Abstract
Thermally degraded engine oil and hydraulic fluid fumes contaminating aircraft cabin air conditioning systems have been well documented since the 1950s. Whilst organophosphates have been the main subject of interest, oil and hydraulic fumes in the air supply also contain ultrafine particles, numerous volatile organic hydrocarbons and thermally degraded products. We review the literature on the effects of fume events on aircrew health. Inhalation of these potentially toxic fumes is increasingly recognised to cause acute and long-term neurological, respiratory, cardiological and other symptoms. Cumulative exposure to regular small doses of toxic fumes is potentially damaging to health and may be exacerbated by a single higher-level exposure. Assessment is complex because of the limitations of considering the toxicity of individual substances in complex heated mixtures.
There is a need for a systematic and consistent approach to diagnosis and treatment of persons who have been exposed to toxic fumes in aircraft cabins. The medical protocol presented in this paper has been written by internationally recognised experts and presents a consensus approach to the recognition, investigation and management of persons suffering from the toxic effects of inhaling thermally degraded engine oil and other fluids contaminating the air conditioning systems in aircraft, and includes actions and investigations for in-flight, immediately post-flight and late subsequent follow up.
Similar content being viewed by others
Background
All modern commercial jet transport aircraft, except for the Boeing 787 Dreamliner, use air compressed within the engine or auxiliary power unit (a smaller engine primarily used during ground operations, APU) as the source of the air used for aircraft ventilation and pressurisation. This air is known as ‘bleed air’ because it is bled off the compression section of the engine or APU. The engine and APU supply air is not filtered. The use of oil bearing seals reliant on pressurised air enables low levels of synthetic engine oils to enter the compressor air during normal engine operation either as background leakage or during transient power or air supply changes [1-8]. In addition, engine oil smoke or fumes can be generated in the engine or APU and contaminate the compressor bleed air supply, due to failure conditions such as failed bearings/seals, improper drainage of oil into the compressor or oil reservoir over-servicing. Hydraulic fluid leaks due to an over-serviced reservoir or ruptured line may also be ingested into the APU or the engine compressor [7]. The hydraulic system fluid reservoir vent is connected to the bleed air system, therefore enabling hydraulic aerosols to enter the cabin air [9]. Bleed air contaminants may include a range of toxic substances, various organophosphates (OP), amines, a complex mixture of thermally degraded components, volatile organic compounds (VOC) including aldehydes and solvents, carbon monoxide, ultrafine particles and de-icing fluids.
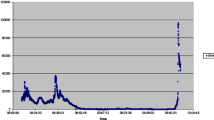
Contaminated aircraft cabin air incidents, commonly identified as ‘fume events’, were first described in military aircraft in the 1950s [10-12]. The onset of fume events coincided with the introduction of synthetic jet engine oils, used in high performance turbine engines [13]. Since then, reports describing aircrew with acute symptoms, followed by chronic neurological, cardiological, respiratory symptoms and other health impacts, correlating with fume events have been published [14-34]. The term Aerotoxic Syndrome associated with exposure to air supply contaminants was first published in 2000 [35], although it is not widely understood by health care professionals. The aviation industry recognises that some people experience acute symptoms following a fume event, although debate continues as to whether exposure to contaminated cabin air can cause long-term symptoms [36-42]. A striking feature of the outcome of fume events is the difference in response observed between passengers and aircrew. While few passengers appear to suffer more than symptoms of irritation following a ‘fume event’, aircrew frequently become systemically unwell and need medical attention. Figure 1 shows this graphically from data collected from publicly available sources. This differential response suggests that the pre-exposure to thousands of hours of low-dose inhalation of engine bleed air increases the vulnerability of aircrew to acute higher dosage during a fume event.
There are no sensors on board transport aircraft and therefore no contaminants are collected at time of exposure. Cabin air monitoring studies have identified the presence of low concentrations of individual bleed air contaminants (e.g. tricresyl phosphates, carbon monoxide, formaldehyde, toluene, benzene) that are well below published chemical exposure limits during “normal” (non-incident) flights [7, 9, 38]. However, few measurements have been undertaken during documented fume events, ground-based exposure limits were not developed for application at altitude or for complex heated mixtures [43-45], and the focus has been on individual substances rather than the complex thermally degraded mixtures [2, 46]. For the assessment of symptomatic aircrew some previous efforts were made to develop guidance material for collation of medical data [47-50], however this was not recent, and it has rarely been undertaken in a timely, systematic or comprehensive manner. More recently the European Committee for Standardization has issued a technical report which recommends medical monitoring at the commencement of aircrew employment and for aircrew and passengers after fume events using a best practice medical protocol [51]. There is an urgent need for a consistent, internationally accepted medical protocol to facilitate the recognition of health effects associated with fume exposure in aircraft cabins and cockpits [26, 52]. The main objective of this paper is to compile present knowledge on causes, frequencies and clinical findings of fume events and to develop, based on this data, a recommended approach to the best reasonable and available observation, measurement and recording of symptoms and signs, and subsequent management of afflicted persons and their health outcomes. We describe a variety of diagnostic tests covering functional or organ related impacts including neurological, neurobehavioral, respiratory, and cardiological symptoms, and data on biomonitoring.
Methods
This protocol is a consensus statement of a group of international experts on cabin air contamination and related health effects, the International Fume Events Task Force. Members (from nine countries) were selected based on clinical, professional and/or academic understanding of specific topics in the field of fume events. A literature search was conducted limited to human subjects and articles in the English language; publications were searched on causes, frequencies, and clinical findings related to aircraft fume events. MEDLINE-Database was searched with PubMed (all fields) from its inception up to 15th June 2022 with the terms aerotoxic syndrome and fume events. Further resources included reference lists of other reviews or research results based on relevant objectives and collections of the authors.
Based on these data, we developed what we consider to be the most reasonable protocol for observation, measurement and recording of symptoms, signs and treatment (if any), and subsequent management of afflicted persons and their health outcomes. This protocol was conducted by various working groups, and in online consensus meetings with the expert panel, and is limited to literature specific to oil and hydraulic fluids because of the more serious health impacts rather than other types of fumes, including electrical, fan failures, exhaust, deicing fluid, and ozone [39]. This medical protocol is an expert-based work-in-progress that will be updated as the field progresses. It is not the intention of this protocol to address the issue of routine monitoring of aircraft cabin air for the presence of toxic fumes.
Results
Chemical constituents in engine oil and hydraulic fluid
The oil and hydraulic fumes consist of a range of hazardous substances; VOCs identified in cabin air quality studies, where quantified, ranged from 100 - 300 + compounds [9, 38, 39, 53]. More than 100 VOC compounds were identified in a bleed air investigation after oil fumes partially incapacitated two pilots [25, 54-56].
Organophosphates are utilised as an anti-wear additive at about 3% in synthetic engine oils. Tricresyl phosphate (TCP) is the most studied OP with proven neurotoxicity [46, 57, 58] and is used in most engine oils [59]. While the focus has been on the ortho isomer tri-ortho-cresyl phosphate (ToCP), the other ortho isomers, (mono- and di-ortho-) are at far higher levels (MoCP—3070 ppm; DoCP – 6 ppm; ToCP—0.005 ppm) in the oils and are 10 and 5 times more toxic [57, 60-62]. TCP used in aviation oils comprises a complex mix of cresols, xylenols and phenols, rather than the ten regularly cited TCP isomers only [57, 58, 61, 63]. Oil exposed to high temperatures was shown to alter the composition of the fresh oil, with higher rates of alkylated cresol phosphates and xylenyl phosphates formed [63], with similar toxicity to the ortho TCP isomers [46, 57, 58, 61, 63, 64]. The toxicity of the ortho isomers of TCP is significantly underestimated [62]. Some studies have reported that the non ortho (meta and para) isomers of TCP, which make up around 99.7% of the TCP blend used, also have neurotoxic properties [26, 57, 65-68]. The ortho isomers of TCP make up 0.3% of the commercial formulation of TCP used in engine oils [61], or up to 0.01% in the oil.
Hydraulic fluids typically contain a mixture of as much as 95% organophosphates, typically dominated by tributyl phosphate (TBP) (20–80%), and phenol isopropyl phosphate (PIP, 3:1), ranging from a low of 2.5% (oil) to a high of 15%, but no TCPs.
Amines such as N-phenyl-1 napthylamine (PAN) are utilised in the synthetic oils as an antioxidant at around 1%. PAN is a skin and respiratory sensitiser. Beta-naphthylamine (BNA) and N-2-naphthylaniline (PBN), are reported as low-level contaminants of PAN.
Thermally degraded complex mixture
When oils are exposed to high temperatures in the engines or APU, a complex mixture of thermally degraded (thermolysis) components is produced. This includes a wide range of volatile and semi-volatile organic compounds (SVOC) [7, 9, 38-40, 53, 59, 69-72]. Oil thermal degradation studies identify an excess of 127 VOCs and SVOCs and many hundreds of additional VOC peaks [73].
Ultrafine Particles are generated when the oils are exposed to high temperatures in the engine or APU [70, 74-76]. Oil contamination of the bleed air results in large increases in UFPs with peak concentrations in the 40–80 nm range [74]. The UFPs in the bleed air may be a "hint" of oil leaks [38]. Hydraulic fluid contamination is associated with increases in fine particle concentrations in the 200–1000 nm range [74].
Carboxylic acids
The base stock of the oils is synthesised from esters and carboxylic acids. These acids have a smell characteristic of dirty socks [55, 72].
CO
Low levels of carbon monoxide (CO) have been reported in cabin air sampling and oil thermal degradation studies [38, 71, 74, 77], showing a strong temperature effect of CO formation. Minimal CO is formed below 3000C but levels are considerably higher with increasing temperature [73, 78].
Air monitoring findings
A number of bleed and cabin air monitoring studies have been undertaken over the last few decades, with overviews available for review [7, 9, 25, 71, 79-81]. Measurements include various organophosphates (TCP, TBP, triphenyl phosphate (TPP), trixylyl phosphate (TXP) and dibutylphenylphosphate (DBPP) and volatile organic compounds (toluene, benzene, formaldehyde, acetaldehyde, valeric acid, hexanoic acid, n-hexane). Virtually all levels identified were below available exposure limits [9, 38, 39, 51, 77, 82], however most were not undertaken during recognised fume events.
Mixed isomers of TCP were commonly identified at low levels in air sampling studies ranging from 23–100% of flights [37, 77, 82]. Traces of meta- and para- TCP isomers were identified in nearly all samples [38, 39]. Higher concentrations of TCP isomers were recorded during taxi out, take-off, climb, descent and landing, compared to the cruise phase of flight [37, 38]. TBP was identified in 73% of all flights in one study [77], and in 100% of samples in another study [38].
UFP measurements in the cabin air identified increased concentrations of UFPs, associated with engine and APU power and air supply changes in phases of flight, and correlating with times when oil seals are less effective enabling oil leakage to occur [76]. Oil contamination of the compressor results in very fine droplets in the bleed air under most operating conditions [75] with increases of UFPs during changes of power and air supply changes [69, 83]. Maximum UFP concentrations under these conditions have been identified at up to 60,000—> 500,000 particles / cm3 in normal flight [76, 77, 83]. UFP concentrations were identified in an EU study between a baseline of 2 × 105 to a peak of 2.8 × 106 particles / cm3 during a confirmed oil fume/smell event, significantly higher than average UFP concentrations (5000 particles / cm3) in indoor offices [40]. Simulated oil leakage studies identified UFPs at least 2 orders of magnitude above background levels [74, 75].
Frequency of fume events
As aircraft have no contaminated air detection systems installed, there have been many attempts to assess how often ‘fume events’ occur. Fume events typically happen when recognisable engine oil or hydraulic fumes contaminate the bleed air stream that supplies the cabin and/or flight deck ventilation system. However, it is not possible to provide an accurate rate of occurrence, due to the three distinct ways in which exposures occur [2, 3, 84]:
-
1. Chronic repeated low dose exposure of aircrew to a complex mixture of oil decomposition products contaminating the ‘bleed air’ supply in normal operations. This is due to current engine/APU air supply designs. These will not be detected or reported.
-
2. Acute contamination events triggering a detectable odour known as a ‘fume event’, although if the rate of contamination increases gradually, this may not trigger an olfactory response.
-
3. Far less frequently a visible haze or smoke.
Fume events can range from a brief familiar occurrence in normal aircraft operations, such as during transient air supply and power changes, to the far less frequent, failure or mechanical events, of which only the latter are more commonly reported.
Other sources of fumes/smoke that can be supplied to the aircraft cabin and flight deck include electrical faults, fan failures, deicing fluid, exhaust, and galley equipment [85, 86]. However, oil and hydraulic fluid are the second most commonly reported type of fumes/smoke that are documented by US airlines, with only electrical fumes being more prevalent [85].
Low level oil leakage is well recognised [1-8, 38, 85, 87-91]. The oil leakage due to engine operating conditions “pollutes the cabin/cockpit air” [92]. The frequency of exposure should be viewed in terms of the design factor enabling low level oil leakage to occur in normal flight operation, rather than failure conditions only [5, 6]. The average number of acute events reported per day on the US fleet via official US databases is 5.3 events [93]. However, the lack of training of aircrew regarding fume events has led to significant under-reporting [25, 49, 84, 85]. Contamination events are widely distributed across almost all common aircraft models.
Biomonitoring
The metabolism and disposition of TCP was studied in animal experiments. Following oral administration, tri-para-cresyl phosphate (TpCP) was absorbed from the intestine, distributed to the fatty tissues, and moderately metabolised to a variety of products of oxidation and dearylation of TCP, which were then excreted in the urine, faeces, bile and expired air [94]. However, in biomonitoring a considerable focus has been placed on ToCP exposure, its toxic metabolite (cresyl saligenin phosphate- CBDP) and its effect on acetyl cholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition [95-98]. ToCP is difficult to detect in blood due to rapid metabolism, but there is an assay for ToCP, although not generally available, based on the active site serine of BChE reacting with the active metabolite of ToCP, cresyl saligenic phosphate [96, 99]. This cresyl phosphate-modified butyrylcholinesterase is a very sensitive biomarker in human plasma for fume event associated ToCP exposure [96, 99], much more sensitive than measurement of acetylcholinesterase. Six of twelve jet airline passengers tested positive for ToCP, without showing toxic symptoms [96].
ToCP is the least present of the three ortho isomers found in aviation blends of TCP, which make up collectively 0.01% of the oil [58, 61, 62]. There is over 600,000 times more mono-ortho-cresyl phosphate (MoCP) than ToCP and 1200 times more di-ortho-cresyl phosphate (DoCP) [62].
An analysis of 332 aircrew urine samples did not detect ToCP (DoCP) metabolites, and only one sample contained very low levels of the meta- and para- TCP isomer metabolites, (DmCP and DpCP) [100]. Metabolites of TBP (DBP) and TPP (DPP) were significantly higher in all urine samples, than in unexposed persons from the general population [100]. This was reported to be due to the release of traces of hydraulic fluids into the cabin air or flame retardants used in the aircraft cabin.
Blood may be taken to assess cholinesterase and where possible neuropathy target esterase (NTE) levels, taking into account the clinical presentation of the person and cost. Cholinesterase inhibition can be assessed in two ways, enzyme activity or mass spectroscopy. At present, the enzyme activity method is the only one that can be readily assessed, while the mass spectroscopy method must be stored locally until the University of Washington mass spectroscopy assessments can be undertaken [66, 101], see Table 1.
The AChE bound to the erythrocytes (red blood cells) correlates with the AChE activity in the neurones. Reduction of AChE activity in isolated erythrocytes may be between 30 to 70% of the individual reference value (baseline). After reaction with OPs the esterase activity mainly recovers within a period of several weeks after new synthesis. Measurement of red cell AChE and plasma BChE activity can be undertaken by standard activity assays or using a ChE Check Mobile Test Kit [102]. Initial blood samples are best taken preferably between 4–24 h following the fume event for BChE, and 4–48 h for AChE. A second sample is required for baseline activity levels. Measurable AChE / BChE inhibition occurs only at higher level OP intoxications, which are not expected in fume events. Lack of inhibition does not mean OP exposure did not take place.
