Abstract
Background
Intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine–pyrimethamine (SP) is widely implemented in sub-Saharan Africa for the prevention of malaria in pregnancy and adverse birth outcomes. However, in areas of intense SP resistance, the efficacy of IPTp may be compromised.
Methods
A cross-sectional study among 915 delivering women (728 analysable live singleton deliveries) was conducted in Fort Portal, western Uganda, to assess associations of reported IPTp use, Plasmodium falciparum infection, maternal anaemia, low birth weight, and preterm delivery, and to estimate the degree of SP resistance as reflected by pfdhfr/pfdhps mutations.
Results
Plasmodium falciparum infection was detected by PCR in 8.9 % and by microscopy of placental blood samples in 4.0 %. Infection was significantly associated with stillbirth, early neonatal death, anaemia, low birth weight, and pre-term delivery. Eighty percent of the women had taken at least one dose of IPTp, and more than half had taken two doses. As compared to women without chemoprophylaxis against malaria, IPTp had no significant influence on the presence of P. falciparum infection (13.8 vs. 9.6 %, P = 0.31). Nor was it associated with reductions in anaemia, low birth weight or preterm delivery. P. falciparum with intense SP resistance (pfdhfr/pfdhps quintuple or sextuple mutations) were observed in 93 % (pfdhps 581G, 36 %), and the additional high resistance allele pfhdr 164L in 36 %.
Conclusions
In Fort Portal, Uganda, reported use of IPTp with SP does not provide an observable benefit. The molecular markers of P. falciparum indicate high grade SP resistance reaching the threshold set by WHO for the discontinuation of IPTp with SP. Alternative approaches for the prevention of malaria in pregnancy are urgently needed.
Similar content being viewed by others
Background
Despite the implementation of intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine–pyrimethamine (SP) in sub-Saharan Africa starting more than two decades ago, malaria in pregnancy continues to be a major public health problem. Pregnant women form a specific risk group for Plasmodium falciparum infection, malaria and related consequences, which include abortion, stillbirth, maternal anaemia, low birth weight (LBW), preterm delivery, and, annually, up to 200,000 infant deaths [1]. In highly endemic regions, primiparae are at particular risk due the lack of specific immunity preventing the placental sequestration of pregnancy-specific P. falciparum strains. Placental sequestration gives rise to local inflammation and also may result in placental infection in the absence of detectable peripheral blood infection [2–4].
Coverage with IPTp is low (<25 %) in African countries with an IPTp policy [5]. Moreover, the effectiveness of IPTp critically depends on parasite sensitivity to the drug, but SP resistance of P. falciparum has spread across Africa and intensified particularly in the East of the continent [6, 7]. In 2012, WHO modified the recommendation of two doses of IPTp with SP during pregnancy in areas of moderate to high transmission towards administration at each scheduled antenatal care (ANC) visit but at least 1 month apart [8]. This accords with the observation of less malaria and better birth outcomes using three or more doses of SP as compared to the standard two-dose regimen [9]. While the effectiveness of this approach has yet to be proven in areas of intense resistance, data from East Africa suggest at least partial failure of IPTp with SP in improving overall pregnancy outcomes [10–13]. In addition, in areas of intense SP resistance in Tanzania, IPTp among infected women was associated with increased placental parasite density and inflammation [14] as well as an increased risk of severe malaria in the offspring [15], and infections with highly resistant parasites were associated with lower birth weight [16].
Resistance to SP is conferred by mutations in the P. falciparum dihydrofolate reductase (pfdhfr) and dihydropteroate synthase (pfdhps) genes: a triple mutation of pfdhfr (108N -5I1-59R) combined with pfdhps mutations 437G and 540E (pfdhfr/pfdhps quintuple mutant) is predictive for SP treatment failure in children, even more so in case of an additional pfdhps 581 mutation (sextuple mutant). pfdhfr 164L is linked with high grade SP resistance [6, 17–19]. Recent work has shown that the effectiveness of IPTp declines with an increasing population prevalence of the pfdhps 540E mutation (representing the pfdhfr/pfdhps quintuple mutation) even though some effect on birth weight remains even at very high prevalence. Increasingly, the pfdhfr/pfdhps sextuple mutation including the pfdhps 581 variant is considered an informative marker on whether IPTp might be compromised or not [20, 21]. In line with that, a recent study from Malawi reported failure of parasite suppression by IPTp in the presence of sextuple-mutant parasites [22].
