Abstract
Background
Certain species of macaques are natural hosts of Plasmodium knowlesi and Plasmodium cynomolgi, which can both cause malaria in humans, and Plasmodium inui, which can be experimentally transmitted to humans. A significant number of zoonotic malaria cases have been reported in humans throughout Southeast Asia, including Thailand. There have been only two studies undertaken in Thailand to identify malaria parasites in non-human primates in 6 provinces. The objective of this study was to determine the prevalence of P. knowlesi, P. cynomolgi, P. inui, Plasmodium coatneyi and Plasmodium fieldi in non-human primates from 4 new locations in Thailand.
Methods
A total of 93 blood samples from Macaca fascicularis, Macaca leonina and Macaca arctoides were collected from four locations in Thailand: 32 were captive M. fascicularis from Chachoengsao Province (CHA), 4 were wild M. fascicularis from Ranong Province (RAN), 32 were wild M. arctoides from Prachuap Kiri Khan Province (PRA), and 25 were wild M. leonina from Nakornratchasima Province (NAK). DNA was extracted from these samples and analysed by nested PCR assays to detect Plasmodium, and subsequently to detect P. knowlesi, P. coatneyi, P. cynomolgi, P. inui and P. fieldi.
Results
Twenty-seven of the 93 (29%) samples were Plasmodium-positive by nested PCR assays. Among wild macaques, all 4 M. fascicularis at RAN were infected with malaria parasites followed by 50% of 32 M. arctoides at PRA and 20% of 25 M. leonina at NAK. Only 2 (6.3%) of the 32 captive M. fascicularis at CHA were malaria-positive. All 5 species of Plasmodium were detected and 16 (59.3%) of the 27 macaques had single infections, 9 had double and 2 had triple infections. The composition of Plasmodium species in macaques at each sampling site was different. Macaca arctoides from PRA were infected with P. knowlesi, P. coatneyi, P. cynomolgi, P. inui and P. fieldi.
Conclusions
The prevalence and species of Plasmodium varied among the wild and captive macaques, and between macaques at 4 sampling sites in Thailand. Macaca arctoides is a new natural host for P. knowlesi, P. inui, P. coatneyi and P. fieldi.
Similar content being viewed by others
Background
Malaria is caused by the protozoan parasite members of the genus Plasmodium and over 250 Plasmodium spp. have been described in mammals, birds, rodents and reptiles [1,2,3]. More than 30 malaria species have been reported in non-human primates and the following have been either naturally acquired or experimentally transmitted to humans by mosquitoes: Plasmodium cynomolgi, Plasmodium knowlesi and Plasmodium inui from Old World monkeys, Plasmodium brasilianum and Plasmodium simium from New World monkeys and Plasmodium schwetzi from chimpanzees [2, 4,5,6,7].
Plasmodium knowlesi was first reported as a significant cause of human malaria in Malaysia in 2004 [8]. Subsequently, naturally-acquired human infections with P. knowlesi were documented in several other countries in Southeast Asia including Thailand [9, 10], Indonesia [11], Philippines [12], Singapore [13], Vietnam [14], Cambodia [15], Laos [16] and Myanmar [17]. Furthermore, travelers have returned to their home countries after visiting Thailand and other Southeast Asian countries with knowlesi malaria [18, 19]. In Thailand, the first locally acquired natural infection with P. knowlesi was reported in 2004, in a patient who had visited the forest in Prachuap Kiri Khan Province, Southern Thailand near the Myanmar border [9]. Subsequently, P. knowlesi infected patients have been reported in Tak, Chantaburi, Yala, Narathiwat, Prachuap Kiri Khan and Ranong Provinces [20,21,22]. These areas are located near the borders of Cambodia, Myanmar and Malaysia. Tourists visiting Ranong Province, and South Western Thailand have also returned to their home countries in Germany [23,24,25] and France [26] with knowlesi malaria. Besides P. knowlesi, naturally acquired human P. cynomolgi infections have recently been reported in Peninsular Malaysia [27], Malaysian Borneo [28, 29] and Cambodia [30] and in a Danish tourist who had visited Peninsular Malaysia and Thailand [31]. Although naturally-acquired human infections with P. inui have not been described, P. inui can cause malaria in humans by blood passage [4] or through mosquito bites in the laboratory [32].