The neuropathy target esterase (NTE) activity in the nervous tissue is correlated with that in lymphocytes. Research based on animal studies suggests that the irreversible inhibition of NTE in the nervous tissue may be the first indicator of the onset of organophosphate-induced delayed peripheral neuropathy (OPIDN). AChE and NTE are different enzymes that serve as biomarkers of intoxication by many OP compounds. Reference values for the NTE activities were 3.01–24.0 nmol phenyl valerate / (min/mg protein) [103].
Heutelbeck et al. reported that AChE activity levels related to OP exposures were unaffected in eleven subjects within five days after fume events, while NTE activities were clustered at low levels, suggesting inhibition of NTE activities [21]. Reduced BChE levels were recorded after a fume event [19].
Urine samples could be taken to assess for specific OPs or their metabolites, and blood samples could be used to assess for VOCs (Table 2). At present, the sampling methods for the OPs and VOCs are very specific, costly and require organisation with specialist laboratories in advance to ensure the process is undertaken appropriately. In this protocol, the data will be presented for completeness, but it is unlikely that this testing can be widely undertaken at present.
Clinical effects described by affected persons after an aircraft fume event
General
Aerotoxic Syndrome encompasses a constellation of symptoms and health disorders [3, 15-23, 26-35, 48, 81, 106-123], and as in many medical conditions with the ‘syndrome’ label, the complete list of symptoms and clinical findings is not necessarily found in any individual case. It is clear that there is considerable individual susceptibility [26]. Symptoms depend on the intensity and duration of exposure, exposure conditions, repeated exposures over time versus a single exposure and the duration of the individual’s service in the industry. Clinical factors such as diet, smoking and alcohol use, age, co-morbidities, concurrent medication, genetically impaired enzyme detoxification and reproductive status also play a role. The total accumulated dose over time is a key factor. Symptoms may be prompted by a single high exposure, repeated or prolonged low-level exposures.
Symptoms and diagnoses
Initial presenting complaints (see Table 3) are commonly and consistently described as foggy thinking, dizziness, recognising an odour in the cabin (commonly described as a ‘dirty socks’ smell), impaired short-term memory and cognitive thinking, fatigue, headache, nausea, tremor, balance impairment, incoordination, breathing difficulties, chest pain, cough, eye, nose, sinus and throat irritation.
Long-term intractable cough, breathing difficulties, central and peripheral nervous system complaints are the common features of those affected by a fume event. In many cases, symptoms are of short duration, but in others may take many hours, days or weeks to resolve. In some cases, particularly those who have experienced more than one fume event, symptoms can continue for months or years and, occasionally, full recovery never occurs. Most individuals report the onset of symptoms to be time correlated with a flight or immediately afterwards, in one of the following timeframes:
-
In-flight (ground or air).
-
Immediate post-flight (within one to two days).
-
Late/subsequent (beyond two days).
A variety of diagnoses have been applied to fume event affected individuals including chronic bronchitis, nasal pathology and sinusitis, vocal cord polyps, irritant induced asthma, interstitial lung disease, cognitive dysfunction, and toxic encephalopathy [26]. Multiple chemical sensitivity is sometimes diagnosed [20, 23, 25, 26, 29-32]. People suffering from the effects of aircraft fume events are commonly misdiagnosed as being anxious, stressed or experiencing other clinical complaints [20, 23-26, 36, 37, 124, 125]. Misdiagnosis occurs because the toxic effects of fume event-associated exposure to various noxae, including thermally degraded aircraft engine oil, are not widely recognised by health care professionals.
Respiratory / cardiac complaints
The respiratory tract is the common portal of entry for cabin air contaminants, although entry through the skin and alimentary tract is also recognised. As it receives the total cardiac output it is systemically more exposed than other organ systems thus, theoretically at least, increasing possible toxicity [126].
Respiratory complaints consistent with lung injury among aircrew are common [2, 14, 17, 19-27, 29-34, 48, 106-108, 112, 114-116, 118, 122, 124]. Granulomata consistent with sarcoidosis has been occasionally reported in pilots and military veterans [116, 127]. A European Commission funded study reported that exposure to engine oil and hydraulic fluid fumes can induce considerable lung toxicity [128].
Cardiac involvement is suggested by irregular heart rate, fatigue, cough, breathlessness, cyanosis, flushing, and an increase in blood pressure. There are no systematic documented reviews of the effect of heart rate and blood pressure after fume events, but cardiac abnormalities have been reported by aircrew [14, 17, 19-22, 25-27, 30-33, 48, 106, 108, 112, 114, 118]. At the pathological level, myocardial and pericardial damage has been reported in acute OP poisoning. Importantly, ECG and echocardiography may be normal.
Neurological complaints
The brain is a recognised OP target organ for toxicity, with the central nervous system (CNS) being particularly vulnerable to toxic insult. A major reason why the brain is a susceptible target organ is that nerve cells must last for a lifetime and are terminally differentiated and cannot, as with most other tissues, repair by cell proliferation [51, 129]. Central nervous system (CNS) effects, reported after exposure to fume events include general incapacity, temporary paralysis, impaired or loss of consciousness, headache, nausea, dysarthria, balance problems, ataxia, cognitive impairment, tunnel or double vision, dilated pupils, nystagmus, and sleep problems, with early symptoms being flu-like. Symptoms of exposure to fume events may also involve the peripheral nervous system (PNS), with motor, sensor and autonomic reactions such as: sweating, loss of temperature control, pallor, flushing and altered taste, tremors, incoordination, muscle weakness, paraesthesia, numbness in limbs and other areas, consistent with peripheral neuropathy.
Although standard neurological testing has often reported negative findings, neurological abnormalities in crew related to fume events have been regularly reported [14, 16, 17, 19-27, 30-34, 48, 81, 106-108, 112, 114, 117, 118, 122, 130-132]. In the case of aircrew, chronic pre-exposure is assumed [2, 3, 26]. Aircrew with a longer flying history appear to be more susceptible than those who have been employed for shorter periods, suggesting a cumulative effect [2, 3, 18, 19, 22, 25, 26, 31]. Repeated low dose exposure to OPs on neuronal cells increase the susceptibility to neurotoxic damage upon further higher dose exposure [26, 133]. The neurological pattern of symptoms reported appear to onset during the flying career and show a temporal relationship with time spent on board aircraft as they onset or worsen when flying and reduce or resolve during days off.
Mechanisms of OP-toxicity
Many of the symptoms (especially the neurobehavioral symptoms) that have been associated with “aerotoxic syndrome” have been documented in agricultural workers, sheep farmers (sheep dipping) and pesticide sprayers as well as veterans of the United States, and other countries who served in the 1990–1991 Persian Gulf War. In these scenarios, organophosphate exposure (i.e. as pesticides or nerve agents) has been discussed as a plausible explanation for the chronic neurologically based symptoms [2, 3, 134-139].
The symptoms associated with the toxicity of OPs involves three main categories [139, 140]:
-
1)
The primary action of OPs at very high doses is the irreversible inhibition of acetylcholinesterase (AChE), resulting in accumulation of acetylcholine and overstimulation of the nicotinic and muscarinic AChE receptors with cholinergic effects. OPs inactivate cholinesterases by attaching an alkyl phosphate group to the hydroxyl group of a serine residue at the enzyme's active site. Recovery from such inhibition generally takes 10–14 days [141]. Cholinergic symptoms depend on the OP compound, dose, frequency, duration and the route of exposure, combined exposure to other chemicals and individual sensitivity and susceptibility [104, 139, 140]. Initial symptoms of mild toxicity include fatigue, dizziness and sweating, sometimes accompanied by headache, inability to concentrate, cognitive dysfunction, weakness, anxiety, tongue and eyelid tremors, miosis and tightness of the chest [140]. If moderate to severe OP toxicity ensues, the so-called “cholinergic crisis” or “cholinergic syndrome” develops, which includes more elevated levels of sweating and salivation, profound bronchial secretion, bronchoconstriction, diarrhoea, muscle tremors and fasciculations, and worsening CNS effects (e.g. dizziness, inhibition of central respiratory centres, convulsions, coma). Death can occur as a result of respiratory failure [142]. Symptoms of moderate and severe poisoning are not consistent with symptoms reported by aircrew, these are high-dose health effects.
-
2)
Organophosphorus ester-induced delayed neurotoxicity (OPIDN) is a central and peripheral axonopathy with the early stage characterised by peripheral effects that recover as peripheral nerves regenerate. The later stage is central, which is more permanent. OPIDN may be caused by single or repeated exposure and is accompanied by a Wallerian type (or dying back) axonal degeneration and secondary demyelination in the most distal portion of the longest tracts in both the central and peripheral nervous system [139]. The clinical picture is manifested by mild sensory disturbances, ataxia, muscle fatigue and twitching, and improvement may require months or years. The neurotoxic effects of tri-ortho-cresyl phosphate (ToCP) and of exposure to other OPs have long been recognised as OPIDN, however the clinical picture being observed does not fit this pattern. Nevertheless, industry risk assessment studies have focused almost entirely on OPIDN as the toxicological endpoint, suggesting the levels of ToCP are too low to cause OPIDN [37, 39, 42, 57, 62, 125, 142, 143].
-
3)
Organophosphorus-induced chronic neurotoxicity (OPICN) is associated with exposure to large acutely toxic or small subclinical doses of OP compounds. Excessive cholinergic activity produces delayed neurodegeneration in various brain areas (cortex, cerebellum, hypothalamus, amygdala) and in the spinal cord, that could explain persistent neuropsychiatric, neurologic and behavioural problems [139]. Clinically, OPICN is manifested by headaches, dizziness, anxiety, apathy, restlessness, labile emotions, anorexia, insomnia, fatigue, inability to concentrate, memory deficits, depression, irritability, confusion, generalised weakness, tremors, respiratory, circulatory and skin problems, with not all people exhibiting all these symptoms [139, 140]. Reports on OPICN occurring in individuals, following long-term, subclinical exposures without previous acute poisoning, have been inconsistent, partially due to the difficulty in defining exposure levels [139]. Symptoms may persist for years after exposure and are distinct from cholinergic and OPIDN effects [139].
-
4)
We suggest a fourth category: non-cholinergic mechanism of OP toxicity, after chronic repeated low-dose exposures. The clinical picture in aircrew is not like nerve gas poisoning and does not match classical OPIDN, although there are clearly features in common. The neurological pattern of symptoms constitute a group of non-localising functional deficits which are consistent with a diffuse toxic encephalopathy as described in Michaelis et al. [26]. The pattern is in many ways directly comparable with the symptoms suffered by farmers from ‘dipper’s flu’ [134, 135]. Chronic repeated low-dose exposure to OPs, the norm with air crew, may have effects at exposure levels below those required to cause lowering of acetylcholinesterase, including oxidative stress, and neuro-inflammation, combined with effects on the known OP targets: motor proteins, neuronal cytoskeleton, axonal transport, neurotrophins and mitochondria [136, 137]. Axonal transport is crucial to maintain brain structures in a healthy state by delivering a number of substances and structures, to and from the neuron cell body. This interferes with the delivery of transmitter substances, neurotrophins and function of mitochondria, and could be the basis for the development of a diffuse subacute encephalopathy [2, 26].
In addition to the direct and delayed OP-induced neurotoxicity effects, concern exists about the potential long-term risks of neurodegenerative disease in aircrew exposed to cumulative low-dose OPs. There is a correlation between exposure to OPs and development of neurodegenerative diseases including Parkinson’s, amyotrophic lateral sclerosis/motor neurone disease and Alzheimer’s disease [136, 139, 144-146]. Cohort studies of aircrew report increased disease rates for motor neurone disease and a twice as high mortality rate of amyotrophic lateral sclerosis (ALS, the most common form of progressive motor neurone disease), when compared to the general population [25, 119, 147, 148].
Neuroimaging
Positron Emission Tomography (PET) was performed in 26 flight attendants, presenting with "neurotoxic complaints" after exposure to contaminated air, associated with fumes from the APU on one or more occasions. PET was abnormal in 12 of these 26 aircrew, with decreased activity in the frontal regions and increased function in occipital areas and the limbic system, consistent with toxic encephalopathy [117].
Diffusion Tensor Imaging (DTI) MRI identified small clusters in the brain in which white matter microstructure was affected in aircrew reporting cognitive impairment and depressive symptoms [28]. Higher cerebral perfusion values in the left occipital cortex and reduced brain activation on an executive function task was observed and cognitive impairment was associated with white matter integrity. Defects in brain white matter microstructure and cerebral perfusion are potential neurobiological substrates for cognitive impairments and mood deficits reported in aircrew [28].
Neurobehavioural and neuropsychological effects
Reported neurobehavioural and neuropsychological health effects associated with fume events include disorientation, dizziness, confusion, lethargy, altered behaviour, personality changes, anxiety, depression, difficulties with problem solving, concentration, memory and writing, and euphoria [14, 16-27, 31-34, 48, 106-109, 111, 112, 114, 117, 118, 122, 131, 132]. In a survey of international aircrew (50 pilots, 970 cabin crew) to establish how many believed they were suffering occupational health problems as a consequence of their working environment, including fume events, 23% reported no time off sick and only 12 aircrew members reported no time off sick and no symptoms. Forty-five percent of the respondents reported confusion and difficulty in thinking, 55% had difficulty concentrating, and 49% had memory loss [114]. Neurobehavioural symptoms were reported as the highest category in 64% of pilots with chronic ill health in a survey of British Aerospace 146 (BAe 146) pilots exposed to acute and chronic oil fumes [26]. Long-term cognitive dysfunction was identified in four cases involving aircrew exposed to oil and hydraulic fumes in fifteen incidents [26]. Slowed information processing speeds, slowed reaction times and executive dysfunctions were identified in pilots and flight attendants [18, 22, 109, 111, 132]. In these studies, the pilots' neuropsychological profile mirrored that seen in other neurotoxic conditions, such as sheep dipper's flu [138].
Peripheral nervous system
In surveys of health symptoms in aircrew, sensory complaints such as paraesthesia, tingling, restless legs, muscular jerking, and numbness are reported in 20–77% of cases [17, 24, 26, 30, 32, 107], consistent with a toxic sensory polyneuropathy. In some of these studies respondents reported that symptoms occurred after exposure to oil or hydraulic fluid leaks and fumes from the aircraft ventilation system and subsequently sought medical attention [17, 32]. In other studies, a temporal relation between the onset of symptoms and exposure to fume events was reported [30]. 93 of 106 pilots (88%) reported that they had been involved in at least one fume event [24]. In a larger study of 274 pilots, 88% were aware of exposure to aircraft contaminated air associated with oil fumes, 34% reported frequent fume event exposures and 18% one or two big events [26]. PNS symptoms were reported in 11 of 15 incidents of which 87% involved maintenance confirmation of oil leakage [26]. Winder and Balouet described sensory symptoms (tingling, numbness) in five of seven pilots [31]. Paraesthesia/tingling feelings of the hands were also reported in almost 30% of 34 flight crew members in one study [17], and in 13 of 38 cases (34%) in another study [30].
Symptoms of small fibre neuropathy, a subtype of polyneuropathy of thin myelinated and unmyelinated nerve fibres, are painful burning paraesthesia and hypersensitivity to touch and temperature changes. There is one study on the prevalence of small fibre neuropathy in aircrew reporting adverse effects associated with fume events [118]. In nearly all patients in this study group, neuropathological investigation by skin biopsy showed that the intra-epidermal nerve fibre density was significantly decreased.
