Abstract
Background
Unilateral diaphragmatic paralysis in patients with univentricular heart is a known complication after pediatric cardiac surgery. Because diaphragmatic excursion has a significant influence on perfusion of the pulmonary arteries and hemodynamics in these patients, unilateral loss of function leads to multiple complications. The current treatment of choice, diaphragmatic plication, does not lead to a full return of function. A unilateral diaphragmatic pacemaker has shown potential as a new treatment option. In this study, we investigated an accelerometer as a trigger for a unilateral diaphragm pacemaker (closed-loop system).
Methods
Seven pigs (mean weight 20.7 ± 2.25 kg) each were implanted with a customized accelerometer on the right diaphragmatic dome. Accelerometer recordings (mV) of the diaphragmatic excursion of the right diaphragm were compared with findings using established methods (fluoroscopy [mm]; ultrasound, M-mode [cm]). For detection of the amplitude of diaphragmatic excursions, the diaphragm was stimulated with increasing amperage by a cuff electrode implanted around the right phrenic nerve.
Results
Results with the different techniques for measuring diaphragmatic excursions showed correlations between accelerometer and fluoroscopy values (correlation coefficient 0.800, P < 0.001), accelerometer and ultrasound values (0.883, P < 0.001), and fluoroscopy and ultrasound values (0.816, P < 0.001).
Conclusion
The accelerometer is a valid method for detecting diaphragmatic excursion and can be used as a trigger for a unilateral diaphragmatic pacemaker.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
The diaphragm has an important role in spontaneous breathing. In particular, inspiration and resting breathing depend on diaphragmatic excursion and contraction [1, 2], a dependence that is especially relevant for small children [3]. For this reason, loss of diaphragmatic function may lead to various forms of dysfunctional breathing, including the need for mechanical ventilation [2]. Diaphragmatic function can be disturbed at different levels. The central nervous system and the phrenic nerve may be damaged or affected (e.g., by surgical injury or trauma), or damage at the neuromuscular junction or the muscle directly may lead to impaired respiration [4]. In children with congenital heart disease, especially univentricular hearts, unilateral diaphragmatic paralysis after heart surgery is prominent in postoperative respiratory disturbance. These patients undergo a multi-step palliation [5], in which the upper and lower venae cavae are connected to the pulmonary arteries so that the oxygen-depleted blood flows passively to the lungs. Spontaneous respiration is important in the pulmonary arterial flow and for the whole Fontan circulation [6,7,8,9], and unilateral diaphragmatic paralysis leads to impairment of both [10, 11].
To date, the only diaphragmatic pacing systems used clinically stimulate both sides of the diaphragm and require an external stimulator. These systems are used in the treatment of spinal cord injury, amyotrophic lateral sclerosis, and Undine syndrome. A diaphragm pacemaker exclusively for unilateral diaphragmatic paralysis does not exist. Attempts have been made to use bilateral systems for these patients [12], but only Kaneko et al., in an experimental trial, have achieved unilateral diaphragm stimulation based on thorax impedance of the contralateral side [13]. The sole available technically feasible approach is stimulation with a fixed rate without sensing or triggering [14]. The first positive results have been achieved for bilateral diaphragm pacemakers in the context of animal experiments [15].
In view of the limitations of current options, it seems obvious that a technical solution is needed that allows triggering of the diaphragmatic excursion and thus unilateral diaphragmatic pacing. The aim of our research is to develop a unilateral diaphragmatic pacemaker for pediatric cardiac patients with unilateral diaphragmatic paralysis to restore a complete return of diaphragm function. Stimulation of the paralytic side should be triggered based on the non-paretic side. In the current study, we investigated whether an accelerometer is a suitable tool for this purpose.
Fluoroscopy [16] and ultrasound (M-mode) [17] are two established methods for evaluating diaphragm excursion, and we compared the results of accelerometer measures against these two approaches and analyzed correlations among them.