The main natural hosts of P. knowlesi, P. cynomolgi and P. inui are long-tailed macaques (Macaca fascicularis) and pig-tailed macaques (Macaca nemestrina) which are found in nature in Southeast Asia [2]. There have also been reports of a P. knowlesi infection in a banded leaf monkey (Presbytis melalophos) in Peninsular Malaysia [33] and a dusky leaf monkey (Semnopithecus obscurus) in Thailand [34]. The other natural hosts of P. cynomolgi are Macaca radiata, Macaca cyclopis, Macaca sinica, Macaca mulatta, Presbytis cristasus and Presbytis entellus [2]. Plasmodium inui also naturally infects many monkey species, including M. cyclopis, M. mulatta, M. radiata, Presbytis cristasus, P. obscurus and Cynopithecus niger [2]. Besides the natural hosts described above, a number of non-human primate hosts can be experimentally infected with P. knowlesi and P. cynomolgi. Experimental hosts of P. knowlesi described to date have included Callithrix jacchus, Cebus spp., Cercocebus fuliginosus, C. cephus, Cynochepalus papio, Hoolock hoolock, M. radiata, M. arctoides, Papio doguera, Papio jubilaeus, Papio papio, Presbytis cristatus, Saimiri sciureus and Semnopithecus entellus [2]. For P. cynomolgi experimental hosts have included M. mulatta, Cercopithecus aethiops, Cebus capucinus and Papio papio [2].
In Thailand, six species of macaques have been identified in nature; M. fascicularis, M. nemestrina, the northern pig-tailed macaque (M. leonina), the rhesus macaque (M. mulatta), the stump-tailed macaque (M. arctoides) and the Assamese macaque (Macaca assamensis) [35,36,37,38,39]. There have been only two studies undertaken to determine the prevalence of malaria in non-human primates in Thailand. Using molecular techniques, P. inui and P. coatneyi were identified in wild-caught M. fascicularis in Ranong Province, near the Myanmar border [36]. Then in 2010, P. inui, P. coatneyi and P. knowlesi were described in wild-caught M. fascicularis and M. nemestrina in Yala and Narathiwat Provinces, in Southern Thailand near the Malaysian border [34]. It is important to determine the geographical range of malaria-positive non-human primates to inform the public of the risks of acquiring zoonotic malaria. The objective of this study was to determine the prevalence of malaria parasites in non-human primates from 4 new locations in Thailand.
Methods
Collection of samples
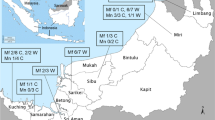
A total of 93 blood samples were collected from captive and wild macaques in 4 areas of Thailand from 2017–July 2019 (Fig. 1). These included 32 samples from captive M. fascicularis at the Krabok Koo Wildlife Breeding Center, Chachoengsao Province (CHA), 32 samples from wild M. arctoides at the Pa La U waterfall, Huahin district, Prachuap Kiri Khan Province (PRA), 25 samples from wild M. leonina at the Khao Yai National Park, Nakornratchasima Province (NAK) and 4 samples from wild M. fascicularis at Chang Island, Mu Ko Ranong National Park, Ranong Province (RAN). The Department of National Parks, Wildlife and Plant Conservation, Thailand, approved the protocol for study in a protected area, collection of the blood samples and released of wild macaques (Permit Number: 0909.204/14187). All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, U.S.A., and were approved by the Animal use and care committee of Kasetsart University Research and Development Institute, Kasetsart University, Thailand (ID: ACKU59-SCI-011).