Other findings
Persons exposed to fume events may also present with a variety of other symptoms and clinical findings. Irritation to the respiratory tract, eyes and skin are commonly reported in association with fume events [2, 14, 17, 19, 20, 22-27, 29-34, 48, 106-108, 112-114, 122]. Many of the substances are recognised as skin and respiratory sensitisers, associated with allergy and asthma symptoms and breathing difficulties [20, 24-27, 29-33, 107, 112, 114, 115, 149-151]. Gastrointestinal symptoms such as nausea, vomiting and cramps are regularly reported by aircrew after fume events [14, 16, 17, 19-27, 29-34, 48, 106-108, 114, 118, 122]. Liver function abnormalities are associated with prolonged or repeated exposure to various substances in the fluids [149, 150, 152, 153].
Chemical sensitivity is often reported associated with fume events [17, 20, 23-27, 29-33, 107, 113, 114]. Some of the substances are classified as suspected of causing harm to fertility or the unborn child or toxic for reproduction [26, 32, 113, 114, 149, 150]. Several contaminants are listed as a human carcinogen (BNA) or suspected carcinogens including, TBP, and PBN [26, 149, 150, 152, 153]. In a study of 5,366 flight attendants in the 2014–2015 Harvard Flight Attendant Health study, a 34%-66% increase in female reproductive cancers including breast cancer is reported, compared to 2,729 controls [112, 113]. Higher rates of cancer in aircrew were reported in aircrew surveys [114], including glioblastoma multiforme (GBM) brain tumours [25, 26]. Many risk factors have been examined as potential contributors to glioma risk, including ionizing radiation, circadian desynchronosis, heritable risk alleles and mobile phones. While these studies [25, 26] identified higher rates of exposure to oil fumes via the aircraft air supply [15, 25, 26, 36, 154], supporting that there could be an occupational link, further epidemiological investigation is warranted.
Sleep disturbances [25, 26, 31, 32, 48, 112, 113], fatigue [17, 19-27, 29-34, 48, 107, 112, 114], changes in visual acuity and eye disorders [14, 16, 17, 20, 21, 24-27, 29-32, 107, 113, 114, 122, 155] and joint pains have often been reported [17, 19, 20, 24-27, 29-32, 48, 107, 113, 114]. The product Safety Data Sheets often list a variety of adverse effects associated with breathing oil or hydraulic fumes including: eye nose and throat irritation and “most important symptoms and effects both acute and delayed—headache, dizziness, drowsiness, nausea and other CNS effects. Shallow respiration, low blood pressure, bluish skin color, convulsions, coma and jaundice” [156]. Other examples include dermatitis, allergic skin reaction, damage to organs through prolonged or repeated exposure, suspected of damaging fertility [156-158].
Emerging areas
Our understanding of the clinical, toxicological and pathological issues that underpin the various presentations of the Aerotoxic Syndrome is progressively improving, particularly over the last twenty years. The following are some of the areas where significant progress has been made or can be made in our understanding of the condition:
-
1)
Ultrafine particles.
Studies have shown that UFPs (less than 100 nm/0.1micron) are more toxic than larger particles [159-163]. Exposure to pollution, fine particles (PM < 2.5–10) and UFPs have various adverse effects on health [164-167]. These include cardiopulmonary [162, 163, 165, 168-170], and neurological effects, including impaired cognitive performance [171, 172]. Exposure to airport and traffic related UFPs may increase the risk of brain, lung and childhood cancers [173-176]. Individual particles are capable of inducing inflammation and oxidative stress [160], suggesting that particle number concentrations, which are dominated by UFPs, may be more indicative of potential health impacts than particle mass concentrations. UFPs have a high alveolar deposition fraction and have the potential to translocate into the blood circulation system.
UFPs up to several hundred thousand particles / cm3 have been identified in both cabin air studies [38, 40, 69, 76, 77, 177] as well as thermally degraded oil and bleed air studies [70, 73-75]. A 2019 review on aircraft exhaust emissions found that the nanoparticles were dominated by nearly intact forms of jet engine lubrication oil [178]. This oil is exposed to temperatures up to 1700 °C in some areas of the engine during the normal lubricant duty cycle leading to more or less complete thermal degradation, with subsequent UFPs and thermally degradation product formation, of which some will enter the cabin air supply [2].
Short term exposures to aviation related ultrafine particles near a major airport was found to be associated with decreased lung function and a prolonged QTc interval in healthy adults [179]. With respect to respiratory irritation, jet engine particles were found to have similar toxicity to diesel exhaust emissions [180]. However, this does not apply to neurotoxicity. The continual presence of ultrafine particles over a typical working lifetime in air crew of up to 20,000 h will predispose them to chronic respiratory problems and will exacerbate the translocation of neurotoxic substances across the blood brain barrier [2].
-
2)
Serum autoantibodies against brain specific proteins.
Brain reactive autoantibodies are present at low levels in the vast majority of human sera [181]. Exposure to OP compounds may lead to damage of the blood–brain barrier [17, 182]. As a result, neural proteins can leak into the blood and elicit an autoimmune response, with an increased level of autoantibodies. Testing autoantibodies against brain neuronal and glial proteins to obtain objective evidence of central nervous system injury has been useful in symptomatic commercial aircrew, and in distinguishing veterans with Gulf War disease from healthy controls [17, 182-184]. In 34 aircrew with CNS related complaints higher autoantibody levels against MAP-2, tubulin, MBP, tau and GFAP than matched controls were found [17]. In a report of a 43-year-old airline pilot suffering from many fume events, who presented with neurological deficits of OPIDN and other symptoms, analysis of the serum confirmed grossly elevated levels of serum autoantibody biomarkers for neuronal cell degeneration compared with a control group [130, 131]. The pilot died without regaining good health; his death could be explained by functional disorders of the brain and the heart in combination with intake of pentobarbital. At autopsy, brain and spinal tissues showed axonal degeneration, spongiosis and demyelination. Peripheral nerves showed T-lymphocyte infiltration and demyelination. T- lymphocytes had also infiltrated the heart muscle tissue (myocarditis). It was concluded that the work environment, clinical condition, histopathology and serum biomarkers for nervous system injury were consistent with organophosphate-induced neurotoxicity.
Unfortunately, this test of autoantibodies, performed by the University of Durham, North Carolina is not available at present. This is remarkable because these autoantibodies are studied in several clinical trials [185]. For instance, autoantibodies against tau protein can reduce pathology and functional decline in animal models of tauopathies and may be beneficial in Alzheimer's disease and Parkinson's disease. So, we hope this method of assessment of aircrew with exposure to toxic compounds will soon be available elsewhere. Procedures for determination of autoantibodies to neural proteins are outlined in the supplement annex.
-
3)
Increased genetic susceptibility to toxic compounds.
Inter-individual variability in response to toxic substances is the norm. For example, there are defined genetic polymorphisms which influence aldehyde dehydrogenase activity, significantly affecting individual tolerance for the effects of ethyl alcohol [186].
The same is the case for organophosphate compounds, although much more complicated. Genetic variability and levels of expression of genes involved in the detoxication of organophosphorus compounds (OPs) such as jet engine anti-wear agents and hydraulic fluids contribute to the variability in sensitivity to exposures to these compounds. Inter-individual genetic variations in the ability to metabolise OPs, may explain why some aircrew and passengers develop symptoms even at low doses, whereas others undergoing the same fume event may remain asymptomatic [66, 187, 188].
The key enzymes that influence individual response to OP exposures include cytochromes P450s (especially CYP450 3A4), carboxylesterase, butyrylcholinesterase (BChE) and paraoxonase-1 (PON1). Key enzymes that protect against increased oxidative stress associated with OP exposures include glutathione S-transferases, superoxide dismutases, and PONs 1, 2, and 3 and others.
With respect to genetic variations in levels/activities of specific proteins, it is important to examine the given variations in detail. Effects measured in vitro will not necessarily translate into the same effects in vivo. A good example is the PON1 genetic variant which inactivates paraoxon (the neurotoxic metabolite of the OP insecticide parathion) via hydrolysis in a test tube. One isomer (Arginine-192; PON1R192) of PON1 does so at a rate approximately seven times faster than another (Glutamine-192; PON1Q192) [189], suggesting that the faster variant is more protective. However, neither variant protects against paraoxon exposure because the in vivo rates of inactivating paraoxon are not sufficient to protect from ill effects [190]. There is little evidence that PON1 hydrolyses the triaryl phosphates added to jet engine oil in vivo.
Some proteins protect by stoichiometric binding to OP compounds, such that higher levels are more protective. This is relevant to carboxylesterase, for example, which varies by at least 18-fold in humans [191]. Also, inter- and intra-individual variations in the levels of BChE have been defined, modulating the effects of OP exposures [192].
High levels of enzymes that modulate the oxidative stress associated with exposure to specific OPs (e.g. glutathione synthetase, glutathione transferases, PONs 1, 2 & 3) may provide some protection against exposures. The CYP450 3A4 enzyme converts several of the triaryl phosphates into metabolites that are potent inhibitors of physiologically crucial enzymes (unpublished resultsFootnote 1). Genetically based diverging levels of CYP 450 3A4 indicate differences in individual hepatic activity of 40-fold [191].
In summary, inter-individual genetic differences can influence susceptibility to the ill effects of OPs in engine oils and hydraulic fluids, whether those effects follow chronic low dose repeated exposures, a higher dose “fume event,” or a combination of the two. The precise mechanisms will need to be validated through animal studies [e.g. [190, 193]] or, when possible, through cell culture model systems.
-
4)
Low-level repeat exposure to mixtures.
Substances that are not toxic individually may become toxic within a chemical mixture of thermally degraded components. Risk assessments based upon single substance evaluations underestimate the toxicity of a mixture. This could occur through exposure to multiple chemicals that cause the same effect, or interactions between chemicals may change the dose response relationships observed for chemicals tested in isolation [194]. Combined exposure to multiple chemicals can lead to health/environmental effects even if single substances in the mixture do not exceed safe levels [195]. Low-dose exposures to mixtures of chemicals in the environment may be combining to contribute to environmental carcinogenesis [196].
-
5)
Chronic low-level exposure to OPs.
Repeat low level exposure to OPs is distinct from acute single exposures. The acetylcholinesterase-based mechanism cannot alone account for the wide variety of adverse consequences of OP exposure that have been described, especially those associated with repeated exposures to levels that produce no overt signs of acute toxicity [137].
As an example, repeated exposures to the nerve agent DFP, at doses that are below the threshold for acute toxicity, can result in alterations in myelin structure and persistent decreases in axonal transport in the rodent brain [197]. Low-level OP exposure may lead to 1) non-cholinergic toxic effects by interference with axonal transport [136, 137], and to the generation of brain-specific autoantibodies that target proteins known to play critical roles in both myelination and axonal transport [17, 182, 183]. An “autoimmune” response might offer one explanation for why OP exposures could lead to chronic symptoms [197]. Axelrad et al. identified that repeat very low dose exposure to certain OPs on neural cells increased the susceptibility (reduced the threshold for toxicity) to neurotoxic damage upon further higher dose exposure [133]. The continual low dose exposure with occasional higher-dose episodes (‘acute on chronic’ mechanism) must therefore be considered for aircrew chronically exposed to fumes [3, 26]. It is typical among aircrew to log in excess of 20,000 h flying time in a career [2]. Over that period, a person would typically inhale 9,000 cubic metres of air (nine million litres). The constant presence of low dose exposures may have considerable cumulative effects. By looking at the various reported levels of OPs in the literature it is possible to make an estimate of their internalised dose of TCP during a working lifetime as shown in Table 4. The concentrations listed and therefore the estimated dose, will be based on the amount of vapour present. However, this is likely to be an underestimate due to recent research which has shown that much of the internalised dose will be in the form of nanosized oil droplets [62, 178].
-
6)
Endocrine disruptors.
OP flame retardants may have effects on the oestrogen receptor, androgen receptor and glucocorticoid receptor [198, 199], by enhancing or blocking the activity of the naturally occurring ligand, such as oestrogen or testosterone. As an example, TCP has endocrine disrupting effects via various hormone pathways including the oestrogen receptor [198-201], which is implicated in breast cancer [202, 203].
-
7)
Causal connection.
How can we move from an observed association to a robust causal inference? Bradford Hill’s seminal paper of 1965 identified nine features of available evidence, which, if present, could help justify a robust causal inference [204-206]. He was careful to point out that if these features of the evidence were absent, then that did not justify concluding that the agent being evaluated was not causing harm [207]. The weight of evidence is suggestive of a causal link between aircraft cabin contamination and health effects in some crew and passengers, see Table 5. We should not be surprised, as the exposure and effects are biologically entirely in keeping with the science of OPs [51, 136, 137, 197]. Further supportive causation evidence is stated in a paper by Harris and Blain: “High doses of a toxic chemical will give rise to an acute toxic response, but prolonged exposure to low concentrations of a toxin may only cause a slowly developing chronic response. The circumstances of exposure and the toxicity of the toxin will determine which of these is the more serious” [129]. In their paper, they quote five cardinal signs on causation in neurotoxicology propounded by Schaumburg [129, 208]:
-
(1) Presence of the suspected agent is confirmed by history and either environmental or clinical chemical analysis. (Fugitive emissions in engine bleed air is recognised and has been demonstrated on many occasions.) – [1, 2, 4-8, 26, 36, 38, 51, 75, 87-92].
-
(2) Severity and temporal onset of the condition are commensurate with duration and level of exposure. (Supported by clinical data). [3, 11, 16, 17, 19, 20, 22, 23, 25-27, 30, 32, 36, 48, 51, 81, 96, 131, 155].
-
(3) The condition is self-limiting and clinical improvement follows removal from exposure: the medical condition of air crew affected by Aerotoxic Syndrome can improve after removal from the cabin environment, though usually slowly and not always completely. [11, 16, 17, 20, 23-26, 32, 33, 131].
-
(4) Clinical features display a consistent pattern of affected organs that correspond to previous cases: there is a consistency of the presenting symptomatology [2, 17, 20, 25, 26, 32, 33, 48, 51, 81, 197].
-
(5) Development of a satisfactory corresponding experimental in vivo or in vitro model is absolute proof of causation: there is ample experimental evidence of the toxicological damage caused by repeated low-dose exposure to OPs [51, 136, 137].
Medical protocol: The documentation of a fume event, clinical history and physical examination
In all reported cases of toxicity following fume event exposures, or Aerotoxic Syndrome, the onset of symptoms may occur in-flight (ground/air) at the time of or following the fume event, immediate post-flight (within one to two days) or later/subsequently beyond two days. The recommended management approach upon presentation to a medical facility is outlined in Fig. 2. Our recommendations for data collection, medical examination and special investigations are set below and formatted to examine each organ system dysfunction individually and are divided into time of presentation: Table 6: In-flight; Table 7: Immediate post-flight; Table 8: Late/Subsequent.
A record of the fume event, with details of technical and engineering follow-up, together with the symptoms and the medical management of people who have been exposed should be documented. Detailed records help to plan further investigations and objectively correlate symptoms and functional disorders in organ systems. The level of detail recorded will depend on the extent and degree of adverse health effects experienced.
In-flight (ground or air) medical protocol
The in-flight report should be made by those assisting people who have been affected by the fume event (crew members and passengers), see Table 6. If a trained health care professional (e.g. doctor, nurse or paramedic) is present, a physical examination is strongly recommended.
Immediate post flight medical protocol
Ideally, the employer or airline should facilitate the investigations recommended in this protocol. However, in the interim, or if there is an inability to undertake the investigations, symptomatic aircrew or passengers should be sent to the Emergency Room at the nearest hospital. The airline should inform the hospital and aircrew of this recommended practice, so that the following data are recorded, see Table 7.