Results
Diaphragm excursion
Baseline measurements demonstrated right hemidiaphragm excursion of 5.01 ± 0.51 mm (n = 7) by fluoroscopy, 0.87 ± 0.13 cm (n = 7) by ultrasound, and 48.33 ± 7.23 mV (n = 7) by accelerometer. The first significant increase in diaphragmatic movement on the right side could be measured by ultrasound (1.73 ± 0.17 cm, P = 0.011) and accelerometer (122.62 ± 13.36 mV, P = 0.007) at stimulation with 0.2 mA. The first significant difference by fluoroscopy (8.61 ± 0.55 mm, P = 0.003) could be detected at 0.3 mA. All measurements showed a continuous increase of the mean value between stimulation with 0.1 mA to the maximum stimulation with 0.5 mA. All three measurement methods offered reliable detection of any diaphragmatic excursions (Fig. 1).
Correlations
All correlation analyses of measurements obtained with the three techniques yielded significant correlations, with a correlation coefficient of 0.816 (P < 0.001) between fluoroscopy and ultrasound measurements (Fig. 2), 0.883 (P < 0.001) between accelerometer and ultrasound measurements (Fig. 3), and 0.80 (P < 0.001) between fluoroscopy and accelerometer values (Fig. 4). These results suggest strong linear correlations of these measurements.
Discussion
Our results demonstrate that diaphragm movement can be reliably recorded with implanted accelerometers. As with clinically established imaging techniques, this system is suitable for determining the excursion of the diaphragm, with accelerometer-based measurement showing a linear correlation with fluoroscopy (P < 0.001) and ultrasound (P < 0.001) Measurements.
Unilateral diaphragmatic pacemaker in Fontan patients
Unilateral diaphragmatic paralysis leads to deterioration of the Fontan circulation [11], as well as to reduced perfusion of the pulmonary artery on the paretic side [10]. Currently, no technique allows unilateral diaphragmatic pacing. The accelerometer accurately detects the movement of the diaphragm, so this signal might act as a trigger for unilateral diaphragm pacing. For these reasons, a contralateral diaphragmatic pacemaker triggered by an accelerometer appears to be a potential therapeutic option for Fontan patients with unilateral diaphragmatic palsy.
Use of the accelerometer in bilateral diaphragmatic pacemakers
Accelerometer-based sensor electronics could be implanted at the highest point of each hemidiaphragm during the implantation of a bilateral thoracic diaphragmatic pacemaker in patients with hypoventilation syndrome (Undine syndrome) [18], to detect the autonomous diaphragm movement. So, an accelerometer may serve as an upgrade to the currently available systems for bilateral diaphragmatic pacing. At the moment, in Undine syndrome patients the bilateral diaphragm pacing most reliably is conducted by direct phrenic nerve stimulation. An accelerometer could be used to check the functionality of the diaphragmatic pacemaker as part of tests/monitoring, with no radiation exposure, in contrast to fluoroscopy. In addition, an implantable accelerometer could be used as a trigger in patients with hypoventilation syndrome to establish a closed-loop system. Hypoventilation or apnea can occur during waking hours as well as during sleep [18, 19], and these apneas with the associated lack of diaphragm movement could be detected by the accelerometer and lead to stimulation.
Use of the accelerometer in animal experiments
An implantable acceleration sensor also could be used in animal experiments involving the question of diaphragm excursion, especially those that investigate diaphragm excursion over a longer period of time in connection with diseases of the diaphragm. All existing methods for evaluating dynamic diaphragm movement—fluoroscopy [16], ultrasound diagnostics using M-mode [17], and dynamic magnetic resonance imaging [20]—show limitations. With ultrasound, limitations include a restricted field of view, the possible impairment of lung air or bowel gas superimposition, and close reliance on operator expertise. Especially in the left half of the diaphragm, ultrasound is limited by the position of the spleen [21, 22]. Fluoroscopy, which is usually used as a gold standard, entails a high radiation exposure [23]. MRI imaging offers the possibility of a three-dimensional representation but is time-consuming and cost-intensive [22].
