Abstract
Background
Uncertainty remains regarding the association between blood lead levels (BLL) and serum uric acid (SUA) with relatively low BLL exposure because of limited data in the adolescent population. We examined the association between BLL and SUA in U.S. adolescents.
Methods
In this cross-sectional study, 8303 adolescents aged 12–19 years from NHANES 1999–2006 were analyzed. BLL was Ln-transformed for analysis for the skewed distribution. Elevated SUA was defined as ≥5.5 mg/dL. Multivariate linear and multiple logistic regression analyses were performed to evaluate the association of BLL with SUA and elevated SUA. Moreover, a generalized additive model (GAM) and a fitted smoothing curve (penalized spline method) were conducted.
Results
The overall mean BLL was 1.3 μg/dL. Multivariate linear regression analyses showed that LnBLL was independently and positively correlated with SUA level (β = 0.13, 95%CI: 0.09–0.17). Multiple logistic analyses showed that LnBLL was associated with a 24% increased prevalence of elevated SUA (OR = 1.24; 95% CI, 1.11–1.38). Analyses using restricted cubic spline confirmed that the associations of LnBLL with SUA and elevated SUA were linear. Subgroup analyses showed that stronger associations between LnBLL and SUA were detected in adolescents with lower levels of education and estimated glomerular filtration rate (eGFR) (all P for interaction < 0.05).
Conclusions
BLL was independently and positively correlated with SUA level and elevated SUA among U.S. adolescents, particularly with lower levels of education and eGFR. The data suggest that there is no “safe” threshold level of exposure to lead.
Graphical abstract

Similar content being viewed by others
Introduction
Lead, a heavy metal, is widely distributed in the environment and remains a major public health challenge [1]. In recent years, lead exposure has declined sharply, particularly in developed countries, but chronic low-dose exposures are still a major public health concern [2, 3]. Lead battery workers are primarily exposed to lead in their places of employment, while the general population are exposed to lead from other sources, such as the drinking water supply [4] and air pollution [5]. Early-life exposure to lead, even at low levels, has been associated with a range of adverse health outcomes, including poor growth in childhood [6], impaired cognitive and behavioral functions [7, 8], and hematopoietic system toxicity [9]. In 1991, the Centers for Disease Control and Prevention (CDC) of the United States advised that blood lead levels (BLL) < 10 μg/dL was the safe range for children. However, subsequently, the CDC emphasized that there was no “safe” threshold for BLL in young children because of its established toxicity even at low levels.
Previous epidemiological studies in adults have reported that BLL exposure was positively associated with serum uric acid (SUA) level among lead-exposed workers [10,11,12]. Association between lead and SUA has been examined in populations encompassing a wide range of lead doses. Despite excessive tubular reabsorption [13] and extrarenal mechanisms of porphyrin metabolism [14], lead exposure causing urate tubular secretion defects is considered to be a major factor involved [15]. As SUA is the final oxidation product of purine metabolism, it has been widely used for evaluating hyperuricemia and gout. An increasing body of epidemiologic evidence shows that elevated SUA or hyperuricemia is associated with a wide variety of adverse health outcomes, including gout, hypertension, obesity, dyslipidemia, diabetes, cardiovascular disease, intellectual disabilities and all-cause mortality [16,17,18,19,20]. However, the association between BLL at a very low level and SUA among adolescents remains unclear because of limited data. In addition, elevated SUA is increasingly prevalent among children and adolescents in the United States [16]. Thus, it is important for researchers to study and understand the association between SUA and continuous lead exposure from the everyday environment.
In the present study, we examined the association between BLL and SUA in a representative sample of US adolescents with relatively low BLL exposure from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2006.