The macaques were trapped and anesthetized intramuscularly with Tiletamine-Zolazepam (2–5 mg/kg body weight) and Xylazine hydrochloride (0.5–2 mg/kg body weight). The blood samples (maximum of 3 ml per animal) were collected using a syringe from the femoral vein of the macaques into a tube with EDTA. From the EDTA tubes, three 40–50 μL of blood from each sample were transferred to Whatman 3 MM filter paper. The samples were initially kept at room temperature and were transported to the Department of Zoology, Faculty of Science, Kasetsart University until molecular analysis. Blood spots on filter papers were transported to the Malaria Research Centre, Universiti Malaysia Sarawak, Kota Samarahan, Sarawak, Malaysia for DNA extraction and molecular analysis.
Analysis of samples
DNA was extracted from blood spots on filter papers with the use of InstaGene (Bio-Rad Laboratories, USA) as described previously [40]. The DNA samples were first examined by nested PCR assays based on the small subunit ribosomal RNA genes of Plasmodium with the aid of genus-specific primers (rPLU1 and rPLU5 in nest 1 amplification, and rPLU3 and rPLU4 in nest 2) as described previously [41]. Plasmodium-positive samples were then examined by nested PCR assays using species-specific primers to detect P. knowlesi, P. coatneyi, P. cynomolgi, P. inui and P. fieldi, as described previously [42]. The products of the PCR amplification were analysed by gel electrophoresis in 2.7% agarose gels and were stained by Sybersafe before being observed under UV light.
Statistical analysis
The Fisher-Freeman-Halton exact test was used to compute the exact probabilities of the prevalence of Plasmodium species between locations and among troops of macaques. The statistical analysis was undertaken using software R (version 3.5.2) and statistical significance was set at P < 0.05.
Results
Out of 93 macaque blood samples examined by nested PCR assays, 27 (29%) were Plasmodium-positive (Table 1). Among wild macaques, all 4 M. fascicularis at RAN were infected with malaria parasites followed by 50% of M. arctoides at PRA and 20% M. leonina at NAK. Only 6.3% of the captive M. fascicularis at CHA were malaria-positive.
All five species of Plasmodium parasites were detected; P. knowlesi, P. inui, P. cynomolgi, P. coatneyi and P. fieldi. The majority of malaria-positive macaques (59.3%) had mono-infections, 33.3% had double and 7.4% had triple infection of Plasmodium spp. Overall, P. inui was the most prevalent species detected, occurring in 35% of the malaria-positive macaques, followed by P. fieldi (30%), P. cynomolgi (20%), P. coatneyi (12.5%) and P. knowlesi (2.5%).
The prevalence of each parasite species varied significantly among the macaque species and sites of collection (Table 1). The P value for the Fisher exact test was 10–5 indicating that the prevalence of each species of Plasmodium species exhibited bias among different locations. The prevalence of each Plasmodium species infection in M. arctoides was higher than that for the other macaques (P = 0.007). and all five species of Plasmodium that were tested for, were detected in these wild M. arctoides.
Discussion
The prevalence of Plasmodium spp. in captive M. fascicularis at the Krabok Koo Wildlife breeding centre at CHA was much lower than that observed in wild macaques from the other 3 provinces. These macaques were reported to be trapped and brought to the centre due to human-monkey conflict. It is unlikely that these M. fascicularis acquired their infections at the breeding centre because if there were vectors of malaria in the vicinity and active transmission of malaria, the prevalence of malaria parasites would have been much higher at this location. A more likely explanation is that the 2 M. fascicularis that were infected had acquired their infections before they were transported to the breeding center. Among the infection rates in wild macaques, all the M. fascicularis studied from RAN had malaria parasites, while half the 32 wild M. arctoides studied at PRA and a quarter of the 25 M. leonina at NAK were infected. However, the current study is a relatively small one where only 4 M. fascicularis at RAN were examined, and future surveillance involving a larger number of samples is needed to determine accurately the prevalence of malaria infection among the wild macaques residing in all these areas. In future studies it would also be preferable to sample wild macaques rather than captive monkeys since this would provide more accurate information on zoonotic malaria parasites that are present in that particular ecosystem and could pose a threat to humans visiting these areas for recreation or hunting.