In Uganda, policy recommendation is at least two doses of IPTp in pregnancy [23]. In the central part of the country, quintuple and sextuple pfdhfr/pfdhps mutations combined were recently found in >90 % of P. falciparum infecting pregnant women at first ANC visit [24], and in the eastern part, more than a quarter of delivering women had evidence of active placental P. falciparum infection despite previous intake of ≥2 doses of SP [13]. In the latter region, IPT of school children with SP did not provide any benefit over placebo [25]. In the present study, the effectiveness of IPTp with SP in the western highland region of Fort Portal was estimated in a cross sectional study looking at effects on infection, anaemia, LBW, and preterm delivery as well as on the pattern of pfdhfr/pfdhps alleles.
Methods
Fort Portal, located at 1500 m altitude, is a community of some 55,000 inhabitants and capital of the western Kabarole district, close to the border of DR Congo. Twenty years ago, an altitude of 1500 m in this district represented a threshold between hypo- and mesoendemic conditions [26]. The 2014 malaria indicator survey reports a prevalence of malaria parasites among children in the larger mid-Western region of 18 % [27]. The Holy Family Virika Hospital in Fort Portal is a private (Catholic Church) not-for-profit health facility and has a bed capacity of 155. It provides services to patients from Kabarole and surrounding districts and thereby supplements the governmental Fort Portal Regional Referral Hospital (330 beds). From February to December 2013, adult women attending Virika Hospital for delivery were asked to participate in the present cross-sectional study and recruited after informed written consent was obtained. The study protocol was reviewed and approved by the Higher Degrees, Research, and Ethics Committee, College of Health Sciences, Makerere University, Kampala, and by the Uganda National Council for Science and Technology.
All women were clinically examined. Fever was defined as an axillary temperature ≥37.5 °C. Obstetric and medical history was documented, as were socio-economic data. Participation in a programme on prevention of mother-to-child-transmission of HIV (PMTCT) was noted. Data on intake of SP or other anti-malarial drugs was verified on ANC cards. Venous peripheral blood was collected into EDTA; blood from the intervillous space was collected with a syringe containing EDTA following incision into the maternal surface of the placenta. Haemoglobin (Hb) was measured by a HemoCue photometer (Ångelholm, Sweden) and anaemia defined as Hb <11.5 g/dL increasing the threshold by +0.5 g/dL to account for altitude [28]. Birth weight and gestational age were assessed within 24 h after delivery. LBW was defined as a birth weight <2500 g and preterm delivery as gestational age <37 weeks applying the simple morphological Finnström score [29]. Malaria parasites were counted microscopically on Giemsa-stained thick blood films per 500 white blood cells for peripheral samples and per 100 high-power fields for placental samples. Following DNA extraction of peripheral blood samples (QIAmp, Qiagen, Germany), semi-nested PCR assays were performed for the diagnosis of P. falciparum and other species [30]. If not otherwise indicated, P. falciparum infection hereinafter refers to infection as detected by PCR. For P. falciparum resistance marker typing, restriction fragment length polymorphisms of PCR-generated amplicons identified mutations of pfdhfr (N51I, C59R, S108N, I164L) and pfdhps (A437G, K540E, A581G) [31]. Isolates with mixed alleles, i.e., both wildtype and mutation present, were considered mutant. Laboratory strains 3D7, HB3 and Dd2 served as controls.
Women were grouped into primiparae, parae 2 and 3, and multiparae (>3 previous deliveries). Geometric mean parasite densities (GMPDs) and 95 % confidence intervals (95 % CIs) were calculated. Continuous variables were compared between groups by t test, analysis of variance, Mann–Whitney U test, and Kruskal–Wallis test as applicable. Associations between categorical variables were identified by χ2 test or Fisher’s exact test, and odds ratios (ORs) were calculated. Multivariate logistic regression with stepwise removal of factors found to be not associated in multivariate analysis (P > 0.05) was used to identify independent predictors of P. falciparum infection. A P value of <0.05 was considered statistically significant.