Undertaking all the examinations and special investigations suggested may not be possible nor medically indicated in every case. Some investigations require specialist laboratories, and there will be practical issues of availability, timing and cost for procedures and tests. Requests for special investigations should be based on clinical indication in each individual case. Despite these caveats, it is strongly recommended that as much data is collected as is practically possible in every case, as described in Tables 1, 2, 6, 7, 9 and 11. Initial medical assessments may also be required for asymptomatic, or relatively asymptomatic persons, especially aircrew who have been exposed to fumes in an aircraft cabin, see Fig. 2. In fact, aircrew should not be cleared back to fly without an assessment. This may also be important in the conduct of clinical and epidemiological studies in order to address why some aircrew react poorly to fumes in the aircraft cabin while others do not. The decision to what extent medical assessment should be undertaken after a fume event, should take into account a variety of factors including: potential exposure to hazardous substances and the duty of care, symptoms may arise at the time of the event, soon after, or on a prospective basis.
It is advisable to provide all crew and passengers with an information leaflet list of acute and chronic symptoms associated with Aerotoxic Syndrome—Table 3.
Respiratory function testing
Routine lung function testing (spirometry) will often yield normal results and symptoms of respiratory tract irritation may be transitory. However, in the early phase spirometry is a simple test measuring basic lung volumes that can be easily performed because it does not require sophisticated equipment. The single breath diffusing capacity for carbon monoxide (DLCO) or nitric oxide (DLNO) are more sensitive tests for gas exchange abnormalities and should be undertaken in all those presenting with respiratory symptoms [209]. Measurement of DLCO and/or DLNO are procedures that detect injuries of lung diffusion but are not available in all medical settings. However, these tests should be arranged in patients with respiratory symptoms, such as cough, shortness of breath, oxygen saturation < 96% and in all those with abnormal spirometric values. The same approach should be applied for exercise testing or ergospirometry. The measurement of expired nitric acid concentration (FeNO), if available is a simple method of assessing pulmonary inflammation. Respiratory orientated exercise testing with measurement of arterial oxygen saturation (oximetry) or arterial blood gas analysis are sensitive for respiratory and cardiac dysfunction and are recommended in cases of acute and especially of persistent respiratory symptoms. Respiratory provocation testing by use of methacholine is sometimes indicated in cases of suspected fume event-induced irritant asthma.
Chest X-rays are routine, and the more sensitive high-resolution computed tomography should be considered. These are seldom performed at the time of injury or soon after. In the presence of persisting respiratory symptoms, radiological examination of the lungs should be arranged.
For recommended testing see Tables 9 and 10.
Cardiac function testing
For those presenting with cardiac symptoms, e.g. irregular or rapid heart rates or chest pain, a chest x-ray and ECG (including 24-h ECG) should be performed. Prompt referral to a cardiologist is indicated for those with severe or continuing symptoms. Cardiac monitoring may be warranted [210]. Echocardiography and stress testing should be considered and in those with possible positional orthostatic tachycardia (POTs) syndrome tilt table testing is indicated. For recommended testing see Tables 9 and 10.
Neuro assessment
Gross neurological status (balance, muscle weakness, numbness, reflexes) can possibly identify balance problems, muscle fasciculations and reduced sensation [118]. In most patients with probable Aerotoxic Syndrome, nerve conduction was found to be unremarkable despite a credible and similar pattern of complaints between patients. However, a few studies report mild sensory polyneuropathy [23, 26, 32]. For recommended testing see Table 11.
Neurocognitive tests that are deemed applicable include the following areas: processing speed, attention and concentration, reaction time to stimuli, sequential reaction time, complex problem solving, short and long term visual and verbal memory and cognitive flexibility/capacity to change direction. For recommended testing see Table 11. If neurobehavioural/ neuropsychological symptoms persist over weeks or months, we recommend a neuropsychological test battery as in Table 12.
Similar and alternative tests have been utilised in other studies with aircrew after a fume event [18, 22, 109, 111]. Tests available may vary in different countries; however, most of the tests listed are universal.
Treatment
Treatment for Aerotoxic Syndrome is symptomatic. Removal from environment may be required for short duration or longer term.
Discussion
During routine flight, continual low-dose (i.e., chronic) exposure to oil fumes, with less frequent acute exposures, is occurring. Aircraft cabin air can become contaminated by hydraulic fluids along with many different volatile lubrication oil compounds and their thermal degradation products.
Prudence requires that we consider more than one contaminant as being involved in the etiology of ill health. There has been a focus on one particular isomer of the OP in the oils, and this has led to the neglect of other contaminants, the combined mixture, and chronic low-level exposures. The latter would be especially relevant in what is described as Aerotoxic Syndrome.
To date, there has been inadequate recognition of both the types and implications of exposures to contaminated cockpit and cabin ventilation air supply that occurs as a feature not only of the design of the systems utilised, but less frequently during abnormal or failure conditions related to these systems. Our proposed medical protocol, combined with a narrative review, provides a better understanding of exposures sourced to the aircraft ventilation air supply, adverse effects reported, and how to respond to people after aircraft contaminated air and fume events. This should also aid in the systematic gleaning of epidemiological data of aviation workers and the public who travel in aircraft.
Although a variety of sources can contaminate the cockpit and cabin air, by-products of oil and hydraulic fluids have been the key focus of concern. The organophosphate TCP, used in engine oils, has been the major focus of concern. Most attention has been given to the tri-ortho isomer ToCP in the oils at very low levels due to its neurotoxic properties associated with OPIDN, a high dose effect of exposure. The neurotoxic properties of the other ortho isomers, at over 600,000 times higher levels in the TCP used in the oils and over 6 million times greater in toxicity than ToCP, have been ignored [62]. Neurotoxicity of the non-ortho isomers of TCP in the oils at around 3%, have also been ignored. As an example, the meta and para isomers of TCP showed demyelination of neurons yet did not cause paralysis [65]. The various OPs in the hydraulic fluids can range from low levels up to 80%. The effects of the complex mixture consisting of a wide range of VOCs associated with thermally degraded engine oils have not been given proper consideration. The other compounds including amines, UFPs, carboxylic acids in the base stock of the oils, and carbon monoxide, all need to be given due attention. While cholinesterase effects of OPs have been shown to take up to 4 h to occur in mice, the effects of CO would be quite rapid.
Various engine bleed air and cabin air monitoring studies have been undertaken during routine flight operations. TCP used in engine oils and the hydraulic fluid OPs have frequently been identified at low levels. A wide range of VOCs, many of which have been reported in oil pyrolysis studies, have been identified both in the cabin and in bleed air monitoring investigations. When detected, the levels identified have almost always been well below occupational exposure limits. Despite such exposure limits being applicable to ground based industrial environments (i.e. workplaces), they have often incorrectly been cited to suggest that the air in aircraft is better than in offices and other ground-based environments, including workplaces. However, the meta and para isomers of TCP, as an example, do not have an exposure limit value. The use of exposure limits and individual substances fails to consider the complex mixture and other toxicity factors associated with the various compounds. Passengers travel in the same environment as the aircrew, yet their exposure is not considered. The findings support that chronic, low-level exposure is occurring in normal operations at background levels as well as during transient fume events.
The frequency of fume events is a continuing debate. The focus has been on the less frequent abnormal or failure conditions, while ignoring that low-level exposure to background levels of oil fumes occurs in normal operation as well as in acute transient exposures and fume events. Therefore, exposures are happening in two distinct ways: 1) continual low-level background exposure and transient exposures associated with phases of flight when oil seals are known to be less effective, and 2) the far less common abnormal or failure events (i.e., acute exposure events). However, lack of training and awareness, lack of comprehensive reporting regulations, under-reporting, and the lack of contaminated air detection systems further hinder the full understanding of how often these exposures and fume events occur. Frequency should now be seen in relation to system design factors that enable exposure to occur, rather than in terms of the number of reports submitted [5, 6].
There have been limited efforts related to biomonitoring for OPs and VOCs. Regarding the OPs, the focus again has been on ToCP, despite the increased levels and higher toxicity of the other mono- and di-ortho isomers of TCP and the complex mixture used in the commercial formulation of TCP in the oils. Urine metabolites of TBP and TPP used in hydraulic fluids were significantly raised over controls, while metabolites of TCP non-ortho isomers were hardly detected. The methodological limitations of identifying these TCP isomers in urine has been acknowledged [211]. Cholinesterase enzyme activity has limitations. Due to the interindividual variability of cholinesterase values, a baseline reference level must be undertaken. Measurable activity levels of AChE and BChE or mass spec measurements of aryl-phosphate bound to the active site serines of Ache or BChE appear to occur only after higher level OP intoxications, which are not generally expected after fume events. BChE may be a more suitable activity enzyme. Development of more accurate mass spectrometry methods, requiring one sample only, is near completion. While VOCs can be investigated in blood after fume events, these tests are costly, very specific, and not readily available.
Aerotoxic Syndrome encompasses a wide constellation of symptoms and health disorders. These include neurological, neurobehavioural, respiratory, cardio, irritant, sensitising, gastrointestinal, rheumatological, fatigue, chemical sensitivity and others. Symptoms can be prompted by a single high dose or repeated, or prolonged low-level exposures. The adverse physiological effects have consistently, but in a non-uniform manner, been reported globally over many decades in association with air supply contamination.
Symptom intensity varies between individuals for a variety of reasons including repeated exposures, intensity and duration of exposures, genetic variability, and related individual susceptibility factors. While neurotoxic effects are of primary concern, there is a wide range of other organ systems that are involved, continuing to show a consistent but diverse pattern. A number of the adverse effects reported are consistent with EU health-based hazard classifications associated with the individual compounds in the oils and fluids above the hazard classification levels [149, 150, 152].
One of the more comprehensive descriptive studies reported the broad range of short and long-term adverse effects in 274 pilots who had flown on the BAe 146 [25, 26]. This aircraft was acknowledged to have higher oil leakage rates than most. Thirteen percent of the 274 pilots reported a range of chronic ill health outcomes and a permanent loss of fitness to fly. This was 37%—433% above a literature search of civilian and military pilot loss of medical certification respectively, for all reasons [25, 26].
It is regularly suggested that the symptom pattern is too diverse and non-specific to be a syndrome, effects are not seen in all those exposed, and symptoms are not consistent with the OP effect of OPIDN. OPIDN is a known high-dose effect from certain OP exposures, yet this is not being seen in aircrew, with chronic low-dose effects being ignored. Additionally, this fails to consider that air monitoring studies and the engine and air supply systems enable low-level contamination in normal flight. The symptoms associated with chronic low-dose exposure to OPs are acknowledged to be non-specific and diffuse, and not associated with cholinergic effects. Inhibition of enzymes at OP levels that do not affect AChE or BChE activity levels, has been shown in vivo by TAP metabolites, including those reported to be linked to cognition [66, 212, 213].
There are emerging areas to be considered, including repeat exposure to low levels of a complex mixture, exposure to UFPs, dose, and effects other than neurotoxicity. Oil exposed to high temperatures generates high levels of UFPs (up to > 500,000 – 2.8 × 106 particles / cm3) to which various substances, including OPs, can adhere. The nanoparticles can then act as a trojan horse when inhaled, crossing the blood–brain barrier with direct access to the brain / CNS [2]. The pattern of exposure is consistent with acute, overlayed on chronic effects of exposure to OPs, with constant low-level dose exposure to nanosized oil droplets significantly raising the internalised dose, with considerable cumulative effects expected. While total dose over time is seen as a key factor, this is generally not considered.
As an example, while TCP measurements are seen as low in the nano or low microgram range, Table 4 suggests that total TCP over a crew’s working lifetime could be far higher at up to > 300 mg based on studies commonly cited, in which fume events were not regarded as occurring. While there is continuing debate about whether any long-term effects are possible and associated with contaminated bleed air, the collection of data in a consistent and standard manner will further support the existing data documenting that such effects are occurring, including signs, symptoms, and diagnoses, many of which were confirmed with oil leakage events [19, 22, 23, 25-27, 29, 31, 120, 151, 155, 214]. There is a range of serious neurodegenerative diseases associated with OPs, and emerging data support disease occurrence in other organs.
It is often cited that there is no causal link between adverse effects reported by aircrew and exposure to the aircraft bleed air contaminants. This relates to the incorrect ToCP/OPIDN argument cited above, the absence of sensor equipment in aircraft to measure exposure levels, and the failure to consider the acute, overlayed on chronic patterns of exposure, the complex nature of the mixtures, and cumulative effects. Also, in vitro / in vivo studies have not been adequately undertaken to reflect the appropriate exposure scenario, considering both inhalation, and chronic low-level effects. In addition, epidemiological data are often ignored.
There has been criticism within the aviation industry that the epidemiological data collected are not robust or specific enough, and that the data lack statistical power. Criticism of evidence is expected from those with an interest in denying connections between exposure and effect [215]. While there is a range of epidemiological study types, none should be ignored [216] in assessing the body of evidence for demonstrating the health effects of exposures. The body of evidence for inferring causation is populated by contributions derived and made available from different epidemiological approaches. These contributions range from the less informative case studies to descriptive analyses comparing observed to expected numbers of illness events across exposed and unexposed groups of people. The contribution to causation of these types of studies is limited, being primarily related to hypothesis generation. Observational studies, including case–control (for rare outcome events) and cohort studies (for more common outcomes), are more influential to discussions about causation. The most influential of the epidemiological study designs to discussions about causation are double-blind randomised controlled trials (RCTs).
Given the above hierarchy of epidemiological studies, to further address question of causation, appropriately designed observational studies would be utilised instead of RCTs because it would not be ethical to undertake RCTs, exposing people to toxic fumes. The most informative of the available observational epidemiological study designs is that of the prospective cohort study. In this design, a large enough group of exposed people is followed up for a long enough time for health outcomes to manifest, and their experience is compared to a large enough unexposed group followed up for an equally long period of time. While this would be ideal, it is inappropriate for addressing Aerotoxic Syndrome for the reason that rare health events never achieve statistical power to demonstrate a difference unless many thousands of people were to be adequately followed up. Feasibility and cost considerations would rule such a design impractical. Therefore, when health outcomes are rare, the optimal design from a cost perspective would be that of the case–control study.
In terms of the evidence to date of health effects associated with contaminated aircraft and fume events, numerous case reports, case-series, and descriptive studies have been undertaken and they should not be ignored.
Importantly, there has been an over-reliance on the lack of exposure data collected during fume events, despite there being no sensors to take measurements. This has been at the expense of failing to consider that the air supply and engine system designs enable exposure to oils to occur in both normal operations (ground and air) and abnormal conditions. There are acknowledged difficulties in identifying the source of oil fumes, due to events often being transient, lack of real time sensors, and maintenance investigation procedures more suited to bigger smoke / failure events. However, positive oil/hydraulic findings have been documented in association with adverse effects and fume events. Of 15 fume events investigated, 87% involved positive maintenance findings of oil fumes, with another said to be likely sourced to oil leakage [26].
The risk assessments thus far have considered effectively ToCP (and not the more plentiful and more toxic mono- and di-ortho isomers) exposure and limited individual substances, which are reported well below exposure limits, yet ignoring the areas identified above. Industrial occupational exposure limits, where they exist, are not protective for aircrew or passengers in aircraft, exposed continually to this complex, thermally degraded mixture.
We consider that prolonged, chronic exposure of aircrew to an aerosol of oil-bearing nanoparticles is a significant feature in the aetiology of the pattern of illness being manifested. Adverse effects continue to occur. Prior attempts to gather epidemiological data have been neither timely, comprehensive, nor systematic. Therefore, this comprehensive medical protocol, set out with a narrative review, is an essential step forward to enable a greater understanding of this specialised environment, with further epidemiological data to be systematically gathered to more precisely explicate risks. The features associated with exposure to oil, other supply air fumes, and adverse effects outlined here is suggestive of a causal link between exposure and health effects in aircrew and passengers. This clearly points to a discreet occupational syndrome that warrants the appropriate gathering of epidemiological data, and the introduction of risk mitigation.
