Abstract
Background
Plasmodium knowlesi is the most common cause of malaria in Malaysia. However, microscopic diagnosis is inaccurate and rapid diagnostic tests (RDTs) are insufficiently sensitive. PCR is sensitive and specific but not feasible at a district level. Loop-mediated isothermal amplification (LAMP) shows potential with only basic requirements. A commercially available LAMP assay, the Eiken Loopamp™ MALARIA Pan Detection kit, is sensitive for Plasmodium falciparum and Plasmodium vivax, but has not previously been evaluated for P. knowlesi. This study aims to determine the sensitivity of this LAMP assay for detecting P. knowlesi infection.
Methods
Study participants included 73 uncomplicated malaria patients with PCR species confirmation: 50 P. knowlesi, 20 P. falciparum and 3 P. vivax. Nineteen malaria-negative, non-endemic area controls were also included. The sensitivity of the Eiken Loopamp™ MALARIA Pan Detection kit (Pan LAMP) for detecting each Plasmodium species was evaluated. Sensitivity and specificity of the Eiken Loopamp™ MALARIA Pf Detection kit (Pf LAMP) for P. falciparum were also determined. The limit of detection for each LAMP assay was evaluated, with results compared to PCR. All P. knowlesi patients were also tested by CareStart™ (Pf/VOM) and OptiMAL-IT™ (Pan/Pf) RDTs.
Results
The sensitivity of the Pan LAMP assay was 100% for P. knowlesi (95% CI 92.9–100), P. falciparum (95% CI 83.2–100), and P. vivax (95% CI 29.2–100). The Pf LAMP was 100% sensitive and specific for P. falciparum detection, with all P. knowlesi samples having a negative reaction. LAMP sensitivity was superior to both RDTs, with only 10 and 28% of P. knowlesi samples testing positive to CareStart™ and OptiMAL-IT™, respectively. Limit of detection using the Pan LAMP for both P. knowlesi and P. vivax was 2 parasites/μL, comparable to PCR. For P. falciparum both the Pan LAMP and Pf LAMP demonstrated a limit of detection of 20 parasites/μL.
Conclusions
The Eiken Loopamp™ MALARIA Pan Detection kit is sensitive for detection of P. knowlesi in low parasitaemia clinical infections, as well as P. falciparum and P. vivax. However, a P. knowlesi-specific field assay in a simpler format would assist correct species identification and initiation of optimal treatment for all malaria patients.
Similar content being viewed by others
Background
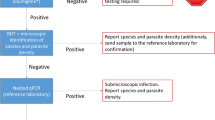
Plasmodium knowlesi has emerged as the most common cause of malaria infection in Malaysia [1–3]. It can cause severe and fatal disease and, due to its rapid clinical course, is important to diagnose and treat early [4–8]. Microscopy is the diagnostic method of choice in Malaysia but has limitations for P. knowlesi as ring stages resemble Plasmodium falciparum and later trophozoites resemble Plasmodium malariae [8–10], hence misidentification is common. P. knowlesi causes fever at low parasitaemia [4, 6] and, therefore, may be undetected by microscopy. Better methods for the diagnosis of knowlesi malaria are needed.
There have been no immunochromatographic rapid diagnostic tests (RDTs) specifically designed for P. knowlesi detection. Commercially available RDTs measuring an antibody response to non-specific pan malarial antigens, combined with the variable cross-reactive response of P. knowlesi to Plasmodium vivax or P. falciparum-designed antigen capture test components have been investigated previously [11–13]. However, the sensitivity of these RDTs for P. knowlesi detection is poor, particularly at low parasitaemia, and they are not currently suitable for clinical use.
Molecular methods, such as PCR, are sensitive and specific [14, 15], but are not feasible in many malaria-endemic areas due to a lack of resources, and do not allow rapid diagnosis for correct initiation of treatment. Loop-mediated isothermal amplification (LAMP) is a promising molecular diagnostic technique that involves nucleic acid amplification and target gene detection in a single step, and is applicable for use in district hospital laboratories as it is sensitive, specific and easy to use. LAMP does not require expensive reagents or equipment and only minimal training is needed [16, 17].