Results
Between February and December 2013, 915 delivering women were recruited and 945 babies (885 singles, 60 twins) were born, of whom 45 (4.9 %) were born dead. The characteristics of the 728 live singleton deliveries with available P. falciparum infection status by PCR are shown in Table 1. Data on chemoprevention was verified by checking ANC cards in 98.3 % (676/688) of these women. Most women originated from the local Kabarole district, and Mutooro ethnicity predominated. Travel to the hospital took a median of approximately 1 h. Almost one third benefited from a coverage programme for hospital costs. Unmarried mothers were common among primiparae but rare in multiparae who at the same time showed comparatively lower levels of formal education than primiparae. Multiparae showed reduced proxy parameters of socio-economic status, e.g., tap water or electricity on the premises.
Almost half of the women had been referred to the hospital for delivery, and 12 % participated in a PMTCT programme. The number of previous antenatal care visits was similar among primiparae and parae 2 and 3 but less in multiparae. Eighty percent of the women had taken at least one dose of IPTp (adding 3.5 % of those taking both IPTp and cotrimoxazole), and more than half had taken two doses. No chemoprophylaxis and daily cotrimoxazole were taken by each 8 % of the women. Cotrimoxazole intake was less common among primiparae whereas IPTp was non-significantly more frequent. Almost two thirds of women stated to have used a bed net in the previous night and one in four women reported to have received treatment for malaria during pregnancy, without differences by parity. Fever was rare. Anaemia (29 %) affected women of all parities. Birth weight increased with increasing parity and this was reflected by a respective trend towards less LBW. Preterm delivery was increased in primiparae (P = 0.03).
Prevalence of Plasmodium falciparum infection
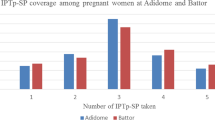
Plasmodium falciparum was detected in peripheral blood by PCR in 8.9 % (65/728) and by microscopy in 2.9 % (20/682). The geometric mean parasite density was 1986/µL (95 % CI, 602–6553). Placental parasitaemia was observed by microscopy in 4.0 % (27/676). 56.9 % (37/65) of the infections were submicroscopic, i.e., reflected by a positive PCR but negative microscopy result of peripheral or placental blood. Irrespective of diagnostic method, infection prevalence slightly and non-significantly declined with increasing parity (Fig. 1). Non-falciparum parasites were rare (seven Plasmodium malariae, one Plasmodium ovale) and not related to parity.
Manifestation of Plasmodium falciparum infection
In all women available for analysis, P. falciparum infection (PCR) was associated with increased odds of stillbirth: it occurred in 4.3 % (31/716) of non-infected and in 10.7 % (8/75) of infected mothers (OR, 2.64; 95 % CI, 1.1–6.3; P = 0.02). This association was pronounced for infections detected by placental microscopy [4.3 % (35/808) vs. 14.7 % (5/34); OR, 3.81; 95 % CI, 1.1–10.8; P = 0.02] but non-significant for submicroscopic infections (OR, 2.33; 95 % CI, 0.6–7.1; P = 0.12).
Among women with live singleton delivery, 13 children died within 24 h of delivery. In 30.8 % (4/13) of these, maternal P. falciparum infection had been observed as compared to 8.5 % (61/715) among mothers of surviving children (OR, 4.77; 95 % CI, 1.0–17.6; P = 0.02). Moreover, P. falciparum infection was associated with each more than doubled odds of anaemia, LBW and preterm delivery (Table 2). Correspondingly, in infected (PCR) as compared to non-infected mothers, median Hb concentration, median birth weight, and mean gestational age were reduced by 1.0 g/dL (P < 0.0001), 130 g (P = 0.03), and 1 week (P = 0.01), respectively. Anaemia and LBW were not increased in women with submicroscopic infection, but preterm delivery showed a respective trend (Table 2).
