Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is a major nosocomial pathogen that causes severe morbidity and mortality worldwide. For the establishment of national strategies to combat MRSA infection in each country, accurate and current statistics characterizing the epidemiology of MRSA are essential. The purpose of this study was to determine the prevalence of MRSA among Staphylococcus aureus clinical isolates in Egypt. In addition, we aimed to compare different diagnostic methods for MRSA and determine the pooled resistance rate of linezolid and vancomycin to MRSA. To address this knowledge gap, we conducted a systematic review with meta-analysis.
Methods
A comprehensive literature search from inception to October 2022 of the following databases was performed: MEDLINE [PubMed], Scopus, Google Scholar, and Web of Science. The review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement. Based on the random effects model, results were reported as proportions with a 95% confidence interval (CI). Analyses of the subgroups were conducted. A sensitivity analysis was conducted to test the robustness of the results.
Results
A total of sixty-four (64) studies were included in the present meta-analysis, with a total sample size of 7171 subjects. The overall prevalence of MRSA was 63% [95% CI: 55–70]. Fifteen (15) studies used both PCR and cefoxitin disc diffusion for MRSA detection, with a pooled prevalence rate of 67% [95% CI: 54–79] and 67% [95% CI: 55–80], respectively. While nine (9) studies used both PCR and Oxacillin disc diffusion for MRSA detection, the pooled prevalences were 60% [95% CI: 45–75] and 64% [95% CI: 43–84], respectively. Furthermore, MRSA appeared to be less resistant to linezolid than vancomycin, with a pooled resistance rate of 5% [95% CI: 2–8] to linezolid and 9% [95% CI: 6–12] to vancomycin, respectively.
Conclusion
Our review highlights Egypt's high MRSA prevalence. The cefoxitin disc diffusion test results were found to be consistent with PCR identification of the mecA gene. A prohibition on antibiotic self-medication and efforts to educate healthcare workers and patients about the proper use of antimicrobials may be required to prevent further increases.
Similar content being viewed by others
Background
Staphylococcus aureus “S. aureus” has long been regarded as one of the most important bacteria responsible for a wide range of diseases, from folliculitis and food poisoning to life-threatening conditions such as endocarditis and necrotizing pneumonia. Methicillin-resistant S. aureus (MRSA), which first appeared in the United Kingdom in 1961, is intrinsically resistant to all beta-lactam antibiotics. The β-lactam resistance of MRSA is caused by the production of a novel penicillin-binding protein (PBP) designated (PBP2a), which, has remarkably reduced binding affinities to β-lactam antibiotics [1]. The acquisition of SCCmec (a mobile genetic element that carries the mecA gene that encodes PBP2a) by a methicillin-sensitive S. aureus (MSSA) strain is one of the mechanisms by which MRSA may spread [2].
MRSA is a major nosocomial pathogen that causes severe morbidity and mortality worldwide. MRSA has now become endemic in many healthcare institutions across the world, and as a result, it has become the main focus of international infection control efforts [2]. It is listed as Priority 1 (High) in the 2017 WHO list of bacteria for which new antibiotics are urgently needed [3]. The CDC has also classified MRSA as a serious threat and therefore listed it in the 2019 Antibiotic Resistance Threat Report [4]. Several studies revealed that MRSA infection was significantly associated with an increased total hospital cost, a prolonged length of hospital stay, and a higher hospital mortality rate [5,6,7,8]. Other studies have found that the control of MRSA is likely to be cost-effective, and any compromises in control are likely to be false economies [9, 10]. The World Health Organization's 2014 global report on antibiotic resistance surveillance provides a global picture of MRSA prevalence. Even though detailed antibiotic resistance data were only available for Europe, America, and Australia, MRSA was reported on all continents. The proportion of MRSA in most countries exceeded 20% and, in some cases, reached 80%. The WHO report on Egypt was dependent on a single study with 122 isolates revealing a prevalence of MRSA at 46% [11]. Lee et al. recommend an empirical antibiotic active against MRSA in patients with presumed severe staphylococcal infections in settings where MRSA prevalence is higher than 20% [2]. So it is critical to estimate the prevalence of MRSA.
There are many different laboratory methods, such as the PBP2a latex agglutination test, the cefoxitin MIC, the cefoxitin disc diffusion (CDD), the oxacillin MIC, and the oxacillin disc diffusion (ODD). The detection of the mecA gene using PCR has long been thought to be the gold standard method [12,13,14].
The emergence and worldwide spread of MRSA represent some of the most important events in the epidemiology of infectious diseases. Unfortunately, in Egypt, limited epidemiological surveys of MRSA infections are carried out; only sporadic studies are performed. Despite these several investigations, the pooled prevalence of MRSA among clinical specimens and its susceptibility to vancomycin and linezolid in Egypt remain unknown, so we conducted this systematic review with meta-analysis to overcome the shortcomings of individual research and to fill this knowledge gap. In addition, we aimed to compare different diagnostic methods for MRSA and determine the pooled resistance rate of linezolid and vancomycin to MRSA. Our article contributes to a better understanding of MRSA epidemiology and provides evidence to guide research, policy, infection control strategies, and antimicrobial stewardship in Egypt.
Methods
Search strategy
A comprehensive literature search, from inception to October 2022, of the following databases: MEDLINE [PubMed], Scopus, Google Scholar, and Web of Science was conducted using the following keywords: "Staphylococcus aureus", "S. aureus", "Methicillin-resistant Staphylococcus aureus", "MRSA", and "Egypt". The review was conducted following the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and was registered in PROSPERO with registration number CRD42022346151. The checklist of items to include when reporting a systematic review or meta-analysis is presented in Table S1.