The Eiken Loopamp™ MALARIA Pan Detection kit (Pan LAMP) is a field-stable kit suitable for use in rural health facilities, designed to detect a non-specific Plasmodium genus target, and has been shown to be sensitive for both P. falciparum and P. vivax, including in clinical samples with low parasite counts [18–20]. However, it has not previously been validated for the detection of P. knowlesi. This study evaluated the sensitivity of the Pan LAMP kit in uncomplicated knowlesi malaria using clinical samples to determine whether it might be a useful diagnostic or surveillance tool. In addition, the limit of detection of this assay was determined for P. knowlesi, P. falciparum and P. vivax samples. Finally, the Eiken P. falciparum-specific LAMP (Pf LAMP) for diagnostic sensitivity and specificity of P. falciparum infection was also evaluated.
Methods
Study sites
This study was conducted with samples collected from three sites in Sabah, Malaysia: Kudat District Hospital, Kota Marudu District Hospital and Queen Elizabeth Hospital in Kota Kinabalu.
Sample collection
Patients with P. knowlesi and P. vivax patients were prospectively enrolled as part of previously reported clinical drug trials in three district hospitals in Sabah, Malaysia [21, 22]. P. falciparum patients were enrolled as part of an ongoing multicentre surveillance study for in vivo artesunate resistance in Sabah.
There were 50 P. knowlesi, 20 P. falciparum and 3 P. vivax patients included in the study with PCR-confirmed mono-infections, all of whom were microscopy positive and enrolled prior to treatment. In addition, 19 healthy human volunteers from a non-endemic country (Australia) with no previous malaria exposure were included as controls after written informed consent. Severe malaria was defined according to WHO 2014 guidelines [23]. Heparinized whole blood was collected and frozen at −80 °C until analysis.
LAMP
The Pan LAMP assay was performed on all clinical malaria samples and negative controls, and the Pf LAMP on all except the P. vivax samples. The LAMP assays were conducted strictly according to the Eiken Loopamp™ MALARIA Pan and Pf Detection kit procedures (Eiken Chemical Company, Japan). Briefly, DNA was extracted using a boil and spin method where 60 μL of thawed, well mixed whole blood was added to a labelled tube containing 60 μL of extraction buffer (400 nM NaCl, 40 mM Tris pH 6.5, 0.4% SDS), vortexed and incubated in a heat block at 95 °C for 5 min. The tubes were centrifuged at 10,000g for 3 min and 30 μL of supernatant was added to 345 μL sterile distilled water. After mixing well, 30 μL was added to the malaria Pan or Pf reaction tube with one negative and one positive control included in each 16 reactions, mixed well by inversion to reconstitute primers in the lid of the reaction tubes and incubated at 65 °C for 40 min in the LA-500 real time turbidimeter (Ref: MVL300—Eiken Chemical Co) before polymerase inactivation at 80 °C for 5 min.
A positive malaria result using the LAMP reaction can be visualized as a magnesium pyrophosphate precipitate detectable by validated turbidimeter. All reactions were also visualized manually by the operator for fluorescence and turbidity, with a subsequent reading by a separate person blinded to previous LAMP readings and PCR results.
PCR
DNA was extracted from 200 μL of blood using Qiagen’s QIAamp DNA Blood mini kit™ as per the manufacturer’s recommendations. Plasmodium species and limit of detection samples were confirmed using a non-multiplexed version of the PCR assay previously described by Padley et al. with primers for P. falciparum, P. vivax, P. malariae, and P. ovale [24] and for P. knowlesi using the PCR method published by Imwong et al. [14]. All limit of detection samples were additionally tested by nested PCR according to the method published by Snounou et al. [25].
RDTs
All P. knowlesi patients in this study had whole blood tested using both the OptiMAL-IT™ RDT (WHO RDT catalogue #710024) containing Plasmodium genus pan-pLDH and P. falciparum-specific pLDH test components, and the CareStart™ Malaria HRP2/pLDH (Pf/VOM) Combo RDT (#G0171) containing a P. falciparum HRP2 and non-P. falciparum pLDH combination as per manufacturer’s recommendations. RDTs were selected for potential combined specificity for P. knowlesi detection [12].
Limit of detection
Samples with known parasite counts per microlitre of whole blood, determined by an expert research microscopist, were used to test the limit of detection of the Pan LAMP assay for P. knowlesi, P. falciparum and P. vivax, and the Pf LAMP assay for P. falciparum. Three samples of each relevant Plasmodium spp. were chosen. Frozen whole blood samples were thawed then diluted in fresh, malaria negative, non-endemic country whole blood to produce parasitaemias equivalent to 2000, 200, 20, 2, and 0.2 parasites/μL with the diluting blood serving as a negative control. These samples then had parasite DNA extracted using the different methods described above for each LAMP assay and conventional PCR, respectively.