Limitations
The limitations of this study include the lack of representation of physiological respiratory parameters in relation to diaphragmatic excursions. Because the experiment was performed with the thorax open, these data could not be validly measured. The study also was relatively brief, so that no conclusions can be drawn about the long-term course of measuring diaphragmatic movement with an accelerometer. These questions should be addressed in additional studies. Furthermore, in our test, the accelerometer was still powered externally, and data acquisition was external. For future applications, these features would need further development into a fully implantable and battery-operated system.
Conclusions
The accelerometer is a valid method for detecting diaphragmatic excursions as a possible trigger for a unilateral diaphragmatic pacemaker.
Methods
Experiments were conducted in accordance with the German Animal Welfare Act and its subsequent statutory acts, which are in accordance with the Council of Europe Convention ETS 123. The competent state agency, the State Office for Nature, Environment and Consumer Protection North Rhine-Westphalia, approved the study (permit: 81-02.04.2020.A392). Our study complied with the Animal Research: Reporting of In Vivo Experiments guidelines 2.0.
Animal preparation and instrumentation
Seven pigs were examined and had a mean weight of 20.7 ± 2.25 kg. Pigs were supplied in-house by the Agriculture Faculty, Rheinische Friedrich-Wilhelms-University Bonn, Königswinter-Vinxel, Germany, were of conventional microbiologic status, and had an acclimatization period of 3 days at our facility.
Premedication before the operation consisted of intramuscularly administered ketamine (20 mg/kg; WDT, Garbsen, Germany) in combination with azaperone (2 mg/kg; Richter Pharma, Wels, Austria) and atropine (0.02 mg/kg; B. Braun, Melsungen, Germany). After adequate sedation was achieved, venous access was implemented with a 1.1-mm outer diameter Jelco® catheter (Smith Medical, Grasbrunn, Germany) in one of the ear veins. Anesthesia induction consisted of piritramide (0.5 mg/kg; Hameln Pharma, Hameln, Germany) and propofol (10 mg/kg; CP Pharma, Burgdorf, Germany). The administration of muscle relaxants was explicitly dispensed with in this experiment. We secured the airway via endotracheal intubation using a straight size 4 Miller blade with a 5.0-mm internal diameter curved, micro-cuffed endotracheal tube (Avanos, Hamburg, Germany). For invasive ventilation, we used a Servo-i (Maquet, Rastatt, Germany) with synchronized intermittent mandatory ventilation with a frequency of ~ 15/min, an inspiratory pressure of 15 cmH2O, and a positive end-expiratory pressure of 5 cmH2O. Anesthesia was maintained with continuous intravenous infusion of propofol (1–5 mg/kg) via a Perfusor®Space (B. Braun, Melsungen, Germany) and continuous intravenous infusion of piritramide (0.2–0.5 mg/kg), supplemented by occasional single doses of ketamine (5–10 mg/kg) or midazolam (0.5 mg/kg; B. Braun, Melsungen, Germany). We monitored the depth of the total intravenous anesthesia with the Narcotrend system via a CompactM-monitor for intraoperative use (Narcotrend, Hannover, Germany) using needle electrodes (Neuroline Twisted Pair Subdermal, 12 × 0.4 mm, Ambu, Ballerup, Denmark) placed at the standard positions according to the manufacturer’s instructions. During the procedure, swine had the electroencephalographic Kugler-stadium [24] D0. This stage corresponds to general anesthesia.
We monitored the animals using an Infinity C500 monitor (Dräger, Lübeck, Germany) for electrocardiogram, pulse oximetry, and invasive blood pressure. For capnometry, we used a Datex-Ohmeda S/5 (Datex-Ohmeda, Duisburg, Germany). We placed an arterial line (2.7 French leadercath, Vygon, Aachen, Germany) in the right femoral artery for continuous blood pressure monitoring and a three-lumen central line (5.5 French, Teleflex, Fellbach, Germany) in the right femoral vein. Regular blood samples were taken to check pH, pCO2, pO2, electrolytes, and hemoglobin. In addition, we placed a 10 Charrière transurethral catheter (Asid Bonz, Herrenberg, Germany) to monitor urine output. Temperature was measured with a 9 French rectal probe (Smiths Medical, Grasbrunn, Germany). Maintenance fluids were infused at a rate of 40–60 mL/kg/h using Ionosteril 1/1 (Fresenius Kabi, Bad Homburg, Germany).