Eligibility criteria
Studies were selected if they fulfilled all of the following criteria: Only primary studies giving statistics on the prevalence, incidence, or proportion of MRSA in Egypt, Clinical specimens collected from patients and studies published in English without time limitation. Studies were excluded if any of the following conditions were met: Studies that were not conducted in Egypt or conducted on Egyptian immigrants, specimens isolated from food, animals, and healthy individuals, studies for which full text was not available, and samples that were partially or totally selected from MRSA culture collections. Case reports, reviews, or conference abstracts were also excluded. Studies were selected based on the aforementioned inclusion and exclusion criteria by two independent authors (F.K.E, M.M). Any disagreement was settled by consensus among all authors.
Data extraction
The data extraction was conducted by three investigators (A.A., H.K., and M.M.) and cross-checked by N.R., M.E., and M.M. From each included study, the following was extracted: the last name of the first author; publication time; region, type of specimen, study period, the total number of S. aureus, number of MRSA, method of detection, and susceptibility to vancomycin and linezolid.
For reports that address MRSA SCCmec genotyping, the number of typeable isolates and their distribution among different SCCmec types were extracted.
Quality assessment
The quality of the included studies was checked by the “Joanna Brigg Critical Appraisal Checklist for Prevalence Studies” by two independent reviewers (N.R. and A.A.) and cross-checked by H.K. and S.E.
Data synthesis
I-squared and Cochran's Q were used to measure the heterogeneity between the studies, and based on the random effects model, results were reported as proportions with a 95% confidence interval (CI). Analyses of the subgroups were conducted based on detection method, sample size, and region. Sensitivity analyses were conducted using the leave-one-out approach to test the robustness of the results. All statistical analyses were performed using Open Meta Analyst (CEBM, University of Oxford, Oxford, UK). Publication bias testing by funnel plot and associated tests was not conducted as they do not produce reliable results for meta-analysis of proportions [15].
Results
Study selection
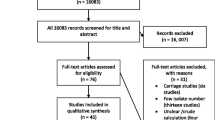
Through database searches, a total of 2264 records were identified. 721 duplicates were removed. The remaining 1543 publications were then evaluated by title and abstract, and 1421 articles were found to be irrelevant and excluded. The remaining 122 articles were evaluated for eligibility by full-text, among which 58 were excluded. And a total of 64 studies fulfilled our inclusion and exclusion criteria and were included in our review (Fig. 1). The characteristics of the included studies and their quality are shown in Tables S2 and 3, respectively.
Pooled MRSA prevalence among clinical isolates in Egypt
The heterogeneity results, total sample size, and pooled proportion of MRSA among all included studies and subgroups are shown in Table 1. A total of 64 studies were included in the present meta-analysis [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83], with a total sample size of 7171 isolates. The overall prevalence of MRSA was 63% [95% CI: 55–70] (Fig. 2), with a high degree of heterogeneity evident by the I-squared test and Cochran's Q test (Table 1). MRSA prevalence was 66% [95% CI: 56–76] and 66% [95% CI: 58–75] in the studies that employed CDD and PCR for MRSA identification, which comprised 34 and 31 investigations, respectively (Figs. 3, 4). However, ODD was employed in 22 studies, with a pooled prevalence of 60% [95% CI: 48–73] (Fig. 5).
Subgroup analysis based on sample size showed that studies with fewer than 50 isolates had higher MRSA prevalence than those between 50 and 100 and those over 100, with pooled MRSA prevalence at 71% [95% CI; 59–83], 69% [95% CI; 59–78], and 55% [95% CI; 45–66] (Figs. S1, S2, and S3), respectively.
MRSA prevalence was reported from only six regions in Egypt, and most of the studies were in the governorates of Cairo and Mansoura, with pooled MRSA prevalences of 67% [95% CI: 54–81] and 59% [95% CI: 40–77] (Figs. S4 and 5), respectively. While the rest of the studies were distributed to Zagazig, Alexandria, Assiut, and Tanta, with pooled MRSA prevalences of 67% [95% CI: 38–95], 61% [95% CI: 47–75], 73% [95% CI: 49–97], and 40% [95% CI: 18–61] (Figs. S6, S7, S8, and S9) respectively.
MRSA prevalence in studies that estimate prevalence by Oxacillin disc compared with PCR or Cefoxitin disc compared with PCR
MRSA prevalence in studies that estimate prevalence by Oxacillin disc compared with PCR or Cefoxitin disc compared with PCR are presented in Table 2. The MRSA prevalence detected by PCR compared with CDD was documented in 15 studies, with a total sample size of 1509 and a pooled resistance rate of 67% [95% CI: 54–79] (Fig. 6) and 67% [95% CI: 55–80] (Fig. 7), respectively. While the MRSA prevalence detected by PCR compared with ODD was documented in 9 studies with a total sample size of 868 and a pooled resistance rate of 60% [95% CI: 45–75] (Fig. 8) and 64% [95% CI: 43–84] (Fig. 9), respectively.
Pooled resistance rate of MRSA clinical isolates to vancomycin and linezolid
The pooled resistance rate of clinically isolated MRSA to vancomycin and linezolid was documented in 21 and 11 studies, with total sample sizes of 1371 and 745, respectively (Table 3). MRSA appeared to be less resistant to linezolid than vancomycin, with a pooled resistance rate of 5% [95% CI: 2 –8] to linezolid and 9% [95% CI: 6 –12] to vancomycin (Figs. 10 and 11), respectively.