Statistical analysis
Data were analysed by using STATA, version 12 (StataCorp LP, College Station, TX, USA). Between-group differences were compared with Wilcoxon–Mann–Whitney test for non-parametric data. PCR results were used as the gold standard. Test sensitivity was defined as true positives/(true positives + false negatives), and test specificity was defined as true negatives/(true negatives + false positives); 95% confidence intervals were calculated using Wilson’s method.
Results
Baseline characteristics
Demographic, clinical and parasitological characteristics of the patients are shown in Table 1. Median (IQR) parasitaemia was 1197/μL (300–3920) and 7246/μL (2299–17,232) for those with P. knowlesi and P. falciparum infection, respectively (p < 0.001).
LAMP
All 50 P. knowlesi samples were positive on the Pan LAMP and negative on the Pf LAMP. The 20 P. falciparum samples were positive in both assays. The 3 P. vivax samples were positive in the Pan LAMP assay. All 19 controls were negative in both assays. This gave a sensitivity of 100% for the Pan LAMP for P. knowlesi (95% CI 92.9–100), P. falciparum (95% CI 83.2–100) and P. vivax (95% CI 29.2–100). When combining results for Plasmodium genus detection overall, the Pan LAMP had both 100% sensitivity (95% CI 95.1–100) and specificity (95% CI 82.4–100). For P. falciparum detection, the Pf LAMP also demonstrated both 100% sensitivity (95% CI 83.2–100) and specificity (95% CI 94.8–100). All manual fluorescence and turbidity readings were 100% concordant between observers and with the LAMP turbidimeter results.
Limit of detection
The limit of detection for P. knowlesi for both the Pan LAMP and specific PCR assays was 2 parasites/μL, with 6/12 and 2/4 (50%) of replicates for each method, respectively, also positive at 0.2 parasites/μL. For P. falciparum, the Pan LAMP limit of detection was 20 parasites/μL, with 8/12 (67%) and 5/12 (42%) of replicates also positive at 2 and 0.2 parasites/μL, respectively, compared to a limit of detection of 2 parasites/μL using specific PCR, with 3/4 (75%) of replicates also positive at 0.2 parasites/μL. For P. vivax the limit of detection for both methods was 2 parasites/μL, with 9/12 (75%) and 2/4 (50%) of replicates also positive at 0.2 parasites/μL for the Pan LAMP and specific PCR assays, respectively. For P. falciparum samples, the Pf LAMP also had a limit of detection of 20 parasites/μL, with 3/6 (50%) of replicates also positive at 2 parasites/μL.
RDTs
For the P. knowlesi samples, the sensitivity of the pan-pLDH test component in the OptiMAL-IT™ RDT was 10% (95% CI 3.3–21.8; 5/50) and the CareStart™ non-P. falciparum pLDH was 28% (95% CI 16.2–42.5; 14/50). Three of the five P. knowlesi samples with a positive pan-pLDH in the OptiMAL-IT™ RDT also tested positive to the P. falciparum-specific pLDH with the same band intensity.
Discussion
The Eiken Loopamp™ MALARIA Pan detection kit is highly sensitive, although inherently non-specific, for the diagnosis of uncomplicated knowlesi malaria, with 100% sensitivity in the population studied, including clinical samples with parasite counts as low as 43 parasites/μL. The limit of detection for P. knowlesi using the Pan LAMP assay was low at 2 parasites/μL, comparable to PCR, and well below the threshold of detection for standard microscopy of approximately 50 parasites/μL [26]. Similar limits of detection were found for P. falciparum and P. vivax, which were also comparable to PCR. Sensitivity of the Pan LAMP for P. knowlesi detection far exceeded that of pLDH-based RDTs (OptiMAL-IT™ and CareStart™), with the sensitivity of these RDTs too low for clinical use, as previously reported [12].
As described with other LAMP systems, the technical benefits of this LAMP assay include its high sensitivity, stability at ambient temperature, and relatively simple use compared to other molecular diagnostic methods [16, 17]. It requires no specialized equipment and minimal training, and is suitable for use in basic health facilities where accurate Plasmodium species confirmation would help to guide public health reporting, disease surveillance and in countries such as Malaysia, monitoring of national malaria elimination goals by 2020 [27].