Conclusion
There is extensive literature supporting exposure to oil, hydraulic and other supply air fumes entering the aircraft breathing air during routine as well as abnormal flight conditions. There is a consistent diffuse pattern of adverse effects documented in aircrew and some passengers. This narrative review and protocol of the documentation of people exposed to fume events related to the aircraft supply air provides the first comprehensive and systematic approach in documenting and gathering further epidemiological data on what is a discreet and emerging occupational health syndrome.
Availability of data and materials
DOIs and hyperlinks have been provided throughout the literature cited where applicable. Additional information is provided in the attached supplement, which entails the full medical protocol / narrative review for those seeking further information.
Change history
28 October 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12940-023-01025-3
Notes
. McDonald, M—Medicinal Chemistry, University of Washington, 2015.
Abbreviations
- AChE:
-
Acetyl cholinesterase
- APU:
-
Auxiliary power unit
- BChE:
-
Butyrylcholinesterase
- BNA:
-
Beta-naphthylamine
- CNS:
-
Central nervous system
- CO:
-
Carbon monoxide
- DoCP:
-
Di-ortho-cresyl phosphate
- MoCP:
-
Mono-ortho-cresyl phosphate
- NTE:
-
Neuropathy target esterase
- OP:
-
Organophosphate
- OPICN:
-
Organophosphorus- induced chronic neurotoxicity
- OPIDN:
-
Organophosphorus ester-induced delayed neurotoxicity
- PAN:
-
N-phenyl-1 napthylamine
- PBN:
-
N-2-naphthylaniline
- PNS:
-
Peripheral nervous system
- SVOC:
-
Semi-volatile organic compounds
- TBP:
-
Tributyl phosphate
- TCP:
-
Tricresyl phosphate
- ToCP:
-
Tri-ortho-cresyl phosphate
- TpCP:
-
Tri-para-cresyl phosphate
- TPP:
-
Triphenyl phosphate
- TXP:
-
Trixylyl phosphate
- UFP:
-
Ultrafine particles
- VOC:
-
Volatile organic compounds
References
Flitney R. A Description Of The Types Of High Speed Rotary Shaft Seals In Gas Turbine Engines And The Implications For Cabin Air Quality. J Bio Phys Chem. 2014;14:85–9. https://doi.org/10.4024/17FL14R.jbpc.14.04.
Howard CV, Johnson DW, Morton J, Michaelis S, Supplee D, Burdon J. Is a Cumulative Exposure to a Background Aerosol of Nanoparticles Part of the Causal Mechanism of Aerotoxic Syndrome ? Nanomed Nanosci Res. 2018;JNAN-139:1–8. https://doi.org/10.29011/JNAN-139. 100039.
Howard CV, Michaelis S, Watterson A. The Aetiology of ‘Aerotoxic Syndrome’ - A Toxico-Pathological Viewpoint. Open Acc J of Toxicol. 2017;1(5):555575. https://doi.org/10.19080/OAJT.2017.01.555575.
Johnson D. Turbine Engine Lubricant and Additive Degradation Mechanisms. Aerospace Engineering: IntechOpen; 2018;1–19 https://doi.org/10.5772/intechopen.82398.
Michaelis S. Implementation of the Requirements for the Provision of Clean Air In Crew And Passenger Compartments Using The Aircraft Bleed Air System. Msc Thesis [MSc]. Cranfield, England: Cranfield University; 2016. https://perma.cc/F8KZ-BWSX. Accessed 2 Dec 2022.
Michaelis S. Aircraft Clean Air Requirements Using Bleed Air Systems. Engineering. 2018;10:142–72. https://doi.org/10.4236/eng.2018.104011.
SAE. Airborne Chemicals in Aircraft Cabins. AIR 4766/2. Warrendale, PA, USA: SAE Aerospace; 2021. https://www.sae.org/standards/content/air4766/2a. Accessed 1 Dec 2022.
de Boer J, Antelo A, van der Veen I, Brandsma S, Lammertse N. Tricresyl Phosphate and the Aerotoxic Syndrome of Flight Crew Members - Current Gaps in Knowledge. Chemosphere. 2015;119:S58–61. https://doi.org/10.1016/j.chemosphere.2014.05.015.
Chen R, Fang L, Liu J, Herbig B, Norrefeldt V, Mayer F, et al. Cabin Air Quality on Non-Smoking Commercial Flights: A Review of Published Data on Airborne Pollutants. Indoor Air. 2021;31(4):926–57. https://doi.org/10.1111/ina.12831.
Kitzes G. Cabin Air Contamination Problems in Jet Aircraft. Aviation Medicine. 1956; 27(1): 53-8. PMCID: 13286221. https://perma.cc/QN4C-CB6R
Loomis T, Krop S. Cabin Air Contamination in RB-57A Aircraft. Maryland, USA: Chemical Corps Medical Laboratory, US Army; 1955. Medical Laboratories Special Report (MLSR) No. 61. https://perma.cc/D6G5-LJG6. Accessed 1 Dec 2022.
Reddall HA. Elimination of Engine Bleed Air Contamination. SAE Technical Paper 550185. Warrendale, PA, USA: Society of Automotive Engineers; 1955. https://doi.org/10.4271/550185. Accessed 1 Dec 2022.
Davidson T, Cooley, T. and Way, J. Air Force Experience with Synthetic Gas Turbine Lubricants. SAE Technical Paper 550080. Warrendale, PA, USA: Society of Automotive Engineers; 1955. https://doi.org/10.4271/550080. Accessed 1 Dec 2022.
Rayman R, McNaughton G. Smoke and Fumes in the Cockpit. Aviation, Space and Environmental Medicine. 1983; 54(8): 738-40. PMCID: 6626083. https://pubmed.ncbi.nlm.nih.gov/6626083/
PCA. Air Safety And Cabin Air Quality in the BAe 146 Aircraft - Report by the Senate Rural and Regional Affairs and Transport References Committee - Final Report. Canberra, Australia: Parliament of the Commonwealth of Australia; October, 2000. http://www.aph.gov.au/binaries/senate/committee/rrat_ctte/completed_inquiries/1999-02/bae/report/report.pdf Accessed 1 Dec 2022.
Montgomery M, Wier GT, Zieve F, Anders MW. Human Intoxication Following Inhalation Exposure to Synthetic Jet Lubricating Oil. Clin Toxicol. 1977;11(4):423–6. https://doi.org/10.3109/15563657708988205.
Abou-Donia MB, Abou-Donia MM, ElMasry EM. Autoantibodies to Nervous System-Specific Proteins are Elevated in Sera of Flight Crew Members: Biomarkers for Nervous System Injury. J Toxicol Environ Health A. 2013;76(6):363–80. https://doi.org/10.1080/15287394.2013.765369.
Delayed CL, Impairment C, Inhalation PIFCA. Delayed Cognitive Impairment and Pilot Incapacitation Following Contaminated Air Inhalation. J Biol Phys Chem. 2014;14:107–10. https://doi.org/10.4024/13CO14M.jbpc.14.04.
Hageman G, Pal TM, Nihom J, MackenzieRoss SJ, van den Berg M. Three Patients with Probable Aerotoxic Syndrome. Clinical Toxicology (Phila). 2019;58(2):139–42. https://doi.org/10.1080/15563650.2019.1616092.
Harper A. A Survey of Health Effects in Aircrew Exposed to Airborne Contaminants. J Occup Health Safety - Austr and New Zealand. 2005;21(5):433–9 (https://perma.cc/R4RA-QQNT).
Heutelbeck ARR, Bornemann C, Lange M, Seeckts A, Müller MM. Acetylcholinesterase and Neuropathy Target Esterase Activities in 11 Cases of Symptomatic Flight Crew Members After Fume Events. J Toxicol Environ Health A. 2016;79(22–23):1050–6. https://doi.org/10.1080/15287394.2016.1219561.
Mackenzie RS. Cognitive Function Following Exposure to Contaminated Air on Commercial Aircraft: A Case Series of 27 Pilots Seen for Clinical Purposes. J Nutritional and Environ Med. 2008;17(2):111–26. https://doi.org/10.1080/13590840802240067.
Mackenzie Ross S, Harper A, Burdon J. Ill Health Following Exposure to Contaminated Aircraft Air : Psychosomatic Disorder or Neurological Injury? J Occup Health Safety - Aust NZ. 2006;22(6):521–8.
Michaelis S. A Survey of Health Symptoms in BALPA Boeing 757 Pilots. J Occup Health Safety -Aust and NZ. 2003;19(3):253–61.
Michaelis S. Health and Flight Safety Implications From Exposure to Contaminated Air in Aircraft [PhD thesis]. Sydney, Australia: University of New South Wales; 2010. http://handle.unsw.edu.au/1959.4/50342. Accessed 1 Dec 2022.
Michaelis S, Burdon J, Howard CV. Aerotoxic Syndrome : A New Occupational Disease ? Public Health Panorama (WHO). 2017;3:198–211 (https://apps.who.int/iris/handle/10665/325308).
Murawski J. Case Study: Analysis of Reported Contaminated Air Events at One Major US Airline in 2009–10. Paper ID: AIAA-2011–5089. Proc 41st Intl Conf on Environ Sys, Am Inst Aeronaut Astronaut, 17–21 July, 2011; Portland. AIAA; 2011. p. 1–11 https://doi.org/10.2514/6.2011-5089.
Reneman L, Schagen SB, Mulder M, Mutsaerts HJ, Hageman G, de Ruiter MB. Cognitive Impairment and Associated Loss in Brain White Microstructure in Aircrew Members Exposed to Engine Oil Fumes. Brain Imaging Behav. 2016;10(2):437–44. https://doi.org/10.1007/s11682-015-9395-3.
Roig J, Domingo C, Burdon J, Michaelis S. Irritant-Induced Asthma Caused by Aerotoxic Syndrome. Lung. 2021;199:165–70. https://doi.org/10.1007/s00408-021-00431-z.
Somers M. Aircrew Exposed to Fumes on the Bae 146: An Assessment of Symptoms. J Occup Health and Safety - Austr New Zealand. 2005;21(5):440–9.
Winder C, Balouet JC. Aircrew Exposure to Chemicals in Aircraft: Symptoms of Irritation and Toxicity. J of Occup Health and Safety - Austr New Zealand. 2001;17:471–83 (https://perma.cc/539Z-K2EL).
Winder C, Fonteyn P, Balouet JC. Aerotoxic Syndrome: A Descriptive Epidemiological Survey of Aircrew Exposed to In-Cabin Airborne Contaminants. J Occup Health Safety - Aust NZ. 2002;18(4):321–38 (https://perma.cc/7C6P-V7PL).
Winder C, Michaelis S. Crew Effects from Toxic Exposures on Aircraft. In: Hocking M, editor. Air Quality in Airplane Cabins and Similar Enclosed Spaces. 4 H. Berlin/Heidelberg, Germany: Springer-Verlag; 2005. p. 223–42 https://doi.org/10.1007/b107246. ISBN: 978–3–540–25019–7.
Bachman G, Santos C, Weiland J, Hon S, Lopez G. Aerotoxic Syndrome: Fuming About Fumes While Flying the Friendly Skies. Clin Toxicol. 2017;55(7):773–4. https://doi.org/10.1080/15563650.2017.1348043.
Winder C, Balouet JC, Aerotoxic Sydrome: Adverse Health Effects Following Exposure to Jet Oil Mist During Commercial Flights. ‘editor’ Eddington I. Towards a Safe and Civil Society: Proceedings of the International Congress on Occupational Health Conference; 2000 4–6 September 2000; Brisbane, Australia: ICOH. https://perma.cc/44EC-EMR4.
COT. Position Paper on Cabin Air. London, England: UK Committee Of Toxicity; 2013. https://cot.food.gov.uk/sites/default/files/cot/cotpospapcabin.pdf. Accessed 2 Dec 2022.
de Ree H, van den Berg M, Brand T, Mulder GJ, Simons R, Veldhuijzen van Zanten B, et al. Health Risk Assessment of Exposure to Tricresyl Phosphates (TCPs) in Aircraft: A Commentary. Neurotoxicology 2014; 45: 209–15 https://doi.org/10.1016/j.neuro.2014.08.011.
Schuchardt S, Bitsch A, Koch W, Rosenberger W. EASA Research Project: CAQ Preliminary Cabin Air Quality Measurement Campaign. Final Report EASA_REP_RESEA_2014_4. Cologne, Germany: European Aviation Safety Agency; 2017. https://www.easa.europa.eu/document-library/research-reports/easarepresea20144. Accessed 1 Dec 2022.
Schuchardt S, Koch W, Rosenberger W. Cabin Air Quality – Quantitative Comparison of Volatile Air Contaminants at Different Flight Phases During 177 Commercial Flights. Build Environ. 2019;148:498–507. https://doi.org/10.1016/j.buildenv.2018.11.028.
European Commission. FACTS: Fresh Aircraft. Deliverable 07 - Final Report: Research Study: Investigation of the Quality Level of the Air Inside the Cabin of Large Transport Aeroplanes and its Health Implication. 2021. https://www.facts.aero/images/Status/FACTS-D7-Final_Report.pdf.
EASA. Cabin Air Quality Assessment of Long-Term Effects of Contaminants. Cologne, Germany: European Aviation Safety Agency; 2020. Available from: https://www.easa.europa.eu/research-projects/cabin-air-quality-assessment-long-term-effects-contaminants; https://www.item.fraunhofer.de/en/r-d-expertise/toxicology/cabin-air-quality.html. Accessed 2 Dec 2022.
COT. Updated Literature on Potential Health Risks from Organophosphate Exposure in Aircraft Cabin Air: Committee on Toxicity. London: Committee of Toxicity; 2022. Available from: https://cot.food.gov.uk/Updated%20literature%20on%20potential%20health%20risks%20from%20organophosphate%20exposure%20in%20aircraft%20cabin%20air. Accessed 5 Dec 2022.
ACGIH. TLVs And BEIs - Threshold Limit Values for Chemical Substances and Physical Agents. Cincinnati, Ohio, USA: American Conference of Governmental Industrial Hygienists; 2015. https://www.acgih.org/publications/digital-pubs/#2015tlv. Accessed 2 Dec 2022.
Watterson A, Michaelis S, Use of Exposure Standards in Aviation. ‘editor’ 2017 International Aircraft Cabin Air Conference; 2019; London, England: Journal of Health and Pollution https://doi.org/10.5696/2156-9614-9.24.191201.
Cone JE. Aircraft Cabin Air Quality Trends Relative to Ground Level Standards. In: Hocking M, editor. Air Quality in Airplane Cabins and Similar Enclosed Spaces. Berlin, Heidelberg: Springer Berlin Heidelberg; 2005. p. 293–315 https://doi.org/10.1007/b107249 ISBN: 978–3–540–31491–2.
Winder C. Hazardous Chemicals on Jet Aircraft: Jet Oils and Aerotoxic Syndrome. Curr Topics Toxicol. 2006;3:65–88 (https://hub.easa.europa.eu/crt/docs/viewcrdattachment/cid_40654/aid_448/fmd_9b32d365f4af18b1381b5c19227bdf9f).
CAA. Information for Health Professionals on Aircraft Fume Events. Civil Aviation Authority - UK Department for Transport/Division 2017. https://www.caa.co.uk/passengers/before-you-fly/am-i-fit-to-fly/guidance-for-health-professionals/aircraft-fume-events/. Accessed 1 Dec 2022.
Harrison R, Murawski J, Mcneely E, Guerriero J, Milton D. Exposure to Aircraft Bleed Air Contaminants Among Airline Workers - A Guide for Health Care Providors. San Francisco, CA, USA: Occupational Health Research Consortium in Aviation (OHRCA); 2009. http://www.ohrca.org/medical-protocols-for-crews-exposed-to-engine-oil-fumes-on-aircraft/. Accessed 1 Dec 2022.
IATA. IATA Guidance for Airline Health and Safety Staff on the Medical Response to Cabin Air Quality Events: Smoke, Fumes/Odours. Montreal, Canada: International Air Transport Association; 2015. https://www.iata.org/contentassets/ccbdc54681c24574bebf2db2b18197a5/guidance-medical-response-cabin-air-events.pdf. Accessed 1 Dec 2022.