At the end of the experiment, animals were euthanized using T61® (tetracaine/mebezonium/embutramide; Intervet, München, Germany) at a dose of 0.5 mL/kg.
Technical set-up
Accelerometer
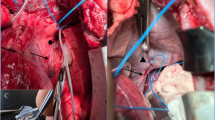
We used an MXR9500 3D accelerometer ICs (MEMSIC Inc., Andover, MA, USA) to record the movement of the diaphragm on the right side. The accelerometer converts diaphragmatic motion proportionally into a change of voltage. These sensors are particularly suitable because of their resolution of 1 mg0 (g0 = 9.81 m/s2, standard acceleration due to gravity), high sensitivity (500 mV/g0), small dimensions (7 × 7 × 1.8 mm3), and low requirement for external circuitry. The additional electronic components were soldered directly onto the IC to reduce the volume of the assembly. A silicone tube with an outer diameter of 1.96 mm (Silastic Rx-Medical Grade tubing, Dow Inc., Midland, MI, USA) bundled the leads, and the entire sensor was mechanically fixed with epoxy resin and finally encapsulated with silicone (Silibone MED ADH 4300 RTV, Bluestar Silicones, Ventura, CA, USA). A polypropylene surgical mesh (Angimesh 9, Angiologica B.M. S.r.l., Pavia, Italy) was glued to the sensor, allowing the surgeon to fix the sensor to the implantation site (Fig. 5). An external power supply provided 3 V to the sensors. The three analog outputs of each sensor were connected to six analog single-ended inputs of the bioamplifier (Powerlab 16/35, ADInstruments Ltd, Sydney, Australia).
All inputs had a sensitivity of 156 µV at ± 5 V full range. Depending on the initial sensor position relative to gravitational acceleration, all sensors were adjusted to an individual offset to achieve maximum signal representability. All channels were low-pass filtered at 50 Hz to suppress electrical hum.
Cuff electrode
We developed and manufactured a simple cuff electrode design for short-term acute experimental setups. Two medical-grade stainless steel electrode contacts were 1.2 mm in diameter and bonded into a silicone tube (Sil-Tec®, 602-305, Technical Products Inc. of Georgia, Lawrenceville, GA, USA) with a center-to-center spacing of 5 mm. This tube has an inner diameter of 1.98 mm, matching the contacted nerve, and is incised over the entire length of 11 mm. Two glued-on wings made of silicone foil with a thickness of 175 µm (NA 501-1, NAGOR, Glasgow, Scotland, UK) allowed for easy handling and positioning on the nerve (Fig. 6).
Intervention set-up
After the pigs were placed in the supine position, a median sternotomy was performed. The chest remained open during the entire experiment. The pleura was opened, and the diaphragm and the right phrenic nerve carefully circumferentially exposed. Subsequently, the silicone-cuff electrode (Fig. 6) was placed at the intended position of the stimulator electrode at the level of the inferior vena cava. To ensure adequate electrode position, a limited neurolysis of around 20 mm was performed by sharp dissection taking care to injure neither the phrenic nerve nor its nutritive vessels. After final positioning of the stimulator electrode its lines were secured to the skin with simple stitches of a size 0 polyester fiber suture. The accelerometer was then implanted at the highest point of the right diaphragmatic dome (Fig. 7).
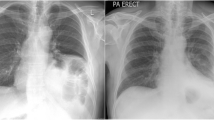
The position was checked by fluoroscopy (Ziehm, Nürnberg, Germany). The cuff electrode was implanted at the right phrenic nerve. For current-controlled direct nerve stimulation by means of a cuff electrode, we used a FE180 stimulator (ADInstruments Ltd, Sydney, Australia). To provoke different patterns of diaphragmatic movement, we varied the amplitude (i.e., 0.1, 0.2, 0.3, 0.5 mA) and kept the pulse width (200 µs), frequency (30 Hz), and stimulation duration (300 ms) fixed.