A complex nature of Plasmodium spp. infections was observed among the wild macaques studied, which is similar to the results of previous studies on wild macaques in Peninsular Malaysia, Malaysian Borneo, Thailand, Singapore, Laos, Cambodia and the Philippines [34, 42,43,44,45,46]. The DNA samples were each collected at single time points and the PCR results may not reflect the actual prevalence or the total number of species of Plasmodium present in each host since it has been observed that the parasitaemia of malaria parasites fluctuate over time within a host [2]. Furthermore, late asexual stage parasites of P. coatneyi sequester, so in a synchronous infection the parasites would only be detected in the blood if the majority of the parasites were at the early trophozoite or ring stage during sample collection [2]. The presence of multiple infections leads to difficulties in accurate identification by microscopy, so molecular methods are essential for identification of the various species of Plasmodium spp. in macaques and other primates.
In the current study, P. inui was the most prevalent Plasmodium spp. parasite detected in M. leonina, similar with the previous studies conducted on wild M. fascicularis and M. nemestrina in Thailand [34], Malaysian Borneo [42], and Peninsular Malaysia [44]. However, the composition of Plasmodium species within macaques at each sampling site was different, and this was also observed in the study on M. fascicularis in the Philippines [45] and in the study by Zhang et al. of regional populations of M. fascicularis across Southeast Asia [46]. The reasons for differences in the number of animals infected with multiple malaria parasites at each site are multifactorial, and probably include differences in host genetics and susceptibility, single time point sampling and differences in the vectors between sites [2].
Macaca arctoides, or stump-tailed macaques, are found in forested areas in continental Southeast Asian countries including Myanmar, Thailand, Vietnam, Laos, Peninsular Malaysia, and in south-western China and north-eastern India [35, 38]. In Thailand, the populations are distributed in the south in peninsular Thailand, and in central and north-western Thailand, mainly in the forests associated with limestone [35, 38]. Macaca arctoides are natural hosts for P. cynomolgi and have been shown experimentally to be susceptible to infection by P. knowlesi and P. coatneyi [2]. In a previous study, no malaria parasites were found in 4 M. arctoides from RAN in southern Thailand [36]. In the current study, M. arctoides at PRA were infected with P. knowlesi, P. coatneyi, P. cynomolgi, P. inui and P. fieldi. Since M. arctoides have previously been reported as natural hosts for P. cynomolgi [2], this is the first documentation of natural infections of M. arctoides with P. knowlesi, P. coatneyi, P. inui and P. fieldi.
Macaques from the following six provinces in Thailand had previously been trapped and studied for malaria parasites by Seethamchai et al. in 2006 [36] and by Putaporntip et al. in 2010; RAN, PRA, Pathalung, Pattani, Yala and Narathiwat [34]. In the current study, macaque samples from CHA and NAK, two provinces which had not been studied previously, were examined by molecular detection assays for malaria parasites. For the other two provinces (RAN and PRA), sampling was undertaken at sites which differed from the previous study in 2006. The macaque collecting site at RAN in the current study was Chang island in the Andaman sea, whereas Seethamchai et al. studied M. fascicularis from the mainland [36]. For PRA, these workers examined semi-wild M. fascicularis at the Wat Khao Takieb temple, while the current study focused on wild M. arctoides at the Pa La U waterfall in Huahin District, 76 km away from the Wat Khao Takieb temple. Therefore, a total of 8 different sites from 6 of the 77 provinces in Thailand have been studied so far to determine the prevalence of malaria parasites in macaques. Further studies, utilizing molecular detection assays and involving more sampling sites and a larger number of monkey blood samples per study site are necessary to determine the geographical range of macaques infected with zoonotic malaria parasites in Thailand and also in other countries in Southeast Asia.