BG Verkehr. Medizinisches Standardverfahren Nach Fume-Events (Standard medical procedure after fume events). Hamburg: BG Verkehr; 2014. Available from: https://www.bg-verkehr.de/redaktion/medien-und-downloads/informationen/branchen/luftfahrt/standard-verfahren-eng.pdf. Accessed 2 Dec 2022.
CEN. Cabin Air Quality on Civil Aircraft - Chemical Compounds - Technical Report. Brussels: CEN CENELEC; 2022. PD CEN/TR 17904:2022. https://www.en-standard.eu/pd-cen-tr-17904-2022-cabin-air-quality-on-civil-aircraft-chemical-compounds/.
Heutelbeck A, Baur X, Belpoggi F, Budnik LT, Burdon J, Gee D, et al., On the Need for a Standardized Human Biomonitoring Protocol for In-Flight Incidents (Called “Fume Events”). ‘editor’ 2nd International DiMoPEx Conference on “Pollution in Living and Working Environments and Health,” DiMoPEx Working Groups Meeting; 2018. 30–31 October 2017; Cesare Maltoni Cancer Research Center, Ramazzini Institute, Bologna, Italy: J Health Pollution https://doi.org/10.5696/2156-9614.8.17.1.
Guan J, Gao K, Wang C, Yang X, Lin C-H, Lu C, et al. Measurements of Volatile Organic Compounds in Aircraft Cabins. Part I: Methodology and Detected VOC Species in 107 Commercial Flights. Build Environ. 2014;72:154–61. https://doi.org/10.1016/j.buildenv.2013.11.002.
Honeywell. Honeywell Engineering Investigation Report - Customer Bleed Air Testing Engine Model Alf502R-5- Engine Serial Number LF 05311. Report No. 21–11156, 3 March 2000; Including Honeywell Air Quality Test For LF 502 Engine, S/N 5311, Test Cell 956 -December,1999. Phoenix: Honeywell; 2000.
ASHRAE. Guideline 28: Air Quality within Commercial Aicraft. Atlanta, GA, USA: ASHRAE; 2021.
SHK. Report Rl 2001: 41e. Incident Onboard Aircraft Se-Dre. Sweden, 12 November 1999. Stockholm, Sweden: Statens Haverikommission (SHK) Board of Accident Investigation; 2001 23 November 2001. https://www.havkom.se/assets/reports/rl2001_41e.pdf. Accessed 5 Dec 2022.
Mackerer CR, Barth ML, Krueger AJ, Roy TA. Comparison of Neurotoxic Effects and Potential Risks From Oral Administration or Ingestion of Tricresyl Phosphate and Jet Engine Oil Containing Tricresyl Phosphate. J Toxicol Environ Health A. 1999;57(5):293–328. https://doi.org/10.1080/009841099157638.
Winder C, Balouet J-C. The Toxicity of Commercial Jet Oils. Environ Res. 2002;89(2):146–64. https://doi.org/10.1006/enrs.2002.4346.
SAE. Supplemental Information for design of Measuring devices For Measurement of Aircraft Cabin Air Quality. AIR 6945. Warrendale, PA, USA: SAE Aerospace; 2021. https://www.sae.org/standards/content/air6945/. Accessed 5 Dec 2022.
Henschler D. Die Trikresylphosphatvergiftung. Experimentelle Klärung von Problemen der Ätiologie und Pathogenese (Tricresyl Phosphate Poisoning. Experimental Clarification of Problems of Etiology and Pathogenesis). Klinische Wochenscrifte. 1958;36(14):663–74. https://doi.org/10.1007/BF01488746 (https://perma.cc/A465-LDSR).
Mackerer C, Ladov E. Mobil Oil Submission (14a): Submission to the Inquiry Into Air Safety - BAe 146 Cabin Air Quality: Vol 3. Canberra, Australia: Parliament of the Commonwealth of Australia; 2000. https://perma.cc/BA88-8XAJ. Accessed 1 Dec 2022.
Howard CV. Inappropriate Use of Risk Assessment in Addressing Health Hazards Posed by Civil Aircraft Cabin Air. Open Access J Toxicol. 2020;4(2):555634. https://doi.org/10.19080/OAJT.2020.04.555634.
Megson D, Ortiz X, Jobst KJ, Reiner EJ, Mulder MFA, Balouet J-C. A Comparison of Fresh and Used Aircraft Oil for the Identification of Toxic Substances Linked to Aerotoxic Syndrome. Chemosphere. 2016;158:116–23. https://doi.org/10.1016/j.chemosphere.2016.05.062.
Bondy HF, Field EJ, Worden AN, Hughes JP. A study on the acute toxicity of the tri-aryl phosphates used as plasticizers. Br J Ind Med. 1960;17(3):190–200. https://doi.org/10.1136/oem.17.3.190.PMCID:PMC1039179.
Aldridge WN. Tricresyl Phosphates and Cholinesterase. Biochem J. 1954;56(2):185–9. https://doi.org/10.1042/bj0560185.
Baker PE, Cole TB, Cartwright M, Suzuki SM, Thummel KE, Lin YS, et al. Identifying Safer Anti-Wear Triaryl Phosphate Additives for Jet Engine Lubricants. Chem Biol Interact. 2013;203(1):257–64. https://doi.org/10.1016/j.cbi.2012.10.005.
Craig PH, Barth ML. Evaluation of the Hazards of Industrial Exposure to Tricresyl Phosphate: A Review and Interpretation of the Literature. J Toxicol Environ Health Part B. 1999;2(4):281–300. https://doi.org/10.1080/109374099281142.
Freudenthal RI, Rausch L, Gerhart JM, Barth ML, Mackerer CR, Bisinger EC. Subchronic Neurotoxicity of Oil Formulations Containing Either Tricresyl Phosphate or Tri-orthocresyl Phosphate. Int J Toxicol. 1993;12(4):409–16. https://doi.org/10.1177/109158189301200410.
Spengler J, Vallarino J, Mcneely E, Estephan H. In-Flight/Onboard Monitoring: ACER’s Component for ASHRAE, 1262, Part 2. Boston, MA, USA: Research in the Intermodal Transport Environment/Aircraft Cabin Environment Research; 2012. https://www.faa.gov/data_research/research/med_humanfacs/cer/media/In-FlightOnboardMonitoring.pdf. Accessed 1 Dec 2022.
FAA. Aircraft Air Quality and Bleed Air Contamination Detection. William J. Hughes Technical Center; 2022. DOT/FAA/TC-21/45. http://www.tc.faa.gov/its/worldpac/techrpt/tc21-45.pdf. Accessed 1 Dec 2022.
SAE, SAE Aerospace Information Report Air 7521: Measurement Data and Reference Values for Compounds Potentially Found in Aircraft Engine Bleed Air. ‘editor’ SAE Aerospace Information Report AIR 7521; 2018; Warrendale: SAE International. https://www.sae.org/standards/content/air7521/.
CAA. CAA Paper 2004/04: Cabin Air Quality. Gatwick Airport, W. Sussex, England: Civil Aviation Authority, UK Department for Transport 2004. https://publicapps.caa.co.uk/docs/33/CAPAP2004_04.PDF. Accessed 1 Dec 2022.
Houtzager M, Noort D, Joosen M, Bos J, Jongeneel R, van Kesteren P, et al. Characterisation of the Toxicity of Aviation Turbine Engine Oils After Pyrolysis (AVOIL). Cologne, Germany: European Aviation Safety Agency; 2017. https://www.easa.europa.eu/document-library/research-reports/easarepresea20152. Accessed 1 Dec 2022.
ASHRAE. ASHRAE Research Project Report 1830-RP: Experimental Characterization of Aircraft Bleed Air Particulate Contamination. Atlanta, GA, USA; 2022.
Jones B, Amiri S, Roth J, Hosni M. The Nature of Particulates in Aircraft Bleed Air Resulting from Oil Contamination. LV-17-C046. 2017 ASHRAE Winter Conference; 28 January - 1 February 2017; Las Vegas, NV, USA. American Society of Heating, Refrigerating, and Air Conditioning Engineers; 2017.
Michaelis S, Loraine T, Howard CV. Ultrafine Particle Levels Measured On-Board Short-Haul Commercial Passenger Jet Aircraft. Environ Health. 2021;20(89):1–14. https://doi.org/10.1186/s12940-021-00770-7.
Crump D, Harrison P, Walton C. Aircraft Cabin Air Sampling Study; Part 1 and 2 of the Final Report. Cranfield, England: Institute of Environment and Health, Cranfield University; 2011. http://dspace.lib.cranfield.ac.uk/handle/1826/5305. http://dspace.lib.cranfield.ac.uk/handle/1826/5306. Accessed 1 Dec 2022.
van Netten C, Leung V. Hydraulic Fluids and Jet Engine Oil: Pyrolysis and Aircraft Air Quality. Arch Environ Health. 2001;56(2):181–6. https://doi.org/10.1080/00039890109604071.
Fox R. Assessing Aircraft Supply Air to Recommend Compounds for Timely Warning of Contamination [PhD]. San Diego, CA, USA: Northcentral University; 2012. https://www.proquest.com/docview/1021859974/2E9451C80D5A4304PQ/1. Accessed 1 Dec 2022.
Fox R, Chase D, Kurlak P, Koerner M, Hagh B, Johnson R, et al., inventors; Honeywell International Inc., assignee. Human Factors Approach to Control Contaminant Concentrations in Aircraft Supply Air from Engine and APU Bleed Air Air and Ground Air Sources, and in Recirculated Air Being Delivered to Aircraft Cabins for the Optimization of User Experience and Energy Consumption. USA patent US9902499B2. 2018. https://worldwide.espacenet.com/patent/search?q=pn%3DUS9902499B2
Hageman G, Mackenzie Ross SJ, Nihom J, van der Laan G. Aerotoxic syndrome: A new occupational disease caused by contaminated cabin air? Advances in Neurotoxicology: Academic Press; 2022. https://doi.org/10.1016/bs.ant.2022.04.001. ISBN: 2468–7480.
Rosenberger W. Effect of Charcoal Equipped HEPA Filters on Cabin Air Quality in Aircraft: A Case Study Including Smell Event Related In-Flight Measurements. Build Environ. 2018;143:358–65. https://doi.org/10.1016/j.buildenv.2018.07.031.
Williams PI, Trembath J. Simultaneous Inboard and Outboard, Inflight Measurements of Ultrafine Particle Concentrations. Aerosol Sci Technol. 2021;55:614–22. https://doi.org/10.1080/02786826.2021.1880544.
Michaelis S. Aircraft contaminated air: A Brief Outline. IJSA. 2022;8(3):249–59. https://doi.org/10.1504/IJSA.2022.10047170.
Anderson J. Sources of Onboard Fumes and Smoke Reported by US Airlines. Aerospace. 2021;8:122. https://doi.org/10.3390/aerospace8050122.
ICAO. Guidelines on Education, Training and Reporting Practices Related to Fume Events. Montréal, Canada: International Civil Aviation Organization; 2015.
Chupp R, Hendricks R, Lattime Sea. NASA/TM-2006–214341. Sealing in Turbomachinery. Cleveland; 2006. https://ntrs.nasa.gov/citations/20060051674. Accessed 5 Dec 2022.
ECHA. Conclusion: Substance Evaluation Conclusion as Required by REACH Article 48 and Evaluation Report for Tris(methylphenyl) phosphate: CAS No 1330–78–5. Helsinki: European Chemical Agency; 2021. https://echa.europa.eu/documents/10162/25602787-d660-8be0-14c3-bcb7d2155f82. Accessed 5 Dec 2022.
ExxonMobil. Jet Engine Oil System- Part 1. 2014. Available from: https://www.exxonmobil.com/en/aviation/knowledge-library/resources/jet-engine-oil-system-1. Accessed 5 Dec 2022.
Lin CH, Horstman R, Bates G, inventors; Boeing, assignee. Seal Assembly And Method For Reducing Aircraft Engine Oil Leakage. patent US2018340546A1 https://worldwide.espacenet.com/patent/search?q=pn%3DUS2018340546A1. 2018.
Homeyer C, Schillinger T, inventors; Rolls Royce, assignee. Bearing Chamber Venting System for an Aircraft Engine and method for providing a Required Pressure Ratio at Bearing Chamber Seals of an Air-Sealed bearing Chamber patent US 2014/0096533 A1: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2014096533A1 2014 April 10.
Lixian LI, Di Matteo M, Hendrick P. Experimental Study to Verify Oil Loss Through the Vent Line of the Aero-Engine Lubrication System. 9TH European Conference For Aeronautics And Space Sciences (EUCASS); 27 June- 1 July; Lille, France. 2022. https://doi.org/10.13009/EUCASS2022-4391.
Shehadi M, Jones B, Hosni M. Characterization of the Frequency and Nature of Bleed Air Contamination Events in Commercial Aircraft. Indoor Air. 2015;26:478–88. https://doi.org/10.1111/ina.12211.
Kurebayashi H, Tanaka A, Yamaha T. Metabolism and Disposition of the Flame Retardant Plasticizer, Tri-p-Cresyl Phosphate, in the Rat. Toxicol Appl Pharmacol. 1985;77(3):395–404. https://doi.org/10.1016/0041-008X(85)90179-6.
Carletti E, Schopfer LM, Colletier JP, Froment MT, Nachon F, Weik M, et al. Reaction Of Cresyl Saligenin Phosphate, the Organophosphorus Agent Implicated in Aerotoxic Syndrome, With Human Cholinesterases: Mechanistic Studies Employing Kinetics, Mass Spectrometry, And X-Ray Structure Analysis. Chem Res Toxicol. 2011. https://doi.org/10.1021/tx100447k.
Liyasova M, Li B, Schopfer LM, Nachon F, Masson P, Furlong CE, et al. Exposure to Tri-o-cresyl Phosphate Detected in Jet Airplane Passengers. Toxicol Appl Pharmacol. 2011;256(3):337–47. https://doi.org/10.1016/j.taap.2011.06.016.
Reinen J, Nematollahi L, Fidder A, Vermeulen NP, Noort D, Commandeur JN. Characterization of Human Cytochrome P450s Involved in the Bioactivation of Tri-ortho-Cresyl phosphate (ToCP). Chem Res Toxicol. 2015;28(4):711–21. https://doi.org/10.1021/tx500490v.
Masson P, Lushchekina S, Schopfer LM, Lockridge O. Effects of Viscosity and Osmotic Stress on the Reaction of Human Butyrylcholinesterase with Cresyl Saligenin Phosphate, a Toxicant Related to Aerotoxic Syndrome: Kinetic and Molecular Dynamics Studies. Biochem J. 2013;454(3):387–99. https://doi.org/10.1042/BJ20130389.
Schopfer LM, Masson P, Lamourette P, Simon S, Lockridge O. Detection Of Cresyl Phosphate-Modified Butyrylcholinesterase In Human Plasma For Chemical Exposure Associated With Aerotoxic Syndrome. Anal Biochem. 2014;461:17–26. https://doi.org/10.1016/j.ab.2014.05.021.
Schindler BK, Weiss T, Schütze A, Koslitz S, Broding HC, Bünger J, et al. Occupational Exposure of Air Crews to Tricresyl Phosphate Isomers and Organophosphate Flame Retardants After Fume Events. Arch Toxicol. 2013;87(4):645–8. https://doi.org/10.1007/s00204-012-0978-0.
Furlong CE. Exposure to Triaryl Phosphates: Metabolism and Biomarkers of Exposure. J Biol Phys Chem. 2011;11:1–11. https://doi.org/10.4024/28FU11A.jbpc.11.04.