Distribution of staphylococcal cassette chromosome mec (SCCmec) types
Only six studies discussed the distribution of SCCmec types in MRSA typeable isolates (Table 4). Three studies: Sobhy et al. [26], El-Baz et al. [19], and Sheneef et al. [55] reported that the MRSA isolates mostly belonged to SCCmec type V (75%, 60%, and 61.5%, respectively). Type Iva, II, and I were the most often reported by Kishk et al. [57], Youssef et al. [52], and Zawahry et al. [76] (63.6%, 56%, and 72%, respectively).
y
Sensitivity analysis
Sensitivity analysis using the leave-one-out approach indicated the combined estimates of overall MRSA prevalence are reliable and are not dependent on any one study; see supplementary file Fig. S10.
For linezolid resistance among MRSA, the absence of Mashaly et al. [56] reduces the overall linezolid resistance by about 2%, i.e., 3% [95% CI: 1–6]. While the absence of Sultan et al. [29] increases the overall linezolid resistance by about 2%, i.e., 7% [95% CI: 3–10] (Fig. S11). For vancomycin resistance among MRSA, the omission of Ibrahim et al. [60] reduces vancomycin resistance by about 2%, i.e., 7% [95% CI: 5–10] (Fig. S12).
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis that highlights the increase in MRSA prevalence in Egypt. According to the current review, the overall prevalence of clinically isolated MRSA in Egypt was 63%, with a pooled resistance rate to vancomycin and linezolid of 9% and 5%, respectively. According to the current review, MRSA prevalence in Egypt is higher than a similar meta-analysis conducted in Iran, which estimated a prevalence of 52.7% among MRSA clinical isolates [48]. Several factors may explain the high MRSA prevalence in Egypt. First, infection control programs are not adequate. Workload, inadequate resources, limited opportunities for infection control training, and insufficient staff were the most common obstacles complained about by healthcare workers against the practice of standard precautions [49, 80]. Second, the inappropriate use of antibiotics and antibiotic self-medication are prevalent in Egypt [81, 84].
Stratified analyses with regard to geographic areas revealed that MRSA prevalence was reported from only six regions in Egypt, and most of the studies were in the governorates of Cairo and Mansoura, with pooled MRSA prevalences of 67% and 59%, respectively. While the rest of the studies were distributed to Zagazig, Alexandria, Assiut, and Tanta, with pooled MRSA prevalences of 67%, 61%, 73%, and 40%, respectively. There may be discrepancies in workloads, resources, and insufficient staff in different regions that could contribute to this variation in MRSA prevalence. Based on our findings, no location had an MRSA frequency of less than 20%, we recommend empirical antibiotics for MRSA coverage if S. aureus infection is suspected. Unless otherwise indicated by the hospital's antibiogram and clinical judgment.
The subgroup analysis based on sample size revealed that studies with sample sizes smaller than 50 isolates had a higher MRSA prevalence than studies with sample sizes between 50 and 99 or above 100 (71%, 69%, and 55%, respectively), which may indicate a bias in smaller sample sizes and emphasize the importance of determining sample sizes based on prespecified and justified calculations.
S. aureus genotyping methods have been developed to study the strain origin, clonal relatedness, and epidemiology of the infection. One of these genotypic methods is SCCmec typing, which could discriminate between hospital-acquired MRSA (HA-MRSA) strains and community-acquired MRSA (CA-MRSA) strains as types I, II, and III occur in HA-MRSA strains while types IV and V occur in CA-MRSA strains [85]. Four out of six studies reported that isolates that harbored IV and V SCCmec types predominated and met the definition of CA-MRSA based on SCCmec types [19, 26, 55, 57]. While two studies reported that isolates that harbored I, II, and III SCCmec types predominated and met the definition of HA-MRSA [52, 76].
The cefoxitin disc diffusion test results were found to be consistent with PCR identification of the mecA gene, similar to previous studies [12, 86,87,88,89]. Both CDD and PCR were at the same point of estimate (66%) of MRSA prevalence in the studies that used CDD and/or PCR for MRSA identification, 34 and 31 studies, respectively. Similarly, in the fifteen studies that co-reported the MRSA prevalence by PCR compared with CDD, it was revealed that both were also at the same estimate (67%).Thus, the CDD test may be an alternative to PCR for the detection of MRSA in resource-constrained settings. In nine studies that used PCR and ODD, the MRSA prevalence rates were 60% and 64%, respectively. This may indicate that the ODD method can be associated with false-positive results. Other studies also reported that the ODD method can be associated with false-positive MRSA [90,91,92].
According to the current review, the pooled resistance rate to vancomycin and linezolid against MRSA was estimated to be 9% and 5%, respectively, which was higher than those reported by the LEADER and ZAAPS programs. The LEADER surveillance programs, which were set up to monitor linezolid resistance in the USA, revealed 0.1% and 0% of linezolid and vancomycin resistance among oxacillin-resistant S. aureus, respectively [93]. On the other hand, the ZAAPS program, which was set up to monitor linezolid resistance worldwide (in non-USA countries), revealed that none of the MRSA isolates were resistant to linezolid [94].
The following measures may be needed to limit further increases in MRSA: First, a national antimicrobial resistance policy is needed in Egypt to understand the emergence, spread, and factors influencing antimicrobial resistance. Second, a prohibition on antibiotic self-medication.Third, efforts to educate healthcare workers and patients about the proper use of antimicrobials. Fourth, rapid molecular diagnostics to support appropriate antimicrobial use. Fifth, antimicrobial stewardship practices should be followed. In addition, more research is required to define the genotypic characteristics of the MRSA strain.