Methods
Study design and population
National Health and Nutrition Examination Survey (NHANES), conducted by the Centers for Disease Control and Prevention (CDC), was an ongoing repeated cross-sectional study designed to assess the lifestyle, health, and nutrition status of non-institutionalized civilian US population through complex, multistage probability sampling [21, 22]. NHANES study protocols were approved by the research ethics review board of the National Center for Health Statistics. Written informed consent was acquired from each participant and obtained from the guardians of participants younger than 18 years of age, and assent was obtained from those aged 12 to 17 years. More detailed information is available at http://www.cdc.gov/nchs/nhanes/nhanes_questionnairees.htm. The NHANES datasets are available on DataDryad (https://doi.org/10.5061/dryad.d5h62).
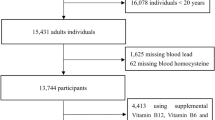
This study used data from the 1999 to 2006 NHANES. Fasting blood samples were collected from those 12 years of age and older. In total, 9493 eligible adolescents aged 12–19 years were enrolled. We excluded participants with missing SUA value (n = 1184) and BLL value (n = 6).
Exposure variable and outcomes
In our study, the exposure variable was BLL. BLL was measured at the CDC’s National Center for Environmental Health, Division of Laboratory Sciences using a Perkin-Elmer model SIMAA 6000 simultaneous multielement atomic absorption spectrometer with Zeeman background correction. The analytical laboratory followed extensive quality control procedures. National Institute of Standards and Technology Standard Reference Materials whole-blood materials were used for external calibration. The limit of detection (LOD) for blood lead was 0.3 μg/dL in NHANES from 1999 to 2004. The LOD for blood lead was 0.25 μg/dL in NHANES from 2003 to 2006. Lead concentration below the level of detection was assigned the limit of detection divided by the square root of 2, as recommended by NHANES. The interassay coefficients of variation ranged from 3.1 to 4.0% for BLL. Details of these assays and quality standards are available at http://cdc.gov/nchs/nhanes.
The outcome variable was SUA. SUA was measured on a Roche Hitachi Model 917 or 704 Multichannel Analyzer in 1999–2001 and a Beckman Synchron LX20 in 2002 using a colorimetric method. Serum creatinine level was measured with the same instruments using the Jaffe kinetic alkaline picrate method. Serum creatinine was corrected to standardize to a “gold” standard reference method as recommended by NHANES. The distribution of creatinine and uric acid results from the two laboratories were compared at the time of transition, and no significant differences were observed.
Estimated glomerular filtration rate (eGFR, milliliters per minute per 1.73 m2) was calculated via the creatinine-based formula of Schwartz: eGFR = k (height in centimeters)/(serum creatinine in milligrams per deciliter), where k is 0.7 in boys and 0.55 in girls [23, 24].
Covariates
We selected the appropriate covariates based on previous studies examining risk factors for SUA as well as adjusting for covariates that, when added to this model, changed the matched odds ratio by at least 10% [25, 26]. Therefore, the following variables were used to construct the fully adjusted model: continuous variables included age (years), body mass index (BMI, kg/m2), blood pressure (BP, mmHg), poverty to income ratio, dietary data [calcium (mg), total monounsaturated fatty acids (TMFA, gm), total polyunsaturated fatty acids (TPFA, gm), total saturated fatty acids (TSFA, gm), total fat (gm), protein (gm)] and laboratory data [blood cadmium (ug/L), serum cotinine (ng/mL), hemoglobin (g/dL), fasting blood glucose (FBG, mg/dL), total cholesterol (TC, mg/dL), triglycerides (mg/dL), high density lipoprotein cholesterol (HDL-C, mg/dL), blood urea nitrogen (BUN, mg/dL), C-reactive protein (CRP, mg/dL)]; categorical variables consisted of race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other Hispanic or other), education (less than high school, high school/equivalent, or greater than high school), physical activity (sedentary, low, moderate, high).
Statistical analysis
The statistical analyses were performed according to the guidelines of the CDC (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx). Sample weights were used for analyses to account for the complex survey design and non-response of NHANES. We calculated the sample weight for the 8 years of data from 1999 to 2006 as WT99–06 = (1/2) × WT99–02 + (1/4) × WT03–04 + (1/4) × WT05–06, where WT99–02 is the variable WTMEC4YR from the NHANES 1999–2000 and NHANES 2001–2002; WT03–04 and WT05–06 were the variable WTMEC2YR from the NHANES 2003–2004 and NHANES 2005–2006 demographic file, respectively [27].