Worek F, Schilha M, Neumaier K, Aurbek N, Wille T, Thiermann H, et al. On-site analysis of acetylcholinesterase and butyrylcholinesterase activity with the ChE check mobile test kit—Determination of reference values and their relevance for diagnosis of exposure to organophosphorus compounds. Toxicol Lett. 2016;249:22–8. https://doi.org/10.1016/j.toxlet.2016.03.007.
Ruhnau P, Müller M, Hallier E, Lewalter J. Ein Validiertes Photometrisches Verfahren Für Die Bestimmung Der Neuropathy Target Esterase Aktivität In Humanen Lymphozyten. A Validated Photometric Assay for the Neuropathy Target Esterase Activity in Human Lymphocytes. Umweltmedizin in Forschung und Praxis. 2001;6:101–3.
Abou-Donia M. Mechanisms of Organophosphorus Ester-Induced Delayed Neurotoxicity: Type-I and Type-II. Annu Rev Pharmacol Toxicol. 1990;30:405–40. https://doi.org/10.1146/annurev.pa.30.040190.002201.
Somkuti SG, Abou-Donia MB. Disposition, Elimination, And Metabolism of Tri-o-cresyl phosphate Following Daily Oral Administration in Fischer 344 Male Rats. Archives of Toxicology. 1990;64(7):572-9. https://doi.org/10.1007/BF01971837.
Cone J. Interim Report No. 1 & 2 - Association Of Professional Flight Attendants Health Survey. San Francisco General Hospital Medical Center. Occupational Health Clinic. September 1983, June 1984. San Francisco: San Francisco General Hospital Medical Centre 1984. https://perma.cc/CDG6-HXE5.
Cox L, Michaelis S. A Survey of Health Symptoms in BAe 146 Aircrew. J of Occup Health and Safety - Aust NZ. 2002;18(4):305–12 (https://stir.ac.uk/9du).
van Netten C. Air Quality and Health Effects Associated with the Operation of BAe 146–200 Aircraft. Appl Occup Environ Hyg. 1998;13(10):733–9. https://doi.org/10.1080/1047322X.1998.10390150.
Coxon L. Neuropsychological Assessment of a Group of BAe 146 Aircraft Crew Members Exposed to Jet Engine Oil Emissions. J of Occup Health and Safety - Aust and New Zealand. 2002;18(4):313–9 (https://researchrepository.murdoch.edu.au/id/eprint/1669/).
Hecker S, Kincl L, McNeely E, van Netten C, Harrison R, Murawski J, et al. Cabin Air Quality Incidents Project Report. Occupational Health Research Consortium in Aviation (OHRCA) and Airliner Cabin Environment Research (ACER); 2014. http://www.ohrca.org/wp-content/uploads/2014/08/finalreport.pdf. Accessed 1 Dec 2022.
Mackenzie Ross SJ, Harrison V, Madeley L, Davis K, Abraham-Smith K, Hughes T, et al. Cognitive Function Following Reported Exposure to Contaminated Air on Commercial Aircraft: Methodological Considerations for Future Researchers. J Biol Phys Chem. 2011;11:180–91. https://doi.org/10.4024/30MA11A.jbpc.11.04.
McNeely E, Gale S, Tager I, Kincl L, Bradley J, Coull B, et al. The Self-Reported Health of US Flight Attendants Compared to the General Population. Environ Health. 2014;13(13):1–11. https://doi.org/10.1186/1476-069X-13-13.
McNeely E, Mordukhovich I, Tideman S, Gale S, Coull B. Estimating the Health Consequences of Flight Attendant Work: Comparing Flight Attendant Health to the General Population in a Cross-Sectional Study. BMC Public Health. 2018;18(346):1–11. https://doi.org/10.1186/s12889-018-5221-3.
Passon D. The International Crew Health Survey. J Biol Phys Chem. 2011;11(201):207. https://doi.org/10.4024/26PA11A.jbpc.11.04.
Burdon J. Lung Injury Following Hydrocarbon Inhalation in Aircrew. J Biol Phys Chem. 2012;12:98–102.
Burdon J, Glanville A. Lung Injury Following Hydrocarbon Inhalation in BAe 146 Aircrew. J Occup Health Safety Aust NZ. 2005;21:450–4. https://doi.org/10.1007/b107244.
Heuser G, Auillera O, Heuser S, Gordon R. Clinical Evaluation of Flight Attendants After Exposure to Fumes in Cabin Air. J Occup Health and Safety - Aust NZ. 2005; 21(5): 455–9. https://perma.cc/QJ8Q-XV6V.
Heutelbeck A. Progress Report: Diagnostics of Health Disorders and Biomonitoring in Aircraft Crewmembers After "Fume Events" - Preliminary Results After Analysing Patient Files. International Aircraft Cabin Air Conference 2017; 19-20 September 2017; Imperial College, London, England. J Health Pollution 2019;S38-S42https://doi.org/10.5696/2156-9614-9.24.191201
Pinkerton L, Hein J, Grajewski B, Kamel F. Mortality from Neurodegenerative Diseases in a Cohort of US Flight Attendants. Am J Ind Med 2016;532–7 https://doi.org/10.1002/ajim.22608.
Diamond M. Cabin Air Quality Monitoring – Organophosphates Sampling during Fume Events in Australia. In: Scholz D, editor. Aircraft Cabin Air International Conference 2021; 15-18 March Virtual. 2021. https://doi.org/10.5281/zenodo.5567739.
Feuillie V, Onboard Fume Events: Short Term Health Consequences in Aircrew -Air France Medical Department, Occupational Health Services. ‘editor’ EASA Workshop, Cologne, 30–31 January 2020; 2020; Cologne: European Aviation Safety Agency. https://www.easa.europa.eu/en/downloads/113142/en.
O’Connor L, Boland M. A Series of Three Aircraft Emergency Public Health Alerts at an International Airport Over a Six Week Period due to Suspected Contaminated Cabin Air. JESPH. 2020;4(3):296–303. https://doi.org/10.26502/jesph.96120101.
Michaelis S. Contaminated Aircraft Cabin Air. J Biological Physics and Chemistry. 2011;11:132–45. https://doi.org/10.4024/24MI11A.jbpc.11.04.
Burdon J. Respiratory Symptoms and Lung Injury After Inhaling Fumes on Aircraft: Toxic Fumes or Hyperventilation? J Biol Phys Chem. 2014;14(4):103–6. https://doi.org/10.4024/21BU14A.jbpc.14.04.
Wolkoff P, Crump DR, Harrison PTC. Pollutant Exposures and Health Symptoms in Aircrew and Office Workers: Is There a Link? Environ Int. 2016;87:74–84. https://doi.org/10.1016/j.envint.2015.11.008.
Erdely A, Hulderman T, Salmen R, Liston A, Zeidler-Erdely PC, Schwegler-Berry D, et al. Cross-Talk Between Lung and Systemic Circulation During Carbon Nanotube Respiratory Exposure. Potential Biomarkers Nano Letters. 2009;9(1):36–43. https://doi.org/10.1021/nl801828z.
Guzman D, Broderick B, Sotolongo A, Osinubi O, Helmer D. A Case of Sarcoidosis in a Veteran With Military-Related Environmental and Occupational Exposure. Chest. 2017;152(4):A830. https://doi.org/10.1016/j.chest.2017.08.862.
He R, Houtzager M, Jongeneel W, Westerink R, Cassee F. In Vitro Hazard Characterization of Simulated Aircraft Cabin Bleed-Air Contamination in Lung Models Using an Air-Liquid Interface (ALI) Exposure System. Environ Int. 2021;156:106718. https://doi.org/10.1016/J.ENVINT.2021.106718.
Harris J, Blain P. Neurotoxicology: What the Neurologist Needs to Know. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 3):iii29–34. https://doi.org/10.1136/jnnp.2004.046318.
Abou-Donia MB, Van de Goot FRW, Mulder MFA. Autoantibody Markers of Neural Degeneration are Associated with Post-Mortem Histopathological Alterations of a Neurologically Injured Pilot. J Biol Phys Chem. 2014;14:34–53. https://doi.org/10.4024/05AB14A.jbpc.14.03.
Hageman G, Pal TM, Nihom J, Ross SJM. Aerotoxic Syndrome: Discussion of Possible Diagnostic Criteria. Clin Toxicol. 2019;58(5):414–6. https://doi.org/10.1080/15563650.2019.1649419.
Reneman L, Schagen SB, Mulder M, Mutsaerts HJ, Hageman G, de Ruiter MB. Cognitive Impairment And Associated Loss In Brain White Microstructure in Aircrew Members Exposed to Engine Oil Fumes. Brain Imaging Behav. 2016;10:437–44. https://doi.org/10.1007/s11682-015-9395-3.
Axelrad JC, Howard CV, McLean WG. The Effects of Acute Pesticide Exposure on Neuroblastoma Cells Chronically Exposed to Diazinon. Toxicology. 2003;185(1–2):67–78. https://doi.org/10.1016/S0300-483X(02)00592-9.
Cherry N, Mackness M, Durrington P, Povey A, Dippnall M, Smith T, et al. Paraoxonase (PON1) Polymorphisms in Farmers Attributing Ill Health to Sheep Dip. Lancet. 2002;359(9308):763–4. https://doi.org/10.1016/S0140-6736(02)07847-9.
Cherry NMM, Mackness B, et al. ‘Dippers’ Flu’ And Its Relationship to PON1 Polymorphisms. Occup Environ Med. 2011;68(3):211–7. https://doi.org/10.1136/oem.2009.052126.
Naughton SX, Terry AV Jr. Neurotoxicity in Acute and Repeated Organophosphate Exposure. Toxicology. 2018;408:101–12. https://doi.org/10.1016/j.tox.2018.08.011.PMCID:PMC6839762.
Terry A Jr. Functional Consequences of Repeated Organophosphate Exposure: Potential Non-Cholinergic Mechanisms. Pharmacol Ther. 2012;134(3):355–65. https://doi.org/10.1016/j.pharmthera.2012.03.001.
Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol. 2010;32(4):452–9. https://doi.org/10.1016/j.ntt.2010.03.004.PMCID:PMC3042861.
Abou-Donia MB. Organophosphorus Ester-Induced Chronic Neurotoxicity. Arch Environ Health. 2003;58(8):484–97. https://doi.org/10.3200/AEOH.58.8.484-497.
Abou-Donia MB. Organophosphate Ester Induced Chronic Neurotoxicity. J Occup Health Saf-Aust NZ. 2005;21(5):408–32.
Davies R, Ahmed G, Freer T. Chronic Exposure to Organophosphates: Background And Clinical Picture. Adv Psychiatr Treat. 2000;6(3):187–92. https://doi.org/10.1192/apt.6.3.187.
Costa LG. Organophosphorus Compounds at 80: Some Old and New Issues. Toxicol Sci. 2018;162:24–35. https://doi.org/10.1093/toxsci/kfx266.
Nowak D, Rakete S, Suojalehto H. Indoor Environment. In: Feary J, Suojalehto H, Cullinan P, editors. Occupational and Environmental Lung Disease [Eur Respir Monogr]. Sheffield: European Respiratory Society; 2020. p. 317–34 https://doi.org/10.1183/2312508X.erm8920.
Merwin SJ, Obis T, Nunez Y, Re DB. Organophosphate Neurotoxicity to the Voluntary Motor System on the Trail of Environment-Caused Amyotrophic Lateral Sclerosis: The Known, the Misknown And the Unknown. Arch Toxicol. 2017;91(8):2939–52. https://doi.org/10.1007/s00204-016-1926-1.
Jokanović M. Neurotoxic Effects of Organophosphorus Pesticides and Possible Association with Neurodegenerative Diseases in Man: A Review. Toxicology. 2018;410:125–31. https://doi.org/10.1016/j.tox.2018.09.009.
Mostafalou S, Abdollahi M. The Link of Organophosphorus Pesticides with Neurodegenerative and Neurodevelopmental Diseases Based on Evidence and Mechanisms. Toxicology. 2018;409:44–52. https://doi.org/10.1016/j.tox.2018.07.014.
Nicholas J, Lackland D, Dosemeci M. Mortality Among US Commercial Pilots and Navigators. J Occup Environ Med. 1998;40(11):980–5. https://doi.org/10.1097/00043764-199811000-00008.
Nicholas JS, Butler GC, Lackland DT, Tessier GS, Mohr J, Hoel DG. Health Among Commercial Airline Pilots. Aviat Space Environ Med. 2001;72(9):821–6 (PMCID: 11565817).
ECHA. CLP Classification and Labelling Database. Helsinki: European Chemicals Agency; 2021. Available from: https://echa.europa.eu/information-on-chemicals/cl-inventory-database. Accessed 1 Dec 2022.
Michaelis S. REACH-CLP Review of Oils Hydraulic & Deicing Fluids: S Michaelis University of Stirling; 2019. https://perma.cc/MD9N-7FM9 Accessed 1 Dec 2022.
High Court of Australia: East West Airlines Ltd V Turner [2010] HCATrans 238 (3 September 2010) & Original Decision: Turner V Eastwest Airlines Limited [2009] NSWDDT , 5 May 2009(2010). http://www.austlii.edu.au/au/other/HCATrans/2010/238.html. http://www.austlii.edu.au/au/cases/nsw/NSWDDT/2009/10.html. Accessed 2 Dec 2022.
European Commission. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures (CLP). (2009). https://eur-lex.europa.eu/legal-content/EN/LSU/?uri=celex%3A32008R1272.
UN. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). New York, Geneva: United Nations; 2017. https://unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev07/English/ST_SG_AC10_30_Rev7e.pdf, https://unece.org/ghs-implementation-0. Accessed 1 Dec 2022.
PCA. BAe Hansard: Oral Evidence - Inquiry Into Air Safety - BAe 146 Cabin Air Quality. Canberra: Parliament Of The Commonwealth Of Australia 1999. https://perma.cc/W3TF-LLBZ.
Otto D. Andrew K Myers: Workers Compensation Board, State of Oregon. WCB Case No. 19–02648 - Opinion and Order. July 31, 2020. Oregon: Workers Compensation Board, State of Oregon; 2020. https://perma.cc/BZ6L-STQL. https://perma.cc/B3PL-R7A7.
ExxonMobil. Material Safety Data Sheet: Mobil Jet Oil II. Antwerpen, Belgium; 2019. https://perma.cc/6YQ8-XL27.
Eastman. Eastman Turbo Oil 2197 Safety Data Sheet. Kingsport, TN: Eastman Chemical Company; 2020. https://perma.cc/R3N2-5WMQ.
Eastman. Skydrol PE-5 - Hydraulic Fluid Safety Data Sheet. Eastman Chemical Company; 2019. https://perma.cc/V5TW-R4VH.
Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, et al. Combustion-Derived Nanoparticles: A Review of Their Toxicology Following Inhalation Exposure. Part Fibre Toxicol. 2005;2:10.
Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. Ultrafine Particulate Pollutants Induce Oxidative Stress and Mitochondrial Damage. Environ Health Perspect. 2003;111(4):455–60. https://doi.org/10.1289/ehp.6000.
Wåhlin P, Palmgren F, Van Dingenen R, Raes F. Pronounced Decrease of Ambient Particle Number Emissions From Diesel Traffic in Denmark After Reduction of the Sulphur Content in Diesel Fuel. Atmos Environ. 2001;35(21):3549–52. https://doi.org/10.1016/S1352-2310(01)00066-8.
Wichmann H, Peters A. Epidemiological Evidence of the Effects of Ultrafine Particle Exposure. Trans R Soc Lond. 2000;A358:2751–69. https://doi.org/10.1098/rsta.2000.0682.
Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, et al. Ambient Particulate Pollutants in the Ultrafine Range Promote Early Atherosclerosis and Systemic Oxidative Stress. Circ Res. 2008;102(5):589–96. https://doi.org/10.1161/CIRCRESAHA.107.164970.
Künzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, et al. Public-Health Impact Of Outdoor And Traffic-Related Air Pollution: A European Assessment. Lancet: Lancet Publishing Group 2000; 356(9232):795–801 https://doi.org/10.1016/S0140-6736(00)02653-2.
Pope CA, Dockery DW. Health Effects of Fine Particulate Air Pollution: Lines That Connect. J Air Waste Manag Assoc. 2006;56(6):709–42. https://doi.org/10.1080/10473289.2006.10464485.
Holgate S, Grigg J, al e. Every Breath We Take: The Lifelong Impact Of Air Pollution. Royal College of Physicians; 2016. https://www.rcplondon.ac.uk/projects/outputs/every-breath-we-take-lifelong-impact-air-pollution. Accessed 2 Dec 2022.
Zhang J, Chen Z, Shan D, Wu Y, Zhao Y, Li C, et al. Adverse effects of exposure to fine particles and ultrafine particles in the environment on different organs of organisms. J Environ Sci. 2022. https://doi.org/10.1016/j.jes.2022.08.013.
Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, et al. Long Term Exposure to Ambient Air Pollution and Incidence of Acute Coronary Events: Prospective Cohort Study and Meta-Analysis in 11 European Cohorts From the Escape Project. BMJ (Online). 2014;348:f7412. https://doi.org/10.1136/bmj.f7412.
Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung S-H, Mortimer K, et al. Air Pollution And Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest. 2019;155(2):409–16. https://doi.org/10.1016/j.chest.2018.10.042.
Bonner JC. Nanoparticles as a Potential Cause of Pleural and Interstitial Lung Disease. Proc Am Thorac Soc. 2010;7(2):138–41. https://doi.org/10.1513/pats.200907-061rm.
Zhang X, Chen X, Zhang X. The Impact of Exposure to Air Pollution on Cognitive Performance. Proc Natl Acad Sci. 2018;115(37):9193–7. https://doi.org/10.1073/PNAS.1809474115.
Carey IM, Anderson HR, Atkinson RW, Beevers SD, Cook DG, Strachan DP, et al. Are Noise and Air Pollution Related to the Incidence of Dementia? A Cohort Study in London, England. BMJ Open. 2018;8(9):e022404. https://doi.org/10.1136/bmjopen-2018-022404.
Lavigne E, Lima I, Hatzopoulou M, Van Ryswyk K, van Donkelaar A, Martin RV, et al. Ambient ultrafine particle concentrations and incidence of childhood cancers. Environ Int. 2020;145:106135. https://doi.org/10.1016/j.envint.2020.106135.
Liao C-M, Chio C-P, Chen W-Y, Ju Y-R, Li W-H, Cheng Y-H, et al. Lung cancer risk in relation to traffic-related nano/ultrafine particle-bound PAHs exposure: A preliminary probabilistic assessment. J Hazard Mater. 2011;190(1):150–8. https://doi.org/10.1016/j.jhazmat.2011.03.017.
Wu AH, Fruin S, Larson TV, Tseng C-C, Wu J, Yang J, et al. Association between Airport-Related Ultrafine Particles and Risk of Malignant Brain Cancer: A Multiethnic Cohort Study. Can Res. 2021;81(16):4360–9. https://doi.org/10.1158/0008-5472.Can-21-1138.
Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The Epidemiology of Glioma in Adults: a “State of the Science" Review. Neuro Oncol. 2014;16(7):896–913. https://doi.org/10.1093/neuonc/nou087.
Li Z, Guan J, Yang X, Lin CH. Source Apportionment of Airborne Particles in Commercial Aircraft Cabin Environment: Contributions From Outside and Inside of Cabin. Atmos Environ. 2014;89:119–28. https://doi.org/10.1016/j.atmosenv.2014.01.042.
Fushimi A, Saitoh K, Fujitani Y, Takegawa N. Identification of Jet Lubrication Oil as a Major Component of Aircraft Exhaust Nanoparticles. Atmos Chem Phys. 2019;19(9):6389–99. https://doi.org/10.5194/acp-19-6389-2019.
Lammers A, Janssen NAH, Boere AJF, Berger M, Longo C, Vijverberg SJH, et al. Effects Of Short-Term Exposures To Ultrafine Particles Near An Airport In Healthy Subjects. Environ Int. 2020;141:105779. https://doi.org/10.1016/j.envint.2020.105779.
Bendtsen KM, Brostrøm A, Koivisto AJ, Koponen I, Berthing T, Bertram N, et al. Airport Emission Particles: Exposure Characterization and Toxicity Following Intratracheal Instillation in Mice. Part Fibre Toxicol. 2019;16(1):1–23. https://doi.org/10.1186/s12989-019-0305-5.
Levin EC, Acharya NK, Han M, Zavareh SB, Sedeyn JC, Venkataraman V, et al. Brain-reactive autoantibodies are nearly ubiquitous in human sera and may be linked to pathology in the context of blood–brain barrier breakdown. Brain Res. 2010;1345:221–32. https://doi.org/10.1016/j.brainres.2010.05.038.
El Rahman HAA, Salama M, Gad El-Hak SA, El-Harouny MA, ElKafrawy P, Abou-Donia MB. A Panel of Autoantibodies Against Neural Proteins as Peripheral Biomarker for Pesticide-Induced Neurotoxicity. Neurotox Res. 2018;33(2):316–36. https://doi.org/10.1007/s12640-017-9793-y.
Abou-donia MB, Conboy LA, Kokkotou E, Jacobson E, Elmasry EM, Elkafrawy P, et al. Screening for Novel Central Nervous System Biomarkers in Veterans with Gulf War Illness. Neurotoxicol Teratol. 2017;61:36–46. https://doi.org/10.1016/j.ntt.2017.03.002.
Abou-Donia MB, Lapadula ES, Krengel MH, Quinn E, LeClair J, Massaro J, et al. Using Plasma Autoantibodies of Central Nervous System Proteins to Distinguish Veterans with Gulf War Illness from Healthy and Symptomatic Controls. Brain Sci. 2020;10(9):610. https://doi.org/10.3390/brainsci10090610.
Hromadkova L, Ovsepian SV. Tau-Reactive Endogenous Antibodies: Origin, Functionality, and Implications for the Pathophysiology of Alzheimer’s Disease. J Immunol Res. 2019;2019:7406810. https://doi.org/10.1155/2019/7406810.PMCID:PMC6811779.
Wüthrich B. Allergic and Intolerance Reactions to Wine. Allergol Select. 2018;2(1):80–8. https://doi.org/10.5414/alx01420e.
Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54(10):1271–94. https://doi.org/10.1016/s0169-409x(02)00066-2.
Lu WJ, Ferlito V, Xu C, Flockhart DA, Caccamese S. Enantiomers of Naringenin as Pleiotropic, Stereoselective Inhibitors of Cytochrome P450 Isoforms. Chirality. 2011;23(10):891–6. https://doi.org/10.1002/chir.21005.
Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The Molecular Basis of the Human Serum Paraoxonase Activity Polymorphism. Nat Genet. 1993;3(1):73–6. https://doi.org/10.1038/ng0193-73.
Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, et al. Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics. 2000;10(9):767–79. https://doi.org/10.1097/00008571-200012000-00002.
Hosokawa M, Endo T, Fujisawa M, Hara S, Iwata N, Sato Y, et al. Interindividual variation in carboxylesterase levels in human liver microsomes. Drug Metab Dispos. 1995;23(10):1022 (PMCID: 8654188).
Delacour H, Dedome E, Courcelle S, Hary B, Ceppa F. Butyrylcholinesterase deficiency. Ann Biol Clin (Paris). 2016;74(3):279–85. https://doi.org/10.1684/abc.2016.1141.
Stevens RC, Suzuki SM, Cole TB, Park SS, Richter RJ, Furlong CE. Engineered recombinant human paraoxonase 1 (rHuPON1) purified from Escherichia coli protects against organophosphate poisoning. Proc Natl Acad Sci U S A. 2008;105(35):12780–4. https://doi.org/10.1073/pnas.0805865105.
IGHRC. The Interdepartmental Group on Health Risks From Chemicals. Chemical Mixtures : A Framework for Assessing Risks to Human Health. Cranfield: IEH, Cranfield University; 2009. http://www.iehconsulting.co.uk/IEH_Consulting/IEHCPubs/IGHRC/cr14.pdf. Accessed 2 Dec 2022.
European Commission. European Commission: Something From Nothing? Ensuring the Safety of Chemical Mixtures. Joint Research Centre: European Commission; 2018. https://data.europa.eu/doi/https://doi.org/10.2760/618648. Accessed 2 Dec 2022.
Goodson WH, Lowe L, Carpenter DO, Gilbertson M, Ali AM, de Cerain Salsamendi AL, et al. Assessing the Carcinogenic Potential of Low-Dose Exposures to Chemical Mixtures in the Environment: The Challenge Ahead. Carcinogenesis. 2015;36(1):S254–96. https://doi.org/10.1093/carcin/bgv039.
Naughton SX, Hernandez CM, Beck WD, Poddar I, Yanasak N, Lin PC, et al. Repeated Exposures to Diisopropylfluorophosphate Result in Structural Disruptions of Myelinated Axons and Persistent Impairments of Axonal Transport in the Brains of Rats. Toxicology. 2018;406–407:92–103. https://doi.org/10.1016/j.tox.2018.06.004.
Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T. In Vitro Endocrine Disruption Potential Of Organophosphate Flame Retardants Via Human Nuclear Receptors. Toxicology. 2013;314(1):76–83. https://doi.org/10.1016/j.tox.2013.09.004.
Liu X, Ji K, Choi K. Endocrine Disruption Potentials of Organophosphate Flame Retardants and Related Mechanisms in H295R And MVIN Cell Lines and in Zebrafish. Aquat Toxicol. 2012;114–115:173–81. https://doi.org/10.1016/j.aquatox.2012.02.019.
Ji X, Li N, Ma M, Rao K, Yang R, Wang Z. Tricresyl phosphate isomers exert estrogenic effects via G protein-coupled estrogen receptor-mediated pathways. Environ Pollut. 2020;264:114747. https://doi.org/10.1016/j.envpol.2020.114747.
Zhang Q, Lu M, Dong X, Wang C, Zhang C, Liu W, et al. Potential Estrogenic Effects of Phosphorus-Containing Flame Retardants. Environ Sci Technol. 2014;48(12):6995–7001. https://doi.org/10.1021/es5007862.
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr Rev. 2009;30(4):293–342. https://doi.org/10.1210/er.2009-0002.
Maharjan CK, Mo J, Wang L, Kim MC, Wang S, Borcherding N, et al. Natural and Synthetic Estrogens in Chronic Inflammation and Breast Cancer. Cancers (Basel). 2021;14(1):206. https://doi.org/10.3390/cancers14010206. PMCID: PMC8744660.
Bradford Hill A. The Environment and Disease: Association or Causation? Proceedings of the Royal Society of Medicine. 1965; 58(5): 295-300. PMCID: 1898525. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1898525/
Vandenbroucke JP, Broadbent A, Pearce N. Causality and Causal Inference in Epidemiology: The Need for a Pluralistic Approach. Int J Epidemiol. 2016;45(6):1776–86. https://doi.org/10.1093/ije/dyv341.
Broadbent A, Vandenbroucke JP, Pearce N. Response: Formalism or pluralism? A reply to commentaries on “Causality and causal inference in epidemiology.” Int J Epidemiol. 2016;45(6):1841–51. https://doi.org/10.1093/ije/dyw298.
Gee D. Associations and Causation : A Bradford Hill Approach to Aerotoxic Syndrome. International Aircraft Cabin Air Conference 2017; London. J Health and Pollution 2019:S32-S7https://doi.org/10.5696/2156-9614-9.24.191201
Schaumburg HH. Human Neurotoxic Disease. In: Spencer P, Schaumburg H, editors. Experimental and Clinical Neurotoxicology. 2nd ed. Oxford: Oxford University Press; 2000. p. 55–82. ISBN: 9780195084771.
Zavorsky GS, Hsia CCW, Hughes JMB, Borland CDR, Guénard H, van der Lee I, et al. Standardisation and Application of the Single-Breath Determination of Nitric Oxide Uptake in the Lung. Eur Respir J. 2017;49:1600962. https://doi.org/10.1183/13993003.00962-2016.
Anand S, Singh S, Nahar Saikia U, Bhalla A, Paul Sharma Y, Singh D. Cardiac Abnormalities In Acute Organophosphate Poisoning. Clin Toxicol. 2009;47(3):230–5. https://doi.org/10.1080/15563650902724813.
Schindler B. Erarbeitung Und Anwendung Einer Analytischen Methode Zur Bestimmung Der Metabolite Von Flammschutzmitteln Auf Der Basis Von Phosphorsäuretriestern In Menschlichen KörperflüSsigkeiten [PhD]: Universität Erlangen-Nürnberg; 2009. https://d-nb.info/995579547/34 Accessed 2 Dec 2022.
Richards PG, Johnson MK, Ray DE. Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol Pharmacol. 2000;58(3):577–83. https://doi.org/10.1124/mol.58.3.577.
Pancetti F, Olmos C, Dagnino-Subiabre A, Rozas C, Morales B. Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: implication for the action of acylpeptide hydrolase. J Toxicol Environ Health B Crit Rev. 2007;10(8):623–30. https://doi.org/10.1080/10937400701436445.
Gomer N. Captain Niels Gomer- Malmo Event: 1999. International Aircraft Cabin Air Conference 2017 2017. Available from: https://zenodo.org/record/4554679; https://vimeo.com/520042108. Accessed 2 Dec 2022.
Soskolne CL, Kramer S, Ramos-Bonilla JP, Mandrioli D, Sass J, Gochfeld M, et al. Toolkit for detecting misused epidemiological methods. Environ Health. 2021;20(1):90. https://doi.org/10.1186/s12940-021-00771-6.
Soskolne C, Andruchow J, Racioppi F. From Theory To Practice In Environmental Epidemiology: Developing, Conducting and Disseminating Health Research. P. 23. World Health Organization, Europe; 2008. https://iris.who.int/handle/10665/347877.
Acknowledgements
The authors would like to thank Judith Anderson (MSc), Department of Air Safety, Health, & Security, Association of Flight Attendants-CWA AFL-CIO for her significant expertise and suggestions. Acknowledgement is also accorded Prof. Dr. med. A. Heutelbeck at the Institut für Arbeits-, Sozial- und Umweltmedizin Universitätsklinikum Jena (UKJ) in Germany for her guidance on the initial importance of this document and its style.
International Fume Events Task Force (IFETF)
The authors of this manuscript make up the IFETF.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Under the leadership of Susan Michaelis and Jonathan Burdon, the primary text, figures and tables were prepared by SM, GH, JB, CVH, XB with supporting activity by CF, LC, DG, JR, TL, AT and CS. All authors (except the late LB) reviewed the text and figures, tables. The supplementary text was primarily prepared by SM, JB, LB, XB, CVH, with supporting input from all other authors. All authors (except the late LB) read and accepted the final text and associated material.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Practical guideline prepared by the International Fume Events Task Force (chaired by S. Michaelis) with working groups from the DiMoPEx COST-Action (Diagnosis, Monitoring and Prevention of Exposure related Noncommunicable diseases, DiMoPEx (www.cost.eu/actions/CA15129) is EU-COST founded action CA 15129, with partners from 27 countries (Late action chair: Lygia Therese Budnik)) and Collegium Ramazzini (The Collegium Ramazzini is an independent, international academy founded in 1982 comprising 180 internationally renowned experts in the fields of occupational and environmental health. (www.collegiumramazzini.org)).
The original article has been updated: the URL link in Reference 216 has been updated to: https://iris.who.int/handle/10665/347877.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Burdon, J., Budnik, L.T., Baur, X. et al. Health consequences of exposure to aircraft contaminated air and fume events: a narrative review and medical protocol for the investigation of exposed aircrew and passengers. Environ Health 22, 43 (2023). https://doi.org/10.1186/s12940-023-00987-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-023-00987-8