Impact of intermittent preventive treatment
IPTp had no significant influence on the presence of P. falciparum infection. Nor was it associated with reductions in anaemia, LBW or preterm delivery (Table 3). Correspondingly, P. falciparum infection in women using IPTp was associated with reductions in median Hb concentration, median birth weight, and mean gestational age of 0.85 g/dL (P = 0.0008), 207 g (P = 0.004), and 1.1 weeks (P = 0.0003), respectively. Stratification by parity did not change these overall findings (Table 4). Moreover, infection prevalence was not reduced in women having taken two doses of IPTp (10.9 %, 41/376) as compared to one dose (6.8 %, 12/176). Timing of last IPTp intake (weeks ago) and infection were not associated (P = 0.93). In women on cotrimoxazole or IPTp plus cotrimoxazole, infection prevalence was substantially reduced but not significantly so (Table 3).
Factors associated with Plasmodium falciparum infection
In univariate analysis, the odds of P. falciparum infection declined with age, presence of electricity in the household, bed net ownership, bed net usage in the preceding night, and Mutooro ethnicity, and increased with referral to hospital for delivery and travel distance to the hospital. In multivariate analysis, referral and travel distance proved to be independent predictors of infection while age and household electricity were negatively associated (Table 5). Further, partly proximate, factors were not associated with infection, including parity, educational level, occupation, district of residence, number of people living in the household, other proxy indicator of socio-economic status, number of antenatal care visits and participation in the PMTCT programme. In the above multivariate model, IPTp did not significantly influence the odds of P. falciparum infection (aOR, 0.59; 95 % CI, 0.25–1.37; P = 0.22).
Molecular markers of drug resistance
Typing of essential pfdhfr and pfdhps alleles was successful for 55 (85 %) isolates. Mutant alleles were found in ≥95 % each for pfdhfr codons 51, 59, and 108 as well as for pfdhps codons 437 and 540 (Fig. 2). In consequence, pfdhfr/pfdhps quintuple and sextuple mutations were observed in 93 % (51/55) of the isolates. The high resistance alleles pfdhfr 164L and pfdhps 581G occurred each in 36 % (18/50; 20/55); pfdhps 581G in 42 % (8/19) occurred together with pfdhfr 164L. pfdhps 581G was associated with increased placental parasitaemia as compared to wildtype parasites, both in the overall group [GMPD, 22/100 high power fields; 95 % CI, 7–70 vs. 4 (2–7), P = 0.01] and among women who had been taking IPTp [25 (7–87) vs. 4 (2–9), P = 0.02].
Discussion
In this highland area of western Uganda, though malaria in pregnancy is comparatively rare, it substantially contributes to mortality and morbidity including stillbirth, early neonatal death, anaemia, LBW and preterm delivery. IPTp, recommended for the prevention of malaria and its consequences, did not show a beneficial effect. One very likely reason is the vast predominance of highly resistant strains of P. falciparum.
The protective efficacy of IPTp with SP against placental malaria has been estimated as roughly 50 % in areas of low to moderate SP resistance and over a decade ago [32]. As a major limitation, the present cross-sectional study lacked power to display an only modest impact of IPTp. Considering the given group sizes and prevalence, the study was powered to detect an effect of IPTp on P. falciparum infection at a magnitude of >75 % reduction. Nevertheless, infection prevalence was actually higher in women having taken two as compared to one dose of SP, and placental parasitaemia, anaemia, and preterm delivery were slightly more common in women having taken IPTp as compared to women without drug-based prevention. A further limitation refers to the validity of reported IPTp use on which the current analysis is based. However, in Tanzania, reported use and detection of plasma sulfa levels matched closely [11] suggesting that reported use is not unreliable per se. It appears, therefore, justifiable to state that IPTp did not fulfil its purpose. Because HIV negativity has yet to be confirmed by molecular means in the study group, participation in a PMTCT programme was considered as a proxy indicator of HIV status. PMTCT participation and cotrimoxazole intake overlapped largely. It is, therefore, not possible to make firm statements on an impact of HIV infection on P. falciparum infection or pregnancy outcomes but the analyses do not provide evidence for respective effects. Also, data on some potentially interfering factors were not available, e.g., syphilis. This should be kept in mind when interpreting the data.