The major question of interest was whether BLL would be associated with SUA after the data was controlled for confounders which might affect SUA. We first examined the distribution of BLL according to the population characteristics listed above. Weighted means, proportions and standard error (Se) were calculated for baseline characteristics using survey sample weights. Because the distribution of values for BLL was strongly skewed toward the upper end, the BLL was Ln-transformed for analysis. Second, we applied multivariate linear regression analysis to evaluate the independent association between LnBLL and SUA. We used four levels of adjustment: Model 1 was adjusted for sociodemographic variables; model 2 was further adjusted for blood biochemical examinations; model 3 was additionally adjusted for dietary data. When model 4 was adjusted for sex, age, BMI, race, hemoglobin, HDL-C and eGFR covariates, the matched odds ratio changed by at least 10%. Third, although there is no universally definition of hyperuricemia in adolescents, previous studies reported that a SUA ≥ 5.5 mg/dL was associated with the risk of hypertenison [16, 28]. Based on this association, the elevated SUA was defined as ≥5.5 mg/dL. We investigated the association of LnBLL with elevated SUA using multivariate binary logistic regression analysis.
The subgroup analyses were performed using stratified multivariate regression analyses. To further characterize the shape of the relationship between BLL and SUA, we used a generalized additive model (GAM) and a fitted smoothing curve (penalized spline method). To ensure the robustness of data analysis, we also did the sensitivity analyses. We converted the LnBLL into a categorical variable, and calculated the P for trend. The purpose was to verify the results of LnBLL as the continuous variable and to observe the possibility of nonlinearity.
All the analyses were performed using the statistical package R (http://www.R-project.org, The R Foundation) and Empower (R) (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA). A two-sided P-value < 0.05 was considered statistically significant.
Results
Based on the inclusion and exclusion criteria, a total of 8303 study participants aged 12–19 years (mean age: 15.5 ± 2.3 years; 50.4% men;) from NHANES 1999–2006 were included in this final data analysis (1999–2002: 2106 subjects; 2001–2002: 2192 subjects; 2003–2004: 2036 subjects; 2005–2006: 1969 subjects). Overall, the mean SUA level was 5.0 mg/dL, and 33.2% had a SUA level ≥ 5.5 mg/dL. The overall mean BLL was 1.3 μg/dL. The weighted distributions of selective participants sociodemographic characteristics and other covariates according to LnBLL quartiles are presented in Table 1. The ranges of LnBLL quartiles 1–4 were < − 0.22, − 0.22-0.18, 0.18–0.64 and ≥ 0.64 μg/dL, respectively. There were significant differences between LnBLL quartiles, except for SBP, TPFA and TSFA. Participants with the highest LnBLL in Q4 (BLL ≥ 1.9 μg/dL) were more likely to be boys, to be non-Hispanic black and Mexican American, to have lower educational levels and physical activity, to be of younger age, to have lower values in BMI, DBP, calcium intake, TMFA intake, TC, triglycerides, HDL-C and CRP, and to have higher values in blood cadmium, serum cotinine, hemoglobin, FBG, SUA, eGFR and BUN than those of the other groups (all P < 0.05).
For those subjects excluded from the analyses due to missing SUA or BLL values, their baseline characteristics were similar to their counterparts who were included in the analyses (Additional file 1: Table S1).