Conclusions
Macaques sampled from all 4 locations in Thailand were infected with malaria parasites. The prevalence of malaria parasites varied among the species of monkeys and the sites of sample collection. This is the first report of natural infections of M. arctoides with P. knowlesi, P. coatneyi, P. inui and P. fieldi. The presence of macaques infected with malaria parasites, some that are transmissible to humans, presents a potential public health risk to the local population.
Availability of data and materials
Not applicable.
Abbreviations
- CHA:
-
Chachoengsao Province
- RAN:
-
Ranong Province
- PRA:
-
Prachuap Kiri Khan Province
- NAK:
-
Nakornratchasima Province
- Pk:
-
P. knowlesi; Pin: P. inui
- Pcy:
-
P. cynomolgi; Pct: P. coatneyi; Pfld: P. fieldi
References
Garnham PCC. Malaria parasites and other haemosporidia. Oxford: Blackwell Scientific Publications; 1966.
Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias: Department of Health, Education and Welfare; 1971.
Faust C, Dobson AP. Primate malarias: diversity, distribution and insights for zoonotic Plasmodium. One Health. 2015;1:66–75.
Das Gupta BM. Transmission of P. inui to man. Proc Natl Inst Sci India. 1938;4:241–4.
Lalremruata A, Magris M, Vivas-Martínez S, Koehler M, Esen M, Kempaiah P, et al. Natural infection of Plasmodium brasilianum in humans: man and monkey share quartan malaria parasites in the Venezuelan Amazon. EBioMedicine. 2015;29:1186–92.
Brasil P, Zalis MG, de Pina-Costa A, Siqueira AM, Júnior CB, Silva S, et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health. 2017;5:e1038–e10461046.
Contacos PG, Coatney GR, Orihel TC, Collins WE, Chin W, Jetner MH. Transmission of Plasmodium schwetzi from the chimpanzee to man by mosquito bite. Am J Trop Med Hyg. 1970;19:190–6.
Singh B, Sung LK, Matusop A, Radhakrishnan A, Shamsul SSG, Cox-Singh J, et al. A large focus of naturally acquired Plasmodium knowlesi infection in human beings. Lancet. 2004;363:1017–24.
Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in human. Thailand Emerg Infect Dis. 2004;10:2211–3.
Jongwutiwes S, Buppan P, Kosuvin R, Seethamchai S, Pattanawong U, Sirichaisinthop J, Putapontip J. Plasmodium knowlesi malaria in humans and macaques, Thailand. Emerg Infect Dis. 2011;17:1799–806.
Lubis IND, Wijaya H, Lubis M, Lubis CP, Divis PCS, Beshir KB, et al. Contribution of Plasmodium knowlesi to multispecies human malaria infections in North Sumatera. Indonesia J Infect Dis. 2017;215:1148–55.
Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, et al. Human infection with Plasmodium knowlesi, the Philippines. Emerg Infect Dis. 2008;14:811–3.
Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Pei SW, et al. Naturally acquired human infection Plasmodium knowlesi. Singapore Emerg Infect Dis. 2008;14:814–6.
den Eede PV, Van HN, Van Overmeir C, Vythilingam I, Duc TN, Hung LX, et al. Human Plasmodium knowlesi infection in young children in central Vietnam. Malar J. 2009;8:249.
Khim N, Siv S, Kim S, Mueller T, Fleischmann E, Singh B, et al. Plasmodium knowlesi infection in humans, Cambodia, 2007–2010. Emerg Infect Dis. 2011;17:1900–2.
Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Lorphachan L, Keomalaphet S, et al. First case of human infection with Plasmodium knowlesi in Laos. PLoS Negl Trop Dis. 2018;12:e0006244.
Jiang N, Chang Q, Sun X, Lu H, Yin J, Zhang Z, et al. Co-infections with Plasmodium knowlesi and other malaria parasites Myanmar. Emerg Infect Dis. 2010;16:476–8.