This assay does however have some limitations. By definition, it is non-specific, with Pan LAMP shown to detect P. falciparum [18] and P. vivax [19], and therefore does not allow differentiation of P. vivax-infected patients requiring additional liver-stage treatment. Nor does it identify co-infection in co-endemic areas [1, 28], hence the Pan LAMP does not offer an improvement in diagnostic specificity. The study design would also be improved by conducting this evaluation in a field setting with fresh blood samples, however an expected decrease in sensitivity for either assay using thawed blood samples was not evident.
In the absence of appropriately sensitive and specific monoclonal antibodies for RDT, a specific P. knowlesi LAMP would aid diagnosis and surveillance in regional areas. Currently three LAMP assays have been designed specifically for P. knowlesi, targeting the β-tubulin [29], AMA-1 [30] and mitochondrial genes [17], respectively, with high sensitivity and specificity reported. However, these assays have not yet been utilized for clinical or surveillance purposes in endemic countries, particularly where PCR is available.
Conclusion
The Eiken Loopamp™ MALARIA Pan Detection kit had excellent sensitivity for the diagnosis of uncomplicated P. knowlesi infection, even in low parasitaemia samples, and may be a suitable tool for malaria surveillance. The limitations of current RDTs and microscopy for P. knowlesi diagnosis means the development of a specific, field-applicable, inexpensive rapid diagnostic tool to improve clinical malaria diagnosis in P. knowlesi-endemic countries, such as Malaysia, remains an important challenge.
References
William T, Jelip J, Menon J, Anderios F, Mohammad R, Mohammad T, et al. Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malar J. 2014;13:390.
Yusof R, Lau Y-L, Mahmud R, Fong M-Y, Jelip J, Ngian HU, et al. High proportion of knowlesi malaria in recent malaria cases in Malaysia. Malar J. 2014;13:168.
William T, Rahman HA, Jelip J, Ibrahim MY, Menon J, et al. Increasing incidence of Plasmodium knowlesi malaria following control of P. falciparum and P. vivax malaria in Sabah, Malaysia. PLoS Negl Trop Dis. 2013;7:e2026.
Barber BE, William T, Grigg MJ, Menon J, Auburn S, Marfurt J, et al. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis. 2013;56:383–97.
William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, et al. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg Infect Dis. 2011;17:1248–55.
Daneshvar C, Davis TME, Cox-Singh J, Rafa’ee MZ, Zakaria SK, Divis PCS, et al. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis. 2009;49:852–60.
Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–71.
Barber BE, William T, Grigg MJ, Yeo TW, Anstey NM. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar J. 2013;12:8.
Lee WC, Chin PW, Lau YL, Chin LC, Fong MY, Yap CJ, et al. Hyperparasitaemic human Plasmodium knowlesi infection with atypical morphology in peninsular Malaysia. Malar J. 2013;12:88.
Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–84.
Barber BE, William T, Grigg MJ, Piera K, Yeo TW, Anstey NM. Evaluation of the sensitivity of a pLDH-based and an aldolase-based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR-confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. J Clin Microbiol. 2013;51:1118–23.
Grigg MJ, William T, Barber BE, Parameswaran U, Bird E, Piera K, et al. Combining parasite lactate dehydrogenase-based and histidine-rich protein 2-based rapid tests to improve specificity for diagnosis of malaria due to Plasmodium knowlesi and other Plasmodium species in Sabah, Malaysia. J Clin Microbiol. 2014;52:2053.
Foster D, Cox-Singh J, Mohamad DS, Krishna S, Chin PP, Singh B. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malar J. 2014;13:60.
Imwong M, Tanomsing N, Pukrittayakamee S, Day NP, White NJ, Snounou G. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol. 2009;47:4173–5.
Divis P, Shokoples S, Singh B, Yanow S. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar J. 2010;9:344.
Britton S, Cheng Q, Grigg MJ, Poole CB, Pasay C, William T, et al. Sensitive detection of Plasmodium vivax using a high-throughput, colourimetric loop mediated isothermal amplification (HtLAMP) platform: a potential novel tool for malaria elimination. PLoS Negl Trop Dis. 2016;10:e0004443.