Table 2 showed the association of SUA with LnBLL using multivariate linear regression analyses. In the crude model, continuous LnBLL was positively correlated with SUA level (β = 0.29, 95%CI: 0.24–0.33, P < 0.001). After adjustment for different confounders, the positive association between LnBLL and SUA was still found in models 1–4. Similarly, in the fully adjusted model (model 3), for every 1 μg/dL increase in LnBLL, estimates from regression coefficients (β) indicated that the change in SUA was 0.13 mg/dL (95%CI: 0.09–0.17). We also converted LnBLL from a continuous variable to a categorical variable (quartiles). Compared to LnBLL < − 0.22 μg/dL (BLL < 0.80 μg/dL), there was a statistically significant higher SUA level for the participants in the second, third and highest LnBLL quartiles. P for trend in all of the models was significant and consistent with the P value when LnBLL was used as a continuous variable, suggesting the linear association between LnBLL and SUA. Table 3 presented the relative odds of having an elevated SUA. The results showed that LnBLL was independently and positively associated with elevated SUA in adolescents. In the fully adjusted model 3, compared to participants in the lowest LnBLL quartile, participants in the highest LnBLL quartile were associated with a 49% increased prevalence of elevated SUA. Also, P for trend in all models was significant.
We further explored the dose-response relation between BLL by a decile approach [≤0.5 (reference), 0.5–0.6, 0.6–0.7, 0.7–0.8, 0.8–0.9, 0.9–1.1, 1.1–1.4, 1.4–1.7, 1.7–2.3, and > 2.3 μg/dL] and SUA and elevated SUA (Additional file 1: Table S2 and Table S3). The overall pattern lent further support for a dose-response relation between BLL levels and SUA and elevated SUA (P for trend < 0.001).
Further analyses using restricted cubic spline confirmed the dose-relationship between LnBLL exposure and SUA (Fig. 1). Figure 1a and b showed that the associations of LnBLL with SUA and elevated SUA were linear.
Dose–response relationship between LnBLL exposure and SUA*. a LnBLL and SUA; b LnBLL and elevated SUA. Abbreviations: BLL, blood lead levels; SUA, serum uric acid. *Adjusted for sex, age, race, education status and physical activity; BMI, SBP, DBP, blood cadmium, serum cotinine, hemoglobin, fasting blood glucose, total cholesterol, triglycerides, HDL-C, eGFR, blood urea nitrogen, C-reactive protein, calcium intake, total monounsaturated fatty acids intake, total polyunsaturated fatty acids intake, total saturated fatty acids intake, total fat intake and total protein intake
In subgroup analysis, we further explored the role of other covariables on the association between LnBLL and SUA. As shown in Fig. 2, the subgroup analysis revealed a highly consistent pattern. Regardless of subgroup, LnBLL was positively associated with SUA. The effect of LnBLL on SUA was more significant at low education levels (< high school: β = 0.16, 95%CI: 0.12–0.20; ≥ high school: β = − 0.03, 95%CI: − 0.12-0.06, P for interaction = 0.002). Compared to subjects with eGFR ≥130 mL/min per 1.73 m2, those with eGFR < 130 mL/min per 1.73 m2 had a higher SUA level (β = 0.19, 95%CI, 0.11–0.27, P for interaction = 0.047) in subjects with high LnBLL. However, the association between LnBLL and SUA were consistent in the following subgroups: sex, age, race, physical activity, BMI and serum cotinine (P for interaction > 0.05 for all covariates).
Subgroup analyses of the effect of LnBLL on SUA. Abbreviations: BLL, blood lead levels; SUA, serum uric acid; BMI, body mass index; eGFR, estimated glomerular filtration rate; CI, confidence interval. Adjusted for sex, age, race, education status and physical activity; BMI, SBP, DBP, blood cadmium, serum cotinine, hemoglobin, fasting blood glucose, total cholesterol, triglycerides, HDL-C, eGFR, blood urea nitrogen, C-reactive protein, calcium intake, total monounsaturated fatty acids intake, total polyunsaturated fatty acids intake, total saturated fatty acids intake, total fat intake and total protein intake, if not be stratified
Discussion
Lead is a multiple-source pollutant well known for its adverse effects, and the main target organs of lead are the hematopoietic, nervous, and renal systems [29]. There are a number of similarities between the renal and vascular effects reported from low-level SUA and those from lead exposure. Tubulointerstitial fibrosis, a classic (although nonspecific) finding in lead exposure, has been observed in the uric acid model in the absence of the urate crystals that are commonly seen in this pathology at higher levels of hyperuricemia. Interestingly, in this cross-sectional study, we found that BLL was independently and positively associated with SUA level and elevated SUA in a representative sample of U.S. adolescents with relatively low BLL exposure. Also, our study confirmed a significant linear relationship between BLL and SUA level. Moreover, the subgroup analyses showed that stronger associations between BLL and SUA were detected in adolescent populations with lower levels of education and eGFR.
