Abstract
Evidence regarding the association between blood lead levels (BLL) and hyperhomocysteinemia (HHcy) in US adults was limited. We aimed to investigate the association of BLL with the risk of HHcy, and to examine possible effect modifiers using US National Health and Nutrition Examination Survey (NHANES) database. We performed a cross-sectional study using data from up to 9,331 participants aged ≥ 20 years of NHANES from 2001 to 2006. BLL was measured by atomic absorption spectrometry. HHcy was defined as plasma homocysteine level > 15 µmol/L. The weighted prevalence of HHcy was 6.87%. The overall mean BLL was 1.9 μg/dL. Overall, there was a nonlinear positive association between Ln-transformed BLL (LnBLL) and the risk of HHcy. The Odds ratios (95% CI) for participants in the second (0.04–0.49 μg/dL), third (0.5–0.95 μg/dL) and fourth quartiles (> 0.95 μg/dL) were 1.12 (95% CI: 0.71, 1.76), 1.13 (95% CI: 0.73, 1.77), and 1.67 (95% CI: 1.07, 2.61), respectively, compared with those in quartile 1. Consistently, a significantly higher risk of HHcy (OR: 1.49; 95% CI: 1.19, 1.88) was found in participants in quartile 4 compared with those in quartiles 1–3. Furthermore, a strongly positive association between LnBLL and HHcy was observed in participants with estimated glomerular filtration rate (eGFR) < 60 mL/min−1/1.73 m−2. Our results suggested that a higher level of BLL (LnBLL > 0.95 μg/dL) was associated with increased risk of HHcy compared with a lower level of BLL (LnBLL ≤ 0.95 μg/dL) among U.S. adults, and the association was modified by the eGFR.
Similar content being viewed by others
Introduction
Hyperhomocysteinemia (HHcy), a metabolic disorder by an abnormally increased level of plasma homocysteine, is defined as plasma homocysteine level > 15 µmol/L1. HHcy is an established risk factor for a multitude of chronic conditions, such as Alzheimer’s disease, congestive heart failure, hearing loss, cancer, and atherosclerosis2,3,4,5,6. Although several factors have been demonstrated to be related to the prevalence of HHcy, including enzyme problems, lacking of cofactors, excessive methionine intake, certain diseases, and ingestion of certain drugs7. Increasing evidences have been suggested that heavy metals exposures may also lead to HHcy8,9,10. However, the potential impacts of heavy metals exposures have gained little attention.
Lead is a toxic metal widely distributed in the environment. In the past two decades, although regulatory actions had dramatically reduced lead exposures in the United States, it still occurs in many environmental settings, such as air pollution, smoking, contact with lead soils, certain foods, and drinking water11,12,13. Environmental low-dose Lead exposures also carried important public health implications14,15. Similarly to HHcy, a direct role of Lead was also observed in cardiovascular disease and cognitive dysfunction16,17. Given the similarities in these health effects, several previous studies have been examined the relationship between BLL and homocysteine10,18,19,20. Some epidemiologic studies had several strengths including prospective designs18. However, those studies have been limited by small sample sizes, lacking of adjustment for potential confounders, or other methodologic limitations. More importantly, few studies have thoroughly investigated the exact shape of the dose–response relationship between lead and HHcy. In addition, the possible modifiers in the association between lead and HHcy have not been comprehensively examined.
Therefore, our objective was to examine the association between blood lead and HHcy in a representative sample of U.S. adults aged ≥ 20 years who participated in the 2001–2006 National Health and Nutrition Examination Survey (NHANES). Additionally, we explored the possible effected modifiers in the association between BLL and HHcy.
Methods
Study population and design
The present study was performed using data from six years (2001–2006) of NHANES. Details of the NHANES had been described elsewhere21,22. In brief, NHANES used a complex multistage and stratified sampling design, and the participants were obtained from a representative of the noninstitutionalized civilian US population. During an interview at home, participants were invited to complete the questionnaires, perform medical examination, provide blood and other biological specimens in the mobile examination center. More detailed information is available on the official website (https://www.cdc.gov/nchs/nhanes/index.htm). The NHANES datasets were available on DataDryad (https://doi.org/10.5061/dryad.d5h62).