Britton S, Cheng Q, Grigg MJ, William T, Anstey NM, McCarthy JS. A sensitive, colorimetric, high-throughput loop-mediated isothermal amplification assay for the detection of Plasmodium knowlesi. Am J Trop Med Hyg. 2016;95:120–2.
Polley S, Gonzalez I, Mohamed D, Daly R, Bowers K, Watson J, et al. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis. 2013;208:637–44.
Vallejo A, Martínez N, González I, Arévalo-Herrera M, Herrera S. Evaluation of the loop mediated isothermal DNA amplification [LAMP] kit for malaria diagnosis in P. vivax endemic settings of Colombia. PLoS Negl Trop Dis. 2015;9:e3453.
Aydin-Schmidt B, Weiping X, Gonzalez I, Polley S, Bell D, Shakely D. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS ONE. 2014;9:e103905.
Grigg M, William T, Menon J, Dhanaraj P, Barber B, Wilkes C, et al. Artesunate-mefloquine versus chloroquine for treatment of uncomplicated Plasmodium knowlesi malaria in Malaysia (ACT KNOW): an open-label, randomised controlled trial. Lancet Infect Dis. 2016;16:180–8.
Grigg M, William T, Menon J, Barber B, Wilkes C, Rajahram G, et al. Efficacy of artesunate-mefloquine for chloroquine-resistant Plasmodium vivax malaria in Malaysia: an open-label, randomized, controlled trial. Clin Infect Dis. 2016;62:1403–11.
WHO. Severe malaria. Trop Med Int Health. 2014;19(Suppl 1):7–131.
Padley D, Moody AH, Chiodini PL, Saldanha J. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann Trop Med Parasitol. 2003;97:131–7.
Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20.
WHO. Microscopy for the detection, identification and quantification of malaria parasites on stained thick and thin blood films in research settings. Geneva: World Health Organization; 2015.
WHO. World Malaria Report 2015. Geneva: WHO; 2015.
Marchand RP, Culleton R, Maeno Y, Quang NT, Nakazawa S. Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and Anopheles dirus mosquitoes, Southern Vietnam. Emerg Infect Dis. 2011;17:1232–9.
Lau Y-L, Fong M-Y, Mahmud R, Chang P-Y, Palaeya V, Cheong F-W, et al. Specific, sensitive and rapid detection of human Plasmodium knowlesi infection by loop-mediated isothermal amplification (LAMP) in blood samples. Malar J. 2011;10:197.
Iseki H, Kawai S, Takahashi N, Hirai M, Tanabe K, Yokoyama N, et al. Evaluation of a loop-mediated isothermal amplification method as a tool for diagnosis of infection by the zoonotic simian malaria parasite Plasmodium knowlesi. J Clin Microbiol. 2010;48:2509–14.
Authors’ contributions
NMA, DB, KP, and MJG conceived and designed this study, KP and AA conducted the assays, TW provided assistance with samples, DB and IJG provided technical equipment and support, KP, NMA and MJG analysed the results, KP, MJG and NMA wrote the initial manuscript. All authors read and approved the final manuscript.
Acknowledgements
Thank-you to all study participants, clinical staff for enrolling and caring for patients and laboratory staff for performing RDT, reading slides and storing samples; particularly Ferryanto Chalfein, Ema Estiana binti Ishak, Redley Yambun, Wilhelmina Nevir, Sitti Saimah binti Sakam and Kelly Nestor. Appreciation to the Director General of Health, Malaysia, for permission to publish this study.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors have reviewed and consent for publication of this manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from all patients or their relatives and approval was granted by the Ethics Committees of the Ministry of Health, Malaysia, and Menzies School of Health Research, Australia.
Funding
This study was funded by the Malaysian Ministry of Health (Grant Number BP00500420, the Asia Pacific Malaria Elimination Network (108-07), and the Australian National Health and Medical Research Council [NHMRC; 1037304 and 1045156; fellowship to NMA (1042072) and scholarship to MJG (1074795)]. This work was supported by FIND, through a grant from the German Government (Kreditanstalt für Wiederaufbau), who provided a turbidimeter and Pf LAMP tubes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Piera, K.A., Aziz, A., William, T. et al. Detection of Plasmodium knowlesi, Plasmodium falciparum and Plasmodium vivax using loop-mediated isothermal amplification (LAMP) in a co-endemic area in Malaysia. Malar J 16, 29 (2017). https://doi.org/10.1186/s12936-016-1676-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-016-1676-9