BLL has been found to be associated with SUA and hyperuricemia in multiple large epidemiologic studies in adults. Eswar Krishnan et al. [17]. used data from 6153 US adults aged 40 years or older and found that low level lead exposure (< 1.21 μmol/L) which was currently considered acceptable in adults was associated with increased prevalence of gout and hyperuricemia. Each doubling of BLL was associated with an unadjusted odds ratio of 1.74 (95%CI, 1.47 to 2.05) for gout and 1.25 (95%CI, 1.12 to 1.40) for hyperuricemia. Haijiang Dai et al. [12]. conducted a cross-sectional study of 2120 Chinese subjects aged ≥20 years and found that continuous lead exposure had an independent impact on SUA for both males and females, although this impact was more pronounced for females than for males. Moreover, the study also showed lead exposure was significantly associated with hyperuricemia for females but not for males.
However, several reports have also yielded some conflicting results. A longitudinal study conducted in Korean lead workers showed that BLL exposure was not associated with SUA [30]. A similar result was also reported by Omae et al. [31]. These conflicting results might be attributed to the differences in cohort characteristics, sample size, and adjustment of confounders. As we know, adults are more likely to have comorbidities, such as hypertension, chronic kidney disease (CKD), even smoking, which can make this relationship between BLL and SUA more complex. While in our study, we reported for the first time the relationship between BLL and SUA in adolescents, a population generally free from smoking, hypertension, diabetes mellitus or CKD. Therefore, they are an ideal population in which to examine this relationship. We found that continuous low level blood lead exposure was independently and positively associated with SUA. Further refinement in national goals for prevention, detection, and removal of lead from the environment should be pursued.
The mechanism driving this association is still unclear. However, several possible reasons could account for the association between BLL and SUA. BLL is an environmental pollutant that exhibits nephrotoxic activity, frequently evidenced by decreased GFR [3]. A review indicated that lead contributes to nephrotoxicity, even at BLL below 5 μg/dL3. BLL exposure could cause tubulointerstitial nephropathy, responsible for hyperuricemia and toxic effects on blood nucleo proteins that alter the metabolism of purine, thereby causing hyperuricemia [32]. The association between BLL and eGFR may be bidirectional [33]. In our study, we found that the effect of BLL on SUA was more pronounced in populations with lower eGFR. A possible reason for theses results is that lower eGFR may lead to an increase in SUA and elevated BLL by excretion disorders [34, 35]. Nevertheless, further studies are warranted to elucidate the mechanism underlying the role of BLL in kidney function. Furthermore, our subgroup analyses also showed that the effect of BLL on SUA was more pronounced in populations with a low education level. There was no association between BLL and SUA among adolescents with more than a high school level of education. The results were consistent in different age groups (the data was not shown in our paper). These findings suggest that life style, behavior or environmental conditions related to lower education has an indirect influence on the association between BLL exposure and SUA. Further research is needed to examine the relationship between education level, BLL and SUA.
Our study has some strengths. First, this study was the first report to explore the association between BLL and SUA in U.S. adolescents with relatively low BLL exposure; Second, we used a representative sample of the general adolescent population of the US from NHANES which applied rigorous quality controls to the procedures. Third, we adjusted for most potential confounders and effect modifiers. Fourth, we handled the target independent variable as both a continuous variable and as a categorical variable. Such an approach can reduce the contingency in the data analysis and enhance the robustness of results.