Study limitations
There are some limitations to our study. First, our results do not fully reflect the prevalence of MRSA in Egypt, as not all regions in Egypt reported the prevalence of MRSA. Second, there was a high degree of heterogeneity among the included studies. Third, the paucity of studies that discriminate between hospital- and community-acquired MRSA. However, our review provides crucial data on the prevalence of MRSA in Egypt and its pooled susceptibility to linezolid and vancomycin that may help to decrease or prevent further increases.
Conclusion
Our findings indicate that MRSA is prevalent in Egypt, with higher pooled resistance to vancomycin and linezolid, and that the cefoxitin disc diffusion test results were consistent with PCR identification of the mecA gene. Thus, the test may be an alternative to PCR for the detection of MRSA. A national antimicrobial resistance policy in Egypt to understand the emergence, spread, and factors influencing antimicrobial resistance may be needed. In addition, a prohibition on antibiotic self-medication and efforts to educate healthcare workers and patients about the proper use of antimicrobials may be required to prevent further increases.
Availability of data and material
All data generated or analyzed during this study are included in this published article [and its supplementary information file].
Abbreviations
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CI:
-
Confidence interval
- PBP:
-
Penicillin-binding protein
- SCCmec:
-
Staphylococcal chromosome cassette mec
- CDD:
-
Cefoxitin disc diffusion
- ODD:
-
Oxacillin disc diffusion
References
Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158(2):513–6. https://doi.org/10.1128/JB.158.2.513-516.1984.
Lee AS, De Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, Harbarth S. Methicillin-resistant staphylococcus aureus. Nat Rev Dis Prim. 2018;4(1):1–23.
WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Saudi Med J. Published 2017. Accessed December 26, 2022. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
CDC. 2019 Antibiotic Resistance Threats Report | CDC. Center for Disease Control. Published 2021. Accessed December 26, 2022. https://www.cdc.gov/drugresistance/Biggest-Threats.html.
Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–9. https://doi.org/10.1086/345476/2/36-1-53-FIG002.GIF.
Zhen X, Lundborg CS, Zhang M, Sun X, Li Y, Hu X, Gu S, Gu Y, Wei J, Dong H. Clinical and economic impact of methicillin-resistant staphylococcus aureus: a multicentre study in China. Sci Rep. 2020;10(1):3900.
Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant staphylococcus aureus. Arch Intern Med. 2002;162(19):2229–35. https://doi.org/10.1001/ARCHINTE.162.19.2229.
Nelson RE, Jones M, Liu CF, Samore MH, Evans ME, Graves N, Lee B, Rubin MA. The impact of healthcare-associated methicillin-resistant staphylococcus aureus infections on post-discharge healthcare costs and utilization. Infect Control Hosp Epidemiol. 2015;36(5):534–42. https://doi.org/10.1017/ICE.2015.22.
Souverein D, Houtman P, Euser SM, Herpers BL, Kluytmans J, Den Boer JW. Costs and benefits associated with the MRSA search and destroy policy in a hospital in the Region Kennemerland, The Netherlands. PLoS One. 2016;11(2):e0148175.
Gould IM, Reilly J, Bunyan D, Walker A. Costs of healthcare-associated methicillin-resistant staphylococcus aureus and its control. Clin Microbiol Infect. 2010;16(12):1721–8. https://doi.org/10.1111/J.1469-0691.2010.03365.X.
WHO. Antimicrobial resistance. Global report on surveillance. World Health Organization.
Anand K, Agrawal P, Kumar S, Kapila K. Comparison of cefoxitin disc diffusion test, oxacillin screen agar, and PCR FOR mecA gene for detection of MRSA. Indian J Med Microbiol. 2009;27(1):27–9. https://doi.org/10.1016/S0255-0857(21)01748-5.
Nair D, Shashindran N, Kumar A, Vinodh V, Biswas L, Biswas R. Comparison of phenotypic MRSA detection methods with PCR for mecA gene in the Background of emergence of oxacillin-susceptible MRSA. Microb Drug Resist. 2021;27(9):1190–4.
Performance Standards for Antimicrobial Susceptibility Testing. M100Ed32 | Performance Standards for Antimicrobial Susceptibility Testing, 32nd Edition. Accessed December 26, 2022. https://clsi.org/standards/products/microbiology/documents/m100/.
Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897–903. https://doi.org/10.1016/J.JCLINEPI.2014.03.003.
Kadry AA, Shaker GH, El-Ganiny AM, Youssef CRB. Phenotypic and genotypic detection of local MRSA isolates. Zagazig J Pharm Sci. 2016;25(1):39–46. https://doi.org/10.21608/ZJPS.2016.38164.
Abdel-Mongy M, Awad T, Mosaed F. Vancomycin resistance among methicillin resistant staphylococcus aureus isolates from neonatal sepsis attending intensive care unit in Shibin El-Kom teaching hospital. Egypt J PurE Appl Microbiol. 2018;12(3):1093–100. https://doi.org/10.22207/JPaM.12.3.07.
Abdelaziz SM, Aboshanab KM, Yassien M, Hassouna NA. Antimicrobial resistance patterns of MDR Staphylococcus aureus clinical isolates involved in the lower respiratory tract infections in Egypt. Arch Pharm Sci Ain Shams Univ. 2019;3(2):294–304. https://doi.org/10.21608/APS.2019.17391.1014.
El-baz R, Rizk DE, Barwa R, Hassan R. Virulence characteristics and molecular relatedness of methicillin resistant Staphylococcus aureus harboring different staphylococcal cassette chromosome mec. Microb Pathog. 2017;113:385–95. https://doi.org/10.1016/J.MICPATH.2017.11.021.