Müller M, Schlagenhauf P. Plasmodium knowlesi in travelers, update 2014. Int Infect Dis. 2014;22:55–64.
Biernat B, Lass A, Pietkiewicz H, Szostakowska B, Wroczynska A, Kuna A, et al. Investigation on the occurrence of Plasmodium knowlesi in travellers returning from the endemic areas of simian malaria. Int Marit Health. 2015;66:168–72.
Putaporntip C, Hongsrimuang T, Seethamchai S, Kobasa T, Limkittikul K, Cui L, et al. Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. J Infect Dis. 2009;199:1143–50.
Sermwittayawong N, Singh B, Nishibuchi M, Sawangjaroen N, Vuddhakul V. Human Plasmodium knowlesi infection in Ranong province, southwestern border of Thailand. Malar J. 2012;11:36.
Nakaviroj S, Kobasa T, Teeranaipong P, Putaporntip C, Jongwutiwes S. An autochthonous case of severe Plasmodium knowlesi malaria in Thailand. Am J Trop Med Hyg. 2015;92:569–72.
Ehrhardt J, Trein A, Kremsner P, Frank M. Plasmodium knowlesi and HIV co-infection in a German traveler to Thailand. Malar J. 2013;12:283.
Orth H, Jensen BO, Holtfreter MC, Kocheril SJ, Mallach S, MacKenzie C, et al. Plasmodium knowlesi infection imported to Germany, January 2013. Euro Surveill. 2013;18:20603.
Froeschl G, Beissner M, Huber K, Bretzel G, Hoelscher M, Rothe C. Plasmodium knowlesi infection in a returning German traveler from Thailand: a case report on an emerging malaria pathogen in a popular low-risk travel destination. BMC Infect Dis. 2018;18:148.
Berry A, Iriart X, Wilhelm N, Valentin A, Cassaing S, Witkowski B, et al. Imported Plasmodium knowlesi malaria in a French tourist returning from Thailand. Am J Trop Med Hyg. 2011;84:535–8.
Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 2014;13:68.
Grignard L, Shah S, Chua TH, William T, Drakeley CJ, Formace KM. Natural human infections with Plasmodium cynomolgi and other malaria species in an elimiation setting in Sabah. Malaysia J Infect Dis. 2019;220:1946–9.
Raja TN, Hu TH, Kadir KA, Mohamad DSA, Divis PCS, Wong LL, et al. Naturally acquired human infections with Plasmodium cynomolgi and Plasmodium knowlesi in Kapit. Malaysian Borneo Emerg Infect Dis. 2020;26:1801–9.
Imwong M, Madmanee W, Suwannasin K, Kunasol C, Peto TJ, Tripura R, et al. Asymptomatic natural human infections with the simian malaria parasites Plasmodium cynomolgi and Plasmodium knowlesi. J Infect Dis. 2019;219:695–702.
Hartmeyer GN, Stensvold CR, Fabricius T, Marmolin ES, Hoegh SV, Nieisen HV, et al. Plasmodium cynomolgi as cause of malaria in tourist to Southeast Asia, 2018. Emerg Infect Dis. 2019;25:1936–9.
Coatney GR, Chin W, Contacos PG, King HK. Plasmodium inui, a quartan-type malaria parasite of Old World monkey transmissible to man. J Parasitol. 1966;52:660–3.
Eyles DE, Laing ABG, Warren MW, Sandosham AA. Malaria parasites of Malayan leaf monkeys of the genus Presbytis. Med J Malaya. 1962;17:85–6.
Putaporntip C, Jongwutiwes S, Thongaree S, Seethamchai S, Grynberg P, Hughes AL. Ecology of malaria parasites infecting Southeast Asian macaques: evidence from cytochrome b sequences. Mol Ecol. 2010;19:3466–76.
Fooden J. The bear macaque, Macaca arctoides: a systematic review. J Hum Evol. 1990;19:607–16.