A total of 15,431 adults aged ≥ 20 years were enrolled for NHANES 2001–2006. Excluded participants with missing blood lead (n = 1625) and missing blood homocysteine (n = 62), and those taking supplemental Vitamin B12, Vitamin B6 and Folic acid might affect homocysteine metabolism (n = 4413). Finally, a total of 9,331 subjects were included in our study (Fig. 1).
Data collection procedures
Blood lead was measured at the Environmental Health Sciences Laboratory of the CDC National Center for Environmental Health (NCEH). Lead levels in whole blood were measured on a Perkin-Elmer model SIMAA 6000 (PerkinElmer, Norwalk, CT) simultaneous multielement atomic absorption spectrometer with Zeeman background correction, The limit of detection was 0.3 μg/dL in NHANES 2001–2004 and 0.25 μg/dL in NHANES 2005–2006. In present study, 0.53% and 0.24% of participants had BLL below the limits of detection in NHANES 2001–2004 and NHANES 2005–2006, respectively. Blood lead levels below the concentration of detection were assigned the limit of detection divided by the square root of 2 according to the recommendation of NHANES. National Institute of Standards and Technology whole-blood standard reference materials were used for external calibration. The interassay coefficients of variation ranged from 3.1% to 4.0% for blood lead.
The outcome variable was homocysteine. Homocysteine was measured by two methods, respectively, including Abbott Homocysteine IMX (HCY) assay (Abbott Diagnostics, Abbott Park, IL) in 2001 and Abbott Axsym system (Abbott Diagnostics, Abbott Park, IL) in 2002–2006. Long-term coefficients of variation for NHANES 2001–2006 were 3%-5% for total homocysteine concentrations. A crossover study was performed on the two methods, the results revealed no significant differences between two methods. Details of these assays and quality standards are available at https://cdc.gov/nchs/nhanes.
Other variables
Standard survey questionnaires were used to collect sociodemographic characteristics, including age, sex, race/ethnicity, marital status, education, physical activity, current smoking, alcohol intake and poverty income ratio. Race/ethnicity was defined as non-Hispanic white, non-Hispanic black, Mexican American, Other Hispanic and other races. Marital status was classified into married, single and living with partner. Educational background was grouped into less than high school, high school and above high school graduation. Physical activity was classified into sedentary, low, moderate and high. Current smoking was described yes or no. Alcohol intake was classified into low and high (< 3 and ≥ 3 drinks per day). Poverty income ratio was defined as the ratio of the midpoint of observed family income category to the official poverty threshold based on US Census Bureau information and classified into two groups (< 1 and ≥ 1). Physical examinations, such as weight and height were conducted following standardized protocol. Body mass index (BMI) was calculated as weight divided by height2 (kg/m2). Concentrations of cotinine were detected by using isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Blood Cadmium levels were measured by using multielement atomic absorption spectrometer with Zeeman background correction. C-reactive protein (CRP) levels were measured by using latex-enhanced nephelometry on a Dade Behring Nephelometer II Analyzer System (BNII). Serum uric acid was measured on a Roche Hitachi Model 917 or 704 Multichannel Analyzer in 2001 and a Beckman Synchron LX20 in 2002 using a colorimetric method. Estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation23. Both serum folate and vitamin B12 levels were determined by using the BioRad Laboratories “Quantaphase II Folate/Vitamin B12” radioassay kit (Bio-Rad Laboratories, Hercules, CA, USA). Dietary intake (Vitamin B12, Vitamin B6 and Folic acid) from foods was assessed using one in-person 24-h dietary recall during 2001–2002 and two 24-h dietary recalls (one in person and one 3–10 days later by telephone) for the 2003–2006 period. To calculate nutrient intake from foods, NHANES employed the US Department of Agriculture’s (USDA) Food and Nutrient Database for Dietary Studies (FNDDS) version 1 for NHANES 2001–2002, version 2 for NHANES 2003–2004, and version 3 for NHANES 2005–200624.