Ali GH, Seiffein NL. Association of some virulence genes in methicillin resistant and methicillin sensitive Staphylococcus aureus infections isolated in community with special emphasis on pvl/mecA genes profiles in Alexandria. Egypt Gene Reports. 2021;25:101334.
Mohamed MM, Bialy AAA, Ahmed AA. Methicillin-resistant Staphylococcus aureus: a challenge for infection control. Menoufia Med J. 2016;29(4):812. https://doi.org/10.4103/1110-2098.202519.
Omar NY, Ali HAS, Harfoush RAH, El Khayat EH. Molecular typing of methicillin resistant staphylococcus aureus clinical isolates on the basis of protein a and coagulase gene polymorphisms. Int J Microbiol. 2014;2014:650328.
Shebl HR, Zaki WK, Saleh AN, Salam SA. Prevalence of MecC gene among methicillin resistant staphylococcus aureus isolated from patients in ain-shams University hospital. J Pure Appl Microbiol. 2020;14(4):2807–13.
Hassan R, Barwa R, El-Sokkary MM, Ashraf D. Virulence characteristics of methicillin resistant Staphylococcus aureus isolated from different clinical sources. J Microbiol N Egypt J Microbiol. 2017;48:14–29.
Samy SM, Tkeli FA, Eisa EA, Amin AM, Dawood HH, Hussein MZ. Genotyping of nosocomial methicillin resistant staphylococcus aureus with tracing the source of infection : a guideline step in infection control strategy at general surgery department of Tanta University Hospital. Egypt J Med Microbiol. 2018;27(4):27–35. https://doi.org/10.12816/0053809.
Sobhy N, Aly F, El Kader OA, Ghazal A, Elbaradei A. Community-acquired methicillin-resistant Staphylococcus aureus from skin and soft tissue infections (in a sample of Egyptian population): analysis of mec gene and staphylococcal cassette chromosome. Brazilian J Infect Dis. 2012;16(5):426–31. https://doi.org/10.1016/J.BJID.2012.08.004.
El Karamany IM, Ibrahim YM, Abouwarda AM, Essam M, Amin MA. Detection of high levels of methicillin and multi-drug resistance among clinical isolates of Staphylococcus aureus. African J Microbiol Res. 2013;7(16):1598–604.
El-Essawy A, Roshdy R, Abu Shady H, Emara A. Prevalence of panton valentine leukocidin gene in methicillin resistant and sensitive staphylococcus aureus, isolated from Egyptian hospitals. Egypt J Bot. 2015;55(1):31–44. https://doi.org/10.21608/EJBO.2015.223.
Sultan AM, Nabiel Y. Association of tsst-1 and pvl with mecA genes among clinical staphylococcus aureus isolates from a Tertiary Care hospital. J Pure Appl Microbiol. 2019;13(2):855–64. https://doi.org/10.22207/JPAM.13.2.21.
El-Bouseary MM, El-Banna TE, Sonbol FI. Prevalence of MRSA among staphylococcus aureus isolates recovered from patients with otitis media. Nat Sci. 2018;16(6):48–55. https://doi.org/10.7537/marsnsj160618.08.
Askar H, Askar H, Badawy W, Hammad E. Aminoglycoside and chlorhexidine resistance genes in Staphylococcus aureus isolated from surgical wound infections. Int Arab J Antimicrob Agents. 2016;6(1):4. https://doi.org/10.3823/784.
Sadaka M, El-Ghazzawy F, Harfoush R, Meheissen M. Evaluation of different methods for the rapid diagnosis of methicillin-resistance in staphylococcus aureus. African J Microbiol Res. 2009;3(2):55.
Metwally L, Gomaa N, Hassan R. Detection of methicillin-resistant staphylococcus aureus directly by loop-mediated isothermal amplification and direct cefoxitin disk diffusion tests. East Mediterr Heal J. 2014;20(04):273–9. https://doi.org/10.26719/2014.20.4.273.
Wali I, Ouda N, El-Seidi E. Mupirocin resistanceamong methicillin resistant staphylococcus aureus isolates in an Egyptian hosptial. Egypt J Lab Med. 2011;2(20):81–92.
Hefzy EM, Hassan GM. Rapid molecular identification of hospital-acquired methicillin resistant staphylococcus aureus (HA-MRSA) lineages. Egypt J Med Microbiol. 2016;25(4):91–7. https://doi.org/10.12816/0037025.
Zaki M, Galeb S, Eid AR, Ahmed D, Mabrouk A, Latif RA. Molecular characterization of Staphylococcus aureus isolated from hospital acquired sepsis in pediatrics, relation to antibiotics, resistance and virulence genes. Germs. 2020;10(4):295. https://doi.org/10.18683/GERMS.2020.1221.
Alfeky AAE, Tawfick MM, Ashour MS, El-Moghazy ANA. High prevalence of multi-drug resistant methicillin-resistant staphylococcus aureus in tertiary Egyptian Hospitals. J Infect Dev Ctries. 2022;16(05):795–806. https://doi.org/10.3855/jidc.15833.
Zaki WK, Hager R. Detection of methicillin resistant Staphylococcus aureus, vancomycin intermediate susceptibility and vancomycin resistance among Staphylococcus aureus isolated from tertiary care hospital. QJM An Int J Med. 2018;111(suppl_1).
Saied T, Elkholy A, Hafez SF, Basim H, Wasfy MO, El-Shoubary W, Samir A, Pimentel G, Talaat M. Antimicrobial resistance in pathogens causing nosocomial bloodstream infections in university hospitals in Egypt. Am J Infect Control. 2011;39(9):e61-65. https://doi.org/10.1016/J.AJIC.2011.04.009.