Plasmodium falciparum infection was detected by microscopy of peripheral and placental blood films in only 3 and 4 %, respectively. Peripheral blood microscopy is notoriously insensitive in pregnant women whereas the sensitivity of PCR assays in detecting placental parasitaemia exceeds 95 % [4]. Submicroscopic infections in pregnancy are common and may contribute substantially to maternal and foetal morbidity [3, 4, 33]. In the present study, however, they did not associate with delivery outcomes, with the potential exemption of a borderline increased risk of preterm delivery. In contrast, malaria in pregnancy per se greatly increased the odds of stillbirth, early neonatal death, anaemia, LBW and preterm delivery. Even if comparatively rare at 9 %, this emphasizes the need for effective prevention of malaria in pregnancy in the study area. Peripheral residence and lacking electricity predicted P. falciparum infection illustrating the poverty-related nature of malaria. Bed net use, even though not significantly associated in multivariate analysis, was stated by almost two thirds of women and roughly halved the odds of infection. This highlights the opportunity and benefits of increasing bed net use among pregnant women in the study area.
Beyond statistical significance, P. falciparum prevalence was greatly reduced in women on cotrimoxazole and absent in those taking both cotrimoxazole and IPTp. This accords with findings from Malawi [34]. A slight superiority of daily cotrimoxazole over IPTp with SP in HIV-infected pregnant women was also observed in Togo [35] whereas the regimens had similar effects in Uganda [36] and Zambia [37]. Data of the present study, although comprising small numbers only, support the policy of using daily cotrimoxazole for malaria prevention in HIV-infected pregnant women instead of SP-IPTp. As a matter of fact, IPTp with SP is not recommended in HIV-infected women receiving daily cotrimoxazole because of additive sulfa toxicity [38].
Resistance marker typing in peripheral blood is reasonably representative of P. falciparum infecting pregnant women [39]. In the present study, pfdhfr/pfdhps quintuple mutants were close to fixation, and sextuple mutants and the high-grade resistance allele pfdhfr 164L occurred in one third of isolates. This accords with recent data from pregnant women in central Uganda [24], with one notable exception: there, only few parasites exhibited the pfdhps 581G mutation even though the proportion increased after IPTp. Consequently, in central Uganda, sextuple mutants made up less than a third of the proportion observed in Fort Portal. Against a background of intense antifolate resistance, this indicates an even higher degree in the present study area. The pfdhps 581G mutation (making up the sextuple mutant) has been considered to halve the protective period provided by a curative dose of SP [14] and to be associated with reduced birth weights and an increased risk of patent infection among mothers taking IPTp [16, 22]. In the present study, it was associated with increased placental parasite density, which accords with recent findings from Malawi [22]. Recent work has shown that this mutation has occurred multiple times on local pfdhps double-mutant backgrounds [40] and emerges in East Africa [41–45]. Moreover, in the present study, the pfdhfr 164L mutation occurred in 36 %, which is the highest figure reported from Africa [6, 18]. A previous study from southwestern Uganda found this high-grade resistance allele in 4 % and 14 % [43]. Even though the molecular data suggest intense SP resistance in the study area, the actual meaning for IPTp is not clear-cut. In central Uganda, despite >98 % pfdhfr/pfdhps quintuple mutants (but at a low prevalence of pfdhps 581G), 50 % of initially P. falciparum infected pregnant women became negative after one or two rounds of IPTp [24]. A current meta-analysis suggests a prevalence threshold of pfdhps 581G at which IPTp no longer protects against LBW of >10.1 % [21]. WHO recently considered the discontinuation of IPTp with SP in case of P. falciparum population prevalences of pfdhps 540E >95 % and pfdhps 581G >10 % [20]. In the present study, these thresholds are basically met (pfdhps 540E, 94.5 %; 581G, 36 %).