Statistical analysis
According to the guidelines of the CDC, two waves of continuous NHANES 2001–2002, 2003–2004 and 2005–2006 surveys were combined and six-year sampling weights were calculated. Because the distributions of blood lead levels (BLL) were highly skewed, the BLL was Ln-transformed (LnBLL) for analysis. We used weighted chi-square test (categorical variables) or weighted linear regression analyses (continuous variables) to calculate for differences among different LnBLL groups (quartiles). Data were presented as mean ± SD or proportions. We constructed multivariate logistic regression models to estimate odd ratios (ORs) for the risk of HHcy associated with LnBLL, after adjusting for the following covariates which were known as traditional or suspected risk factors for HHcy: Model I was adjusted for age, sex, BMI, race, education status, physical activity, marital status, poverty-to-income ratio, current smoking and alcohol intake. Model II was adjusted for all covariables in Model I plus adjusted for serum cotinine, blood cadmium, Serum Vitamin B12, Serum folate, Serum uric acid, eGFR, CRP, Vitamin B12 intake, Vitamin B6 intake and Folic acid intake. Additionally, to address for potential non-linear relationship between BLL and HHcy, a generalized additive model (GAM) and smooth curve fitting (penalized spline method) were conducted. Finally, to evaluate effect modification, the subgroup analyses were assessed for the variables: age, sex, BMI, race, education status, physical activity, marital status, current smoking, alcohol intake, eGFR.
We used the statistical package R software version 3.4.2 (https://www.R-project.org, The R Foundation) and Empower (R) version 2.17.8 (https://www.empowerstats.com; X&Y Solutions, Inc., Boston, MA) in the present analyses. A P value < 0.05 was considered statistically significant.
Research ethics
NHANES study protocols were approved by the research ethics review board of the National Center for Health Statistics. Methods were carried out following the STROBE statement. Written informed consents were obtained from all participants in the study.
Results
Baseline characteristics of selected participants
Based on the inclusion and exclusion criteria, a total of 9,331 participants were enrolled, the mean age of the study participants was 44.4 years. Among the participants, 51.3% were men, 67.3% were Non-Hispanic White, 13.2% were Non-Hispanic Black, 9.4% were Mexican American, 4.9% were Other Hispanic, and 5.2% Other races. The mean concentrations of BLL and homocysteine were 1.9 μg/dL and 9.1 μmol/L, respectively. The prevalence of HHcy was 6.87%. The weighted distributions of population characteristics and other covariates according to LnBLL quartiles (< 0.04, 0.04–0.49, 0.5–0.95 and > 0.95 μg/dL) were shown in Table 1. There were significant differences between LnBLL quartiles, except for serum Vitamin B12 and Vitamin B6 intake. Participants in the highest quartile of LnBLL (BLL > 0.95 μg/dL) were older, more likely to be male, non-Hispanic black and Mexican American, to have lower poverty-to-income ratio, educational levels and physical activity, higher alcohol intake and lower folic acid intake, they also had higher values in homocysteine, blood cadmium, serum cotinine, serum uric acid and lower values in BMI, serum folate, eGFR and CRP than those of the other groups (all P < 0.05).
Association of lead exposure with HHcy
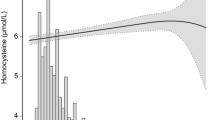
The association between BLL and risk of HHcy was presented in Fig. 2. Overall, the risk of HHcy increased with BLL. The ORs and 95% CIs for these three equations were listed in Table 2. In the fully adjusted model (model II), when LnBLL was assessed as quartiles, compared with participants in quartile 1 of LnBLL, the adjusted ORs for participants in quartiles 2–4 were 1.12 (95% CI: 0.71, 1.76), 1.13 (95% CI: 0.73, 1.77), and 1.67 (95% CI: 1.07, 2.61), respectively. Consistently, a significantly higher risk of HHcy (OR: 1.49; 95% CI: 1.19, 1.88) was found in participants in quartile 4 compared with participants in quartiles 1–3. Conversely, a significantly lower risk of HHcy (OR: 0.67; 95% CI: 0.53, 0.84) was found in participants in quartiles 1–3 compared with participants in quartile 4. Additionally, multiple linear regression coefficients of homocysteine in association with LnBLL were presented in Supplementary Information Table 1, the results remained similar as in Table 2.