El-Sweify MA, Raheel AS, Abu-Ata HN, El-Hadidy GS, Hessam WF. Identification of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) causing hospital-acquired infections in Suez Canal University Hospitals, Egypt by detection of its major virulence determinants. Microbes Infect Dis. 2021;2(4):715–24. https://doi.org/10.21608/MID.2020.41062.1057.
Elsayed N, Ashour M, Amine AEK. Vancomycin resistance among Staphylococcus aureus isolates in a rural setting. Egypt Germs. 2018;8(3):134. https://doi.org/10.18683/GERMS.2018.1140.
Barakat GI, Nabil YM. Correlation of mupirocin resistance with biofilm production in methicillin-resistant Staphylococcus aureus from surgical site infections in a tertiary centre. Egypt J Glob Antimicrob Resist. 2016;4:16–20. https://doi.org/10.1016/J.JGAR.2015.11.010.
Bendary MM, Solyman SM, Azab MM, Mahmoud NF, Hanora AM. Characterization of methicillin resistant staphylococcus aureus isolated from human and animal samples in Egypt. Cell Mol Biol (Noisy-le-grand). 2016;62(2):94–100. https://doi.org/10.14715/cmb/2016.62.2.16.
Hashem RA, Yassin AS, Zedan HH, Amin MA. Fluoroquinolone resistant mechanisms in methicillin-resistant Staphylococcus aureus clinical isolates in Cairo. Egypt J Infect Dev Ctries. 2013;7(11):796–803. https://doi.org/10.3855/jidc.3105.
Ahmed EF, Gad GFM, Abdalla AM, Hasaneen AM, Abdelwahab SF. Prevalence of methicillin resistant staphylococcus aureus among Egyptian patients after surgical interventions. Surg Infect. 2014;15(4):404–11. https://doi.org/10.1089/SUR.2013.212.
Sonbol FI, Abdelaziz AA, El-banna TES, Farag O. Detection and characterization of staphylococcus aureus and methicillin-resistant S. aureus (MRSA) in ear infections in Tanta, Egypt. J Adv Med Pharm Res. 2022;3(2):36–44.
Elbargisy RM. Distribution of leukocidins, exfoliative toxins, and selected resistance genes among methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strains in Egypt. Open Microbiol J. 2022;16(1).
Askari E, Soleymani F, Arianpoor A, Tabatabai SM, Amini A, Nasab MN. Epidemiology of mecA-methicillin resistant staphylococcus aureus (MRSA) in Iran: a systematic review and meta-analysis. Iran J Basic Med Sci. 2012;15(5):1010.
Refeai SA, Kamal NN, Ghazawy ERA, Fekry CM. Perception and barriers regarding infection control measures among healthcare workers in Minia City, Egypt. Int J Prev Med. 2020;11:11.
El-Baz AM, Yahya G, Mansour B, El-Sokkary MMA, Alshaman R, Alattar A, El-Ganiny AM. The link between occurrence of class I integron and acquired aminoglycoside resistance in clinical MRSA isolates. Antibiot. 2021;10(5):488.
Shady AH, El-Essawy A, Salama M, El-Ayesh A. Detection and molecular characterization of vancomycin resistant staphylococcus aureus from clinical isolates. African J Biotechnol. 2016;11(99):16494–503. https://doi.org/10.4314/ajb.v11i99.
Youssef CRB, Kadry AA, El-Ganiny AM. Investigating the relation between resistance pattern and type of Staphylococcal cassette chromosome mec (SCCmec) in methicillin-resistant Staphylococcus aureus. Iran J Microbiol. 2022;14(1):56–66. https://doi.org/10.18502/ijm.v14i1.8802.
El-Jakee JK, Atta NS, Samy AA, Bakry MA, Elgabry EA, Kandil MM, Gad El-Said WA. Antimicrobial resistance in clinical isolates of staphylococcus aureus from bovine and human sources in Egypt. Glob Vet. 2011;7(6):581–6.
EL-gayar MH, Aboulwafa MM, Aboshanab KM, Haleem Hassouna NA. Virulence characters of some methicillin resistant staphylococcus aureus Isolates. Arch Clin Microbiol. 2014;5((4)):0–0.
Sheneef A, Goda AM, Ftohy TE, Ezz R, El-Sharkawy ED, Ibrahim M. Staphylococcal cassette chromosome mec typing of community-acquired methicillin-resistant staphylococcus aureus isolates in sohag CA-MRSA, SSTIs, PVL, SCCmec *Corresponding Author. Egypt J Med Microbiol. 2017;26(2):111–7.
Mashaly M, El-Mashad N, El-deeb H. Detection of VanA type vancomycin resistance among MRSA isolates from an emergency hospital in Egypt. Comp Clin Path. 2019;28(4):971–6. https://doi.org/10.1007/S00580-018-2858-3/TABLES/1.
Kishk RM, Mandour MF, Saleh RM. Staphylococcal Cassette Chromosome mec (SCCmec) gene typing in detection of methicillin-resistant staphylococcus aureus: toward precise detection in health care facility. Open J Med Microbiol. 2019;09(03):127–37. https://doi.org/10.4236/OJMM.2019.93013.
Hassan RH, Eldegla H, Elmorsy F, Eldars WM. Clinical and microbiological characteristics of healthcare-associated infections in a tertiary care pediatric hospital. Egypt Pediatr Assoc Gaz. 2017;65(4):127–31. https://doi.org/10.1016/J.EPAG.2017.09.001.