Some limitations of our study should be noted. First, the main limitations of this study was its observational nature. Second, as a cross-sectional design, it had less power to infer the causal association between BLL and SUA. Further prospective follow-up studies are needed to verify these findings. Third, further selection bias might have occurred because participants with missing BLL and SUA values were excluded. However, for the excluded population, their baseline characteristics were similar to their counterparts who were included in the analyses. Fourth, the BLL indicate recent exposure, but do not necessarily reflect the total bone lead burden which can provide a more valuable measure of internal dosage.
Conclusion
In summary, continuous low level blood lead exposure was independently and positively associated with SUA in U.S. adolescents, and this impact was more pronounced in populations with lower levels of education and eGFR. The data suggest that there is no “safe” threshold level of exposure to lead. Further refinement in national goals for prevention, detection, and removal of lead from the environment should be pursued.
Availability of data and materials
The datasets are available on https://doi.org/10.5061/dryad.d5h62.
Abbreviations
- BLL:
-
Blood lead levels
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- CRP:
-
C-reactive protein
- DBP:
-
Diastolic blood pressure
- eGFR:
-
estimated glomerular filtration rate
- FBG:
-
Fasting blood glucose
- HDL-C:
-
high density lipoprotein cholesterol
- SBP:
-
Systolic blood pressure
- SUA:
-
Serum uric acid
- TC:
-
Total cholesterol
- TMFA:
-
Total monounsaturated fatty acids
- TPFA:
-
Total polyunsaturated fatty acids
- TSFA:
-
Total saturated fatty acids
References
Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990-2010: findings from the global burden of disease study 2010. Lancet. 2013;381(9882):1987–2015.
Hu H. Bone lead as a new biologic marker of lead dose: recent findings and implications for public health. Environ Health Perspect. 1998;106(Suppl 4):961–7.
Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006;70(12):2074–84.
Schock MR, Hyland RN, Welch MM. Occurrence of contaminant accumulation in lead pipe scales from domestic drinking-water distribution systems. Environ Sci Technol. 2008;42(12):4285–91.
Renaudin M, Leblond S, Meyer C, Rose C, Lequy E. The coastal environment affects lead and sodium uptake by the moss Hypnum cupressiforme used as an air pollution biomonitor. Chemosphere. 2018;193:506–13.
Afeiche M, Peterson KE, Sanchez BN, et al. Prenatal lead exposure and weight of 0- to 5-year-old children in Mexico city. Environ Health Perspect. 2011;119(10):1436–41.
Boucher O, Muckle G, Jacobson JL, et al. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the environmental contaminants and child development study in Nunavik. Environ Health Perspect. 2014;122(3):310–6.
Liu J, Liu X, Wang W, et al. Blood lead concentrations and children's behavioral and emotional problems: a cohort study. JAMA Pediatr. 2014;168(8):737–45.
Jacob B, Ritz B, Heinrich J, Hoelscher B, Wichmann HE. The effect of low-level blood lead on hematologic parameters in children. Environ Res. 2000;82(2):150–9.
Wang VS, Lee MT, Chiou JY, et al. Relationship between blood lead levels and renal function in lead battery workers. Int Arch Occup Environ Health. 2002;75(8):569–75.
Alasia DD, Emem-Chioma PC, Wokoma FS. Association of lead exposure, serum uric acid and parameters of renal function in Nigerian lead-exposed workers. Int J Occup Environ Med. 2010;1(4):182–90.
Dai H, Huang Z, Deng Q, et al. The effects of Lead exposure on serum uric acid and Hyperuricemia in Chinese adults: a cross-sectional study. Int J Environ Res Public Health. 2015;12(8):9672–82.
Emmerson BT, Mirosch W, Douglas JB. The relative contributions of tubular reabsorption and secretion to urate excretion in lead nephropathy. Aust NZ J Med. 1971;1(4):353–62.
Emmerson BT, Ravenscroft PJ. Abnormal renal urate homeostasis in systemic disorders. Nephron. 1975;14(1):62–80.
