2014), the 25th plan of Central South University).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
TS carried out the study design, performed data collection and analysis, and wrote the manuscript; WW, GL analyzed the patients’ information; TS and LT carried out the enzyme-linked immunosorbent and PCR assays; LW participated in statistical analysis of the data; RC, ZYW measured lipid concentrations; MH, YPR provided funding support, conceived the study, participated in its design and coordination, and provided critical revision. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Second Xiangya Hospital Investigational Review Board.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shen, T., Wu, W.M., Du, W.H. et al. Positive association between serum apolipoprotein M levels and hepatitis B virus DNA load in HBeAg-negative chronic hepatitis B. Lipids Health Dis 15, 210 (2016). https://doi.org/10.1186/s12944-016-0384-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-016-0384-3