Dose–response relationship between LnBLL exposure and HHcy*. Abbreviations: BLL, blood lead level; HHcy, hyperhomocysteinemia; eGFR, estimated glomerular filtration rate. Solid line represents the smooth curve fit between variables. Dotted lines represent the 95 of confidence interval from the fit.*Adjusted for age, sex, BMI, race, education status, physical activity, marital status, poverty-to-income ratio, current smoking, alcohol intake, serum cotinine, blood cadmium, Serum Vitamin B12, Serum folate, Serum uric acid, eGFR, C-reactive protein, Vitamin B12 intake, Vitamin B6 intake and Folic acid intake.
Stratified analysis by potential effect modifiers
We further performed stratified analyses to assess the effect of LnBLL on HHcy in various subgroups. None of the variables, including age (< 40, 40- < 60, and ≥ 60 y; P-interaction = 0.582), sex (male and female; P-interaction = 0.775), race (Non-Hispanic White, Non-Hispanic Black, Mexican American and Other Race; P-interaction = 0.286), education (< High school and ≥ High school; P-interaction = 0.162), marital status (Married, Single and Living with partner; P-interaction = 0.805), physical activity (Sedentary, Low, Moderate and High; P-interaction = 0.962), BMI (< 25, 25–< 30 and ≥ 30 kg/m2; P-interaction = 0.492), alcohol intake (< 3 and ≥ 3 drinks per day; P-interaction = 0.103), and current smoking (no and yes; P-interaction = 0.554) significantly modified the association between LnBLL and HHcy (Fig. 3). However, there was a significant interaction between LnBLL and eGFR (< 60 and ≥ 60 mL/min−1/1.73 m−2) on HHcy. A stronger positive association between LnBLL and HHcy was found in participants with eGFR < 60 mL/min−1/1.73 m−2 (OR: 2.30; 95% CI: 1.57, 3.38) compared with eGFR ≥ 60 mL/min−1/1.73 m−2 (OR: 1.19; 95% CI: 0.88, 1.61; P-interaction = 0.005).
Subgroup analyses of the effect of LnBLL on HHcy. Abbreviations: BLL, blood lead levels; HHcy, hyperhomocysteinemia; BMI, body mass index; eGFR, estimated glomerular filtration rate; OR, odd ratios; CI, confidence interval. *Adjusted for age, sex, BMI, race, education status, physical activity, marital status, poverty-to-income ratio, current smoking, alcohol intake, serum cotinine, blood cadmium, Serum Vitamin B12, Serum folate, Serum uric acid, eGFR, C-reactive protein, Vitamin B12 intake, Vitamin B6 intake and Folic acid intake, except for the stratifying variable.
Discussion
In this large cross-sectional study of a representative sample of U.S. adults who participated in NHANES 2001–2006, BLL was independently and positively associated with HHcy after adjusting other covariates. Moreover, we found a nonlinear relationship between BLL and HHcy. Higher LnBLL concentrations (> 0.95 μg/dL, quartile 4) was associated with a higher risk of HHcy compared with lower concentrations (≤ 0.95 μg/dL, quartiles 1–3). Furthermore, our study expanded the results of previous studies by demonstrating that the positive relation of BLL with HHcy was more pronounced in participants with eGFR < 60 mL/min−1/1.73 m−2.