Emmerson PT, Howard-Flanders P. Preferential sensitization of anoxic bacteria to x-rays by organic nitroxide-free radicals. Radiat Res. 1965;26(1):54–62.
Loeffler LF, Navas-Acien A, Brady TM, Miller ER, Fadrowski JJ. Uric acid level and elevated blood pressure in US adolescents: National Health and nutrition examination survey, 1999-2006. Hypertension. 2012;59(4):811–7.
Krishnan E, Lingala B, Bhalla V. Low-level lead exposure and the prevalence of gout: an observational study. Ann Intern Med. 2012;157(4):233–41.
Kramer CK, von Muhlen D, Jassal SK, Barrett-Connor E. A prospective study of uric acid by glucose tolerance status and survival: the rancho Bernardo study. J Intern Med. 2010;267(6):561–6.
Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115(19):2526–32.
Lin JD, Lin PY, Lin LP, et al. Serum uric acid, hyperuricemia and body mass index in children and adolescents with intellectual disabilities. Res Dev Disabil. 2009;30(6):1481–9.
Curtin LR, Mohadjer LK, Dohrmann SM, et al. The National Health and Nutrition Examination Survey: Sample Design, 1999-2006. Vital Health Stat 2. 2012;(155):1–39.
Patel CJ, Pho N, McDuffie M, et al. A database of human exposomes and phenomes from the US National Health and nutrition examination survey. Sci Data. 2016;3:160096.
Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am. 1987;34(3):571–90.
Schwartz GJ, Haycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr. 1976;88(5):828–30.
Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14.
Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343(25):1826–32.
Scinicariello F, Abadin HG, Murray HE. Association of low-level blood lead and blood pressure in NHANES 1999-2006. Environ Res. 2011;111(8):1249–57.
Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–52.
Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM. Lead toxicity update. A brief review. Med Sci Monit. 2005;11(10):A329–36.
Weaver VM, Jaar BG, Schwartz BS, et al. Associations among lead dose biomarkers, uric acid, and renal function in Korean lead workers. Environ Health Perspect. 2005;113(1):36–42.
Omae K, Sakurai H, Higashi T, Muto T, Ichikawa M, Sasaki N. No adverse effects of lead on renal function in lead-exposed workers. Ind Health. 1990;28(2):77–83.
Niamane R, El HS, Bezza A, Lazrak N, Hajjaj-Hassouni N. Lead-related gout. A case report. Joint Bone Spine. 2002;69(4):409–11.
Sanders AP, Mazzella MJ, Malin AJ, et al. Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014. Environ Int. 2019;131:104993.
Lin JL, Lin-Tan DT, Hsu KH, Yu CC. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N Engl J Med. 2003;348(4):277–86.
Eswar K. Reduced Glomerular Function and Prevalence of Gout: NHANES 2009–10. PLoS One. 2012.
Acknowledgments
A special thanks to all of the NHANES participants who freely gave their time to make this and other studies possible. We also gratefully thank for Richard Xu of Whiting School of Engineering, Johns Hopkins University assistance in editing this manuscript.
Funding
None
Author information
Authors and Affiliations
Contributions
GH (substantial contributions to conception and design, drafting the article); GH, GJ and ST (acquisition of data, or analysis and interpretation of data); PZ and LH (revising it critically for important intellectual content); GH, GJ, ST, PZ and LH (final approval of the version to be published and agreement to be accountable for all aspects of the work). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NHANES study protocols were approved by the research ethics review board of the National Center for Health Statistics. Written informed consent was acquired from each participant and obtained from the guardians of participants younger than 18 years of age, and assent was obtained from those aged 12 to 17 years.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Characteristics of the included and excluded population. Table S2. Association of SUA with BLL levels-a dose-response analysis. Table S3. Association of elevated SUA with BLL levels-a dose-response analysis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hu, G., Jia, G., Tang, S. et al. Association of low-level blood lead with serum uric acid in U.S. adolescents: a cross-sectional study. Environ Health 18, 86 (2019). https://doi.org/10.1186/s12940-019-0524-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-019-0524-0