Seethamchai S, Putaporntip C, Malaivijinond S, Cui L, Jongwutiwes S. Malaria and Hepatocystis species in wild macaques, Southern Thailand. Am J Trop Med Hyg. 2008;78:646–53.
Boonratana R, Das J, Yongcheng L, Htun S, Timmins RJ. Macaca leonina. The IUCN Red List of Threatened Species. 2008; e.T39792A10257933.http://dx.doi.org/https://doi.org/10.2305/IUCN.UK.2008.RLTS.T39792A10257933.en.
Htun S, Timmins RJ, Boonratan R, Das J. Macaca arctoides. The IUCN Red List of Threatened Species 2008. e.T12548A3354519. http://dx.doi.org/https://doi.org/10.2305/IUCN.UK.2008.RLTS. T12548A3354519.en
Richardson M, Mittermeier RA, Rylands AB, Konstant B. Macaca nemestrina. The IUCN Red List of Threatened Species. 2008:T12555A3356892.http://dx.doi.org/https://doi.org/10.2305/IUCN.UK.2008.RLTS.T12555A3356892.en.
Cox-Singh J, Mahayet S, Abdullah MS, Singh B. Increased sensitivity of malaria detection by nested polymerase chain reaction using simple sampling and DNA extraction. Int J Parasitol. 1997;27:1575–7.
Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiology studies. Am J Trop Med Hyg. 1999;60:687–92.
Lee KS, Divis PCS, Zakaria SK, Matusop A, Julin RA, Conway DJ, et al. Plasmodium knowlesi reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog. 2011;7:e1002015.
Lim I. Identification and molecular characterization of simian malaria parasites in wild monkeys of Singapore. M.Sc. Thesis, National University of Singapore. 2011:184.
Akter R, Vythilingam I, Khaw LT, Qvist R, Lim YA, Sitam FT, et al. Simian malaria in wild macaques: first report from Hulu Selangor district, Selangor. Malaysia Malar J. 2015;14:386.
Gamalo LE, Dimalibot J, Kadir KA, Singh B, Paller VG. Plasmodium knowlesi and other malaria parasites in long-tailed macaques from the Philippines. Malar J. 2019;18:147.
Zhang X, Kadir KA, Quintanilla-Zariñan LF, Villano J, Houghton P, Du H, et al. Distribution and prevalence of malaria parasites among long-tailed macaques (Macaca fascicularis) in regional populations across Southeast Asia. Malar J. 2016;15:450.
Acknowledgements
We would like to thank all staff of the Department of National Parks, Wildlife and Plant Conservation, Thailand for their assistance in trapping macaques and Dr. Chantha Wongautong from the Department of Statistics, Faculty of Science, Kasetsart University for statistical analysis support.
Funding
The study was supported by grants from the Thailand Research Fund (MRG 6080260) and Universiti Malaysia Sarawak (Grant no. 05-FA052000-0606–0002).
Author information
Authors and Affiliations
Contributions
WF and BS conceived the study and managed its implementation, analysed the results and wrote the manuscript. WF and DT identified study locations and collected blood samples. CU and KA, supervised by BS, undertook the molecular detection of malaria parasites and data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The Department of National Parks, Wildlife and Plant Conservation, Thailand, approved the protocol for study in a protected area, collection of the blood samples and release of wild macaques (Permit Number: 0909.204/14187). All procedures were approved by the Animal Use and Care Committee of Kasetsart University Research and Development Institute, Kasetsart University, Thailand (ID: ACKU59-SCI-011).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fungfuang, W., Udom, C., Tongthainan, D. et al. Malaria parasites in macaques in Thailand: stump-tailed macaques (Macaca arctoides) are new natural hosts for Plasmodium knowlesi, Plasmodium inui, Plasmodium coatneyi and Plasmodium fieldi. Malar J 19, 350 (2020). https://doi.org/10.1186/s12936-020-03424-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-020-03424-0