The association between BLL and homocysteine had been examined in several previous studies8,10,13,18,25,26. In the United States, a sample of 1,140 adults who were 50–70 years of age, participated in the Baltimore Memory Study found a positive association of BLL with homocysteine, however, no association was found between tibia lead and homocysteine10. A prospective cohort study in 2301 American patients with a median follow-up of 3.9 y found that an interquartile range (IQR) increment in BLL (3 μg/dL) was associated with a 6.3% higher homocysteine concentration18. Guallar et al.13 performed a cross-sectional study among 4,447 persons aged ≥ 40 years who participated in the 1999–2002 NHANES and found that a positive linear association between BLL and homocysteine. However, our study found that the dose–response relationship between BLL and homocysteine was not linear (Supplementary Information Fig. 1) in the full adjusted model (model II). We speculated that several residual confoundings may conceal the true association between BLL and HHcy. For example, dietary intake and serum levels of folic acid, vitamin B12 and vitamin B6 status may be more likely to inflect homocysteine levels27; eGFR has been shown not only to increase homocysteine concentrations but also association with BLL28,29. Our results agreed with earlier reports in other NHANES (2003–2004) study20, which revealed a nonlinear increase in the risk of HHcy prevalence with increase BLL. This study found that, in the fully adjusted models compared with participants in quartile 1 (BLL ≤ 1.0 μg/dL), the significant increased risk of HHcy was found in participants in quartile 4–3 and 4–4 (BLL ≥ 3.5 μg/dL; OR: 2.6; 95% CI: 1.4, 4.8 and 3.2; 95% CI: 1.7, 5.9, respectively), not in quartile 2 to quartile 4–2.
The pathophysiology of the association between BLL and homocysteine has not yet been totally explicated. A primary mechanism for lead toxicity is inhibition heme and hemoglobin synthesis30, thus directly affecting the function of cystathionine β-synthase, which is a unique heme-containing in transsulfuration to cysteine of homocysteine, leading to an accumulation of homocysteine31. Additionally, BLL has high electron-sharing affinities for the free sulfhydryI group of proteins, homocysteine itself also contains a sulfhydryl group, so BLL may inhibit the metabolism of homocysteine by this pathway30,32. Moreover, in the remethylation pathway of homocysteine through the enzyme methionine synthase with vitamin B12, folate, and MTHFR, when the MTHFR enzyme mutates might cause Lead to bind more to active sites, and cause impaired enzyme function33.
In the present study, a strong association between BLL and HHcy was found in participants with low eGFR levels (< 60 mL/min−1/1.73 m2). Chronic lead exposure may contribute to chronic kidney disease, indicated by low eGFR34,35. In the Malmö Diet and Cancer Study-Cardiovascular Cohort, for example, BLL has been shown to decrease eGFR in participants of the prospective study aged 46 to 67 years34. Similarly to lead, elevated homocysteine level was also associated with an accelerated decline in renal function in the general population36. Conversely, a inverse relationship between the eGFR and plasma homocysteine level was present throughout the whole range of renal function37. Therefore, eGFR may modify the relation between BLL and homocysteine. However, the causal mechanisms are not fully understood, further studies focused on the suggested pathway are warranted.
Study limitations
There were several limitations in our study. Firstly, taking into account the present study was a cross-sectional study, the directionality of the association between BLL and HHcy cannot be conclusively established. Although we made an effort to adjust for as many potential confounders as possible, bias due to unmeasured confounding may still remain. Secondly, the biologic residence time of Lead in blood was measured in few days, single BLL may be limited to assess the association between BLL and HHcy. However, it has been reported that BLL can be considered as a steady-state mixture from both acute external Lead exposure and the internal stores of Lead, and can be associated with plasma homocysteine levels 4–6 years later25,38. Thirdly, the gene interaction could not be considered in present study, which was already known to be associated with the level of homocysteine (e.g., MTHFR gene). Further studies with different genotypes related to Lead and homocysteine metabolism are warranted to better investigate the association between Lead exposure and HHcy.