Hashem NM, Hosny AEDMS, Abdelrahman AA, Zakeer S. Antimicrobial activities encountered by sulfur nanoparticles combating Staphylococcal species harboring sccmecA recovered from acne vulgaris. AIMS Microbiol 2021;7(4):48.
Ibrahim ESH, El-Baghdady K, Abd El-All SM, Warda MAA, Prince AM, Ibrahim M. Prevalence of multidrug resistance in the Egyptian methicillin-resistant staphylococcus aureus isolates. African J Biol Sci. 2020;16(1):43–52. https://doi.org/10.21608/AJBS.2020.80481.
Hafez EE, Al Sohaimy SA, El Saadani MA. The effect of the mecA gene and its mutant form on the response of S. aureus to the most common antibiotics. Int J Immunol Stud. 2009;1(1):106.
Mostafa SI. Molecular typing of methicilin resistant Staphylococcus aureus by spa gene polymorphism. African J Microbiol Res. 2013;7(9):755–9. https://doi.org/10.5897/AJMR12.1430.
Sleem AS, Ajlan SE, Zaher EM, Elmahdy E. Phenotypic and genotypic detection of antimicrobial resistance and virulence factors among staphylococcus aureus clinical isolates. Microbes Infect Dis. 2022;3(4):910–9. https://doi.org/10.21608/MID.2022.155274.1366.
Ali A, Sayed N, Hassan R. Study of vancomycin susceptibility pattern among Staphylococcus aureus isolated from superficial incisional surgical site infections. Microbes Infect Dis. 2022. https://doi.org/10.21608/mid.2022.115351.1232.
Alzahraa Hareidy FR, Azmy AF, Kamel NM, Moawad AS, Omran ME, Alzahraa HF. Egyptian Bee (Apis Mellifera) propolis: a promising antibacterial agent for combating antibiotic resistance and biofilm formation of multidrug-resistant staphylococcus aureus. Azhar Int J Pharm Med Sci. 2022;2(1):30–47. https://doi.org/10.21608/AIJPMS.2021.69608.1058.
Ahmed SH, Ahmed AS, Mohamed WA, Elfeky MA, Deaf EA, Badary MS, Hetta HF. Nosocomial vancomycin and methicillin resistant staphylococcal infections in intensive care units in Assiut University Hospitals. Egypt J Med Microbiol. 2011;20(2):127–40.
Fahim NAE. Prevalence and antimicrobial susceptibility profile of multidrug-resistant bacteria among intensive care units patients at Ain Shams University Hospitals in Egypt—a retrospective study. J Egypt Public Health Assoc. 2021;96(1):1–10. https://doi.org/10.1186/s42506-020-00065-8.
El Gemezy E, Serry F, Kadry A. Antimicrobial susceptibility of Staphylococcus aureus clinical isolates and prevalence of MRSA in ICUs of Mansoura University Hospitals. Zagazig J Pharm Sci. 2016;25(2):93–7.
Taher S, Roshdy H. Prevalence of panton-valantine leucocidin genes among staphylococcus aureus isolates in Mansoura University Hospitals. EJMM-Egyptian J Med Microbiol. 2009;18(4):97–108.
Abdelraheem WM, Khairy RMM, Zaki AI, Zaki SH. Effect of ZnO nanoparticles on methicillin, vancomycin, linezolid resistance and biofilm formation in Staphylococcus aureus isolates. Ann Clin Microbiol Antimicrob. 2021;20(1):1–11. https://doi.org/10.1186/S12941-021-00459-2/TABLES/3.
Taha AE, Badr MF, El-Morsy FE, Hammad E, Susceptibility A. Methicillin-resistant staphylococcus aureus in an Egyptian University Hospital. J Pure Appl Microbiol. 2019;13(4):2111–22. https://doi.org/10.22207/JPAM.13.4.23.
Elkhyat AH, Makled AF, Albeltagy AM, Keshk TF, Dawoud AM, Hamed A, El-Soud A. Prevalence of vanA gene among methicillin resistant S. aureus strains isolated from burn wound infections in Menoufia University Hospitals. Egypt J Med Microbiol. 2020;29(3):97–104.
Rashwan NM, Daif EA, AbdulMoez FA, Afifi NA, Ghandour AM. Screening of nosocomial methicillin-resistant staphylococcus aureus [MRSA] in the intensive care units of Assiut university hospital. Egyptian J Med Microbiol. 2006;15(4):797–805.
Salem-Bekhit MM. Phenotypic and genotypic characterization of nosocomial isolates of staphylococcus aureus with reference to methicillin resistance. Trop J Pharm Res. 2014;13(8):1239–46. https://doi.org/10.4314/tjpr.v13i8.7.
Rizk N, Zaki SA. Heterogeneous vancomycin intermediate resistance within methicillin-resistant Staphylococcus aureus clinical isolates in Alexandria province. Egypt Int J Antimicrob Agents. 2007;2(29):S519-20.
Al Zawahry YA, Abdel-Shafi S, Zaki M, El-Serwy H. Phenotypic and genotypic investigation of methicillin resistant staphylococci species isolated from children with sepsis in Egypt. Egypt J Bot. 2018;58(1):11–22. https://doi.org/10.21608/EJBO.2017.1506.1118.
Ahmed SH, Tolba STM, El-Zawahry YA. Evaluation of the role of bla genes in beta lactam and methicillin resistant staphylococcus aureus. Egypt J Bot. 2019;59(1):29–38. https://doi.org/10.21608/EJBO.2018.4221.1187.
Shrief R, El Kholy RM, Rizk MA, Zaki MES. Prevalence of tetracycline resistant genes in staphylococcus aureusisolates from surgical site infections Egypt. Biosci Biotechnol Res Asia. 2019;16(2):221–8. https://doi.org/10.13005/BBRA/2739.