Measurement method
To evaluate the diaphragm excursion by fluoroscopy (Fig. 8), a virtual tangent was drawn across the highest point of the diaphragm, and the diaphragm excursion was measured at a right angle in millimeters. At minimum of three diaphragmatic excursions were recorded, and the mean value was taken.
Diaphragmatic excursion by ultrasound was assessed with a subxiphoid plane and in M-mode as described by Epelman et al. [25]. For this purpose, we used an ultrasound Philips HD 15 (Philips, Amsterdam, The Netherlands) with an S5–2.5 MHz sector array transducer (Fig. 9).
The diaphragm excursion was displayed in three planes by using the accelerometer (Fig. 10). For the final representation of the diaphragm excursion by means of the accelerometer, the individual accelerometer vectors (x, y, z) were taken, and the magnitude of vectors was calculated to represent a movement in the three-dimensional space. All measurements were taken simultaneously.
Parallel evaluation of the left diaphragm was not performed because of the more difficult imaging window involved and the difficult surgical access to the left phrenic nerve. Baseline measurements were taken under the ventilation setting with an inspiratory pressure of 15 cmH2O, a positive end-expiratory pressure of 5 cmH2O, and a breathing rate of 15/min.
Data analysis
Data are presented as mean ± standard error of the mean. Statistical analysis was performed with IBM SPSS Statistics Version 28. Three measurements were always averaged for statistical evaluation. The statistical significance of changes from baseline values within each group was tested with analysis of variance (ANOVA) for repeated measures. Differences between groups were analyzed by one-way ANOVA comparing several groups. When values did not show a normal distribution, ANOVA for nonparametric values (Kruskal–Wallis test) was used. A linear regression calculation was performed to test the correlation of accelerometer, ultrasound M-Mode, and fluoroscopy values. Correlation coefficients (r) ≥ 0.7 and ≤ − 0.7 were assumed to indicate a correlation between these variables. Statistical significance was accepted at P ≤ 0.05 after multiple testing.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gibson GJ. Diaphragmatic paresis: pathophysiology, clinical features, and investigation. Thorax. 1989;44(11):960–70.
Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168(1):10–48.
Saikia D, Mahanta B. Cardiovascular and respiratory physiology in children. Indian J Anaesth. 2019;63(9):690–7.
McCool FD, Manzoor K, Minami T. Disorders of the diaphragm. Clin Chest Med. 2018;39(2):345–60.
de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96(5):682–95.
Penny DJ, Redington AN. Doppler echocardiographic evaluation of pulmonary blood flow after the Fontan operation: the role of the lungs. Br Heart J. 1991;66(5):372–4.
Redington AN, Penny D, Shinebourne EA. Pulmonary blood flow after total cavopulmonary shunt. Br Heart J. 1991;65(4):213–7.
Hsia TY, Khambadkone S, Redington AN, Migliavacca F, Deanfield JE, de Leval MR. Effects of respiration and gravity on infradiaphragmatic venous flow in normal and Fontan patients. Circulation. 2000;102(19):III148–53.
van der Woude SFS, Rijnberg FM, Hazekamp MG, Jongbloed MRM, Kenjeres S, Lamb HJ, Westenberg JJM, Roest AAW, Wentzel JJ. The influence of respiration on blood flow in the Fontan circulation: insights for imaging-based clinical evaluation of the total cavopulmonary connection. Front Cardiovasc Med. 2021;8: 683849.
Stevenson JG. Effect of unilateral diaphragm paralysis on branch pulmonary artery flow. J Am Soc Echocardiogr. 2002;15(10 Pt 2):1132–9.
Hsia TY, Khambadkone S, Bradley SM, de Leval MR. Subdiaphragmatic venous hemodynamics in patients with biventricular and Fontan circulation after diaphragm plication. J Thorac Cardiovasc Surg. 2007;134(6):1397–405.
Onders RP, Elmo M, Kaplan C, Katirji B, Schilz R. Extended use of diaphragm pacing in patients with unilateral or bilateral diaphragm dysfunction: a new therapeutic option. Surgery. 2014;156(4):776–84.