Conclusions
Our study revealed a nonlinear association between BLL and HHcy in a U.S. adults, and the association was modified by the eGFR. Additional studies are warranted to confirm our findings in prospective cohorts and to elucidate the potential mechanisms underlying the relationship between Lead and HHcy.
References
Moll, S. & Varga, E. A. Homocysteine and MTHFR mutations. Circulation 132, e6–e9 (2015).
Seshadri, S. et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 346, 476–483 (2002).
Cooke, G. E. et al. Plasma atherogenic markers in congestive heart failure and Posttransplant (heart) patients. J. Am. Coll. Cardiol. 36, 509–516 (2000).
Martinez-Vega, R. et al. Folic acid deficiency induces premature hearing loss through mechanisms involving cochlear oxidative stress and impairment of homocysteine metabolism. Faseb J. 29, 418–432 (2015).
Qin, X. et al. MTHFR C677T and MTR A2756G polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensive chinese adults. Nutr. J. 11, 2 (2012).
Sreckovic, B. et al. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab Syndr. 11, 179–182 (2017).
Kim, J., Kim, H., Roh, H. & Kwon, Y. Causes of Hyperhomocysteinemia and its Pathological Significance. Arch. Pharm. Res. 41, 372–383 (2018).
Pollack, A. Z. et al. Blood lead, cadmium and mercury in relation to homocysteine and C-reactive protein in women of reproductive age: A panel study. Environ. Health. 16, 84 (2017).
Cai, R. et al. Effects of blood lead and cadmium levels on homocysteine level in plasma. Eur. Rev. Med. Pharmacol. Sci. 21, 162–166 (2017).
Schafer, J. H., Glass, T. A., Bressler, J., Todd, A. C. & Schwartz, B. S. Blood lead is a predictor of homocysteine levels in a population-based study of older adults. Environ. Health Perspect. 113, 31–35 (2005).
Renaudin, M., Leblond, S., Meyer, C., Rose, C. & Lequy, E. The coastal environment affects lead and sodium uptake by the Moss Hypnum Cupressiforme used as an air pollution biomonitor. Chemosphere 193, 506–513 (2018).
Schock, M. R., Hyland, R. N. & Welch, M. M. Occurrence of contaminant accumulation in lead pipe scales from domestic drinking-water distribution systems. Environ. Sci. Technol. 42, 4285–4291 (2008).
Guallar, E. et al. Confounding of the relation between homocysteine and peripheral arterial disease by lead, cadmium, and renal function. Am. J. Epidemiol. 163, 700–708 (2006).
Ekong, E. B., Jaar, B. G. & Weaver, V. M. Lead-related nephrotoxicity: A review of the epidemiologic evidence. Kidney Int. 70, 2074–2084 (2006).
Hu, H. Bone lead as a new biologic marker of lead dose: Recent findings and implications for public health. Environ Health Perspect. 106(Suppl 4), 961–967 (1998).
Cheng, Y. et al. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: The normative aging study. Am. J. Epidemiol. 153, 164–171 (2001).
Schwartz, B. S. et al. Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with neurobehavioral test scores in South Korean Lead Workers. Am. J. Epidemiol. 153, 453–464 (2001).
Bakulski, K. M. et al. Lead exposure, B Vitamins, and plasma homocysteine in men 55 years of age and older: The VA normative aging study. Environ. Health Perspect. 122, 1066–1074 (2014).
Krieg, E. J. & Butler, M. A. Blood lead, serum homocysteine, and neurobehavioral test performance in the third national health and nutrition examination survey. Neurotoxicology 30, 281–289 (2009).
Lee, Y. M. et al. Association of homocysteine levels with blood lead levels and micronutrients in the US General Population. J. Prev. Med. Public Health. 45, 387–393 (2012).
Curtin, L. R. et al. The national health and nutrition examination survey: Sample design, 1999–2006. Vital. Health Stat. 2, 1–39 (2012).