Fikry A, Abd ER, Thabet A, Samir A, Abo El-Yazeed H, El-Amry KF, Deif HN. Bacteriological and molecular comparative study between staphylococcus aureus isolated from animals and human. J Appl Vet Sci. 2021;6(2):50–8. https://doi.org/10.21608/JAVS.2021.159379.
Salem MR, Youssef MRL. Health care providers’ perspectives for providing quality infection control measures at the neonatal intensive care unit, Cairo University Hospital. Am J Infect Control. 2017;45(9):e99-102. https://doi.org/10.1016/J.AJIC.2017.03.013.
El-Hawy RM, Ashmawy MI, Kamal MM, Khamis HA, Abo El-Hamed NM, Eladely GI, Abdo MH, Hashem Y, Ramadan M, Hamdy DA. Studying the knowledge, attitude and practice of antibiotic misuse among Alexandria population. Eur J Hosp Pharm. 2017;24(6):349–54. https://doi.org/10.1136/EJHPHARM-2016-001032.
Bassyouni RH, Dwedar RA, Farahat MG, Kamel Z, Elwekel MA, Alves D, Mario N. Protective effect of hamamelitannin against biofilm production by methicillin-resistant staphylococci isolated from blood of patients at intensive care units. Microbiol Res J Int. 2015;10(5):1–8. https://doi.org/10.9734/BMRJ/2015/15477.
Abdelmawgoud YE, El-Latif WA, Fawzy NK, Elnagdy SM. Prevalence of inducible clindamycin resistance and nanotechnological control of staphylococcus aureus clinical isolates. Egypt J Bot. 2022;62(1):73–84. https://doi.org/10.21608/EJBO.2021.47561.1576.
Elsayed AA, Darwish SF, Zewail MB, Mohammed M, Saeed H, Rabea H. Antibiotic misuse and compliance with infection control measures during COVID-19 pandemic in community pharmacies in Egypt. Int J Clin Pract. 2021;75(6):e14081.
David MZ, Daum RS. Community-associated methicillin-resistant staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616. https://doi.org/10.1128/CMR.00081-09.
Soodabeh R, Mojtaba M, Saeed S, Maryam T, Zahra F. Comparison of mecA gene-based PCR with CLSI cefoxitin and oxacillin disc diffusion methods for detecting methicillin resistance in Staphylococcus aureus clinical isolates. African J Microbiol Res. 2013;7(21):2438–41. https://doi.org/10.5897/ajmr2013.2525.
Mathews AA, Thomas M, Appalaraju B, Jayalakshmi J. Evaluation and comparison of tests to detect methicillin resistant S. aureus. Indian J Pathol Microbiol. 2010;53(1):79–82.
Pourmand MR, Hassanzadeh S, Mashhadi R, Askari E. Comparison of four diagnostic methods for detection of methicillin resistant Staphylococcus aureus. Iran J Microbiol. 2014;6(5):341–4.
Koupahi H, Jahromy SH, Rahbar M. Evaluation of different phenotypic and genotypic methods for detection of methicillin resistant staphylococcus aureus (MRSA). Iran J Pathol. 2016;11(4):370.
Torimiro N, Torimiro SEA. Antibiotic resistance profile in community-associated staphylococcus aureus strains isolated from a Nigerian peri-urban community. African J Biotechnol. 2016;11(94):16071–6. https://doi.org/10.4314/ajb.v11i94.
Pillai MM, Latha R, Sarkar G. Detection of methicillin resistance in staphylococcus aureus by polymerase chain reaction and conventional methods: a comparative study. J Lab Physicians. 2012;4(02):083–8. https://doi.org/10.4103/0974-2727.105587.
Pournajaf A, Ardebili A, Goudarzi L, Khodabandeh M, Narimani T, Abbaszadeh H. PCR-based identification of methicillin–resistant Staphylococcus aureus strains and their antibiotic resistance profiles. Asian Pac J Trop Biomed. 2014;4:S293–7. https://doi.org/10.12980/APJTB.4.2014C423.
Flamm RK, Mendes RE, Hogan PA, Streit JM, Ross JE, Jonesa RN. Linezolid surveillance results for the United States (LEADER surveillance program 2014). Antimicrob Agents Chemother. 2016;60(4):2273–80. https://doi.org/10.1128/AAC.02803-15.
Mendes RE, Hogan PA, Jones RN, Sader HS, Flamm RK. Surveillance for linezolid resistance via the Zyvoxw Annual Appraisal of Potency and Spectrum (ZAAPS) programme (2014): Evolving resistance mechanisms with stable susceptibility rates. J Antimicrob Chemother. 2016;71(7):1860–5. https://doi.org/10.1093/jac/dkw052.
Acknowledgements
None
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ahmed Azzam designed and planned this investigation. The retrieval and screening of studies were handled by Fatma Khaled Elagezy and May Mohsen and cross-checked by all the other authors. Ahmed Azzam, Heba Khaled, and May Mohsen were in charge of the data collection and analysis, which was cross-checked by Neveen Refaey, Mohammed AlSaifi, and Maha Mosa. All authors contributed to the data interpretation and manuscript writing. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Azzam, A., Khaled, H., Mosa, M. et al. Epidemiology of clinically isolated methicillin-resistant Staphylococcus aureus (MRSA) and its susceptibility to linezolid and vancomycin in Egypt: a systematic review with meta-analysis. BMC Infect Dis 23, 263 (2023). https://doi.org/10.1186/s12879-023-08202-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08202-2