Kaneko S, Jacobs G, Matsushita S, Nose Y. A new approach to respiratory assist for phrenic nerve paralysis. Trans Am Soc Artif Intern Organs. 1985;31:301–4.
Le Pimpec-Barthes F, Legras A, Arame A, Pricopi C, Boucherie JC, Badia A, Panzini CM. Diaphragm pacing: the state of the art. J Thorac Dis. 2016;8(Suppl 4):S376–86.
Siu R, Abbas JJ, Hillen BK, Gomes J, Coxe S, Castelli J, Renaud S, Jung R. Restoring ventilatory control using an adaptive bioelectronic system. J Neurotrauma. 2019;36(24):3363–77.
Leal BE, Gonçalves MA, Lisboa LG, Linné LMS, Tavares MGS, Yamaguti WP, Paulin E. Validity and reliability of fluoroscopy for digital radiography: a new way to evaluate diaphragmatic mobility. BMC Pulm Med. 2017;17(1):62.
Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319–29.
Trang H, Samuels M, Ceccherini I, Frerick M, Garcia-Teresa MA, Peters J, Schoeber J, Migdal M, Markstrom A, Ottonello G, Piumelli R, Estevao MH, Senecic-Cala I, Gnidovec-Strazisar B, Pfleger A, Porto-Abal R, Katz-Salamon M. Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J Rare Dis. 2020;15(1):252.
Schenk M. Ondine syndrome. Dtsch Med Wochenschr. 2010;135(24):24.
Cicero G, Mazziotti S, Blandino A, Granata F, Gaeta M. Magnetic resonance imaging of the diaphragm: from normal to pathologic findings. J Clin Imaging Sci. 2020;10:1.
Magder S, Malhotra A, Hibbert KA, Hardin CC. Cardiopulmonary monitoring: basic physiology, tools, and bedside management for the critically ill. Cham: Springer; 2021.
Laghi FA Jr, Saad M, Shaikh H. Ultrasound and non-ultrasound imaging techniques in the assessment of diaphragmatic dysfunction. BMC Pulm Med. 2021;21(1):85.
Ricoy J, Rodríguez-Núñez N, Álvarez-Dobaño JM, Toubes ME, Riveiro V, Valdés L. Diaphragmatic dysfunction. Pulmonology. 2019;25(4):223–35.
Hess R, Kugler J. Elektroencephalographie In Klinik Und Praxis. Eine Einfuhrung. 1967;Schwabe & CO:Switzerland.
Epelman M, Navarro OM, Daneman A, Miller SF. M-mode sonography of diaphragmatic motion: description of technique and experience in 278 pediatric patients. Pediatr Radiol. 2005;35(7):661–7.
Acknowledgements
The authors thank Jan Dauvergne and Christina Oetzmann von Sochaczewski for their invaluable technical assistance. We thank Andrea Pieper and Kerstin Hagemeister for their support in organizing the animal experiments.
Funding
Open Access funding enabled and organized by Projekt DEAL. The present study was supported by the kinderherzen Fördergemeinschaft Deutsche Kinderherzzentren e.V. (www.kinderherzen.de).
Author information
Authors and Affiliations
Contributions
TK, UH, and BB proposed the study and were responsible for the study protocol. TK analyzed the data and prepared the figures. RR, TK, and AK were responsible for the experimental devices and equipment and measurement accuracy. TK designed the animal care protocol and supervised animal care during the whole study. All authors participated in planning and performing the experiments. TK, UH, RR, and BB wrote the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Experiments were conducted with approvals from the competent state agency, the State Office for Nature, Environment and Consumer Protection North Rhine-Westphalia (permit: 81-02.04.2020.A392).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kratz, T., Ruff, R., Koch, T. et al. Proof of concept of an accelerometer as a trigger for unilateral diaphragmatic pacing: a porcine model. BioMed Eng OnLine 22, 55 (2023). https://doi.org/10.1186/s12938-023-01119-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12938-023-01119-6