Patel, C. J. et al. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci. Data. 3, 160096 (2016).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Hamner, H. C., Cogswell, M. E. & Johnson, M. A. The association of acculturation factors with biochemical indices of folate status among mexican Americans 60 years of age or older in the post-fortification era: National health and nutrition examination survey (NHANES) 2001–2006. J Nutr Gerontol Geriatr. 30, 50–71 (2011).
Kim, K. N., Lee, M. R., Lim, Y. H. & Hong, Y. C. Blood lead levels, iron metabolism gene polymorphisms and homocysteine: A gene-environment interaction study. Occup. Environ. Med. 74, 899–904 (2017).
Chia, S. E. et al. Association of blood lead and homocysteine levels among lead exposed subjects in vietnam and Singapore. Occup. Environ. Med. 64, 688–693 (2007).
Jacques, P. F., Selhub, J., Bostom, A. G., Wilson, P. W. & Rosenberg, I. H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 340, 1449–1454 (1999).
Lewerin, C., Ljungman, S. & Nilsson-Ehle, H. Glomerular filtration rate as measured by serum Cystatin C is an important determinant of plasma homocysteine and serum methylmalonic acid in the elderly. J. Intern. Med. 261, 65–73 (2007).
Wu, M. T. et al. A Delta-Aminolevulinic Acid Dehydratase (ALAD) polymorphism may modify the relationship of low-level lead exposure to uricemia and renal function: The normative aging study. Environ. Health Perspect. 111, 335–341 (2003).
Flora, S. J., Mittal, M. & Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 128, 501–523 (2008).
Banerjee, R., Evande, R., Kabil, O., Ojha, S. & Taoka, S. Reaction mechanism and regulation of cystathionine beta-synthase. Biochim. Biophys. Acta. 1647, 30–35 (2003).
Quig, D. Cysteine metabolism and metal toxicity. Altern. Med. Rev. 3, 262–270 (1998).
Yakub, M. et al. Polymorphisms in MTHFR, MS and CBS genes and homocysteine levels in a Pakistani population. PLoS ONE 7, e33222 (2012).
Harari, F. et al. Blood lead levels and decreased kidney function in a population-based cohort. Am. J. Kidney Dis. 72, 381–389 (2018).
Kim, Y. & Lee, B. K. Associations of blood lead, cadmium, and mercury with estimated glomerular filtration rate in the korean general population: Analysis of 2008–2010 Korean National Health and nutrition examination survey data. Environ. Res. 118, 124–129 (2012).
Levi, A. et al. Elevated serum homocysteine is a predictor of accelerated decline in renal function and chronic kidney disease: A historical prospective study. Eur. J. Intern. Med. 25, 951–955 (2014).
van Guldener, C., Stam, F. & Stehouwer, C. D. Homocysteine metabolism in renal failure. Kidney. Int. Suppl. 78, S234–S237 (2001).
Hu, H., Shih, R., Rothenberg, S. & Schwartz, B. S. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect. 115, 455–462 (2007).
Acknowledgements
The authors thank to all of the NHANES participants who gave freely of their time to make this and other studies possible.
Author information
Authors and Affiliations
Contributions
M.L. participated in literature search, study design, data collection, data analysis, data interpretation, and wrote the manuscript. L.H., W.Z., T.W., L.Z. conceived of the study, and participated in its design, coordination, data collection and analysis. Z.Z. revised manuscript language. H.B. and X.C. participated in study design and provided the critical revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, M., Hu, L., Zhou, W. et al. Nonlinear association between blood lead and hyperhomocysteinemia among adults in the United States. Sci Rep 10, 17166 (2020). https://doi.org/10.1038/s41598-020-74268-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74268-6
- Springer Nature Limited
This article is cited by
-
Blood Homocysteine Levels Mediate the Association Between Blood Lead Levels and Cardiovascular Mortality
Cardiovascular Toxicology (2024)
-
Association and mediation analyses among multiple metals exposure, plasma folate, and community-based impaired estimated glomerular filtration rate in central Taiwan
Environmental Health (2022)
-
Landscape of cardiometabolic risk factors in Chinese population: a narrative review
Cardiovascular Diabetology (2022)