What then could be alternatives for the prevention of malaria in pregnancy in the study area? IPTp with mefloquine has disappointed expectations [46] and is not recommended [20]. WHO advises that in areas where IPTp-SP is discontinued because of resistance, access of pregnant women to long-lasting insecticide treated nets and to prompt diagnosis and effective treatment should be ensured. In the study area, bed net use can in fact be increased. Diagnosis, preferentially with a sensitive antigen capture test [4], preceding case management requires an easily accessible health system and an alert population. The same applies to the concept of intermittent screening and treatment [47]. Moreover, for both approaches, the issue of asymptomatic but still deleterious infections remains unsolved. Artemisinin-based combination therapy (ACT) is recommended for the treatment of malaria in pregnancy [38] but their use in IPTp has not been evaluated and may be complicated by the necessity of a 3-day regimen.
Conclusion
Malaria in pregnancy in the area of Fort Portal, western Uganda, is comparatively rare but contributes significantly to stillbirth, anaemia, LBW, and preterm delivery. The molecular markers of P. falciparum show a very high degree of SP resistance, and reach the threshold set by WHO for the discontinuation of IPTp with SP. In line with that, IPTp with SP did not provide an observable benefit. Alternative approaches for the prevention of malaria in pregnancy are urgently needed.
References
Desai M, ter Kuile FO, Nosten F, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104.
Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–17.
Mockenhaupt FP, Bedu-Addo G, von Gaertner C, Boyé R, Fricke K, Hannibal I, et al. Detection and clinical manifestation of placental malaria in southern Ghana. Malar J. 2006;5:119.
Mockenhaupt FP, Ulmen U, von Gaertner C, Bedu-Addo G, Bienzle U. Diagnosis of placental malaria. J Clin Microbiol. 2002;40:306–8.
van Eijk AM, Hill J, Larsen DA, Webster J, Steketee RW, Eisele TP, et al. Coverage of intermittent preventive treatment and insecticide-treated nets for the control of malaria during pregnancy in sub-Saharan Africa: a synthesis and meta-analysis of national survey data, 2009–11. Lancet Infect Dis. 2013;13:1029–42.
Naidoo I, Roper C. Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol. 2013;29:505–15.
Mockenhaupt FP, Bedu-Addo G, Eggelte TA, Hommerich L, Holmberg V, von Oertzen C, et al. Rapid increase in the prevalence of sulfadoxine–pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis. 2008;198:1545–9.
WHO. WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine–pyrimethamine (IPTp-SP). Geneva: World Health Organization; 2013. http://www.who.int/malaria/publications/atoz/policy_brief_iptp_sp_policy_recommendation/en/. Accessed 15 July 2015.
Kayentao K, Garner P, van Eijk AM, Naidoo I, Roper C, Mulokozi A, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine–pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604.
Menendez C, Bardaji A, Sigauque B, Romagosa C, Sanz S, Serra-Casas E, et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One. 2008;3:e1934.
Harrington WE, Mutabingwa TK, Kabyemela E, Fried M, Duffy PE. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin Infect Dis. 2011;53:224–30.
Mosha D, Chilongola J, Ndeserua R, Mwingira F, Genton B. Effectiveness of intermittent preventive treatment with sulfadoxine–pyrimethamine during pregnancy on placental malaria, maternal anaemia and birthweight in areas with high and low malaria transmission intensity in Tanzania. Trop Med Int Health. 2014;19:1048–56.
Arinaitwe E, Ades V, Walakira A, Ninsiima B, Mugagga O, Patil TS, et al. Intermittent preventive therapy with sulfadoxine–pyrimethamine for malaria in pregnancy: a cross-sectional study from Tororo. Uganda. PLoS One. 2013;8:e73073.
Harrington WE, Mutabingwa TK, Muehlenbachs A, Sorensen B, Bolla MC, Fried M, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci USA. 2009;106:9027–32.
Harrington WE, Morrison R, Fried M, Duffy PE. Intermittent preventive treatment in pregnant women is associated with increased risk of severe malaria in their offspring. PLoS One. 2013;8:e56183.
Minja DT, Schmiegelow C, Mmbando B, Boström S, Oesterholt M, Magistrado P, et al. Plasmodium falciparum mutant haplotype infection during pregnancy associated with reduced birthweight, Tanzania. Emerg Infect Dis. 2013;19:1446–54.
Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, et al. Molecular markers for failure of sulfadoxine–pyrimethamine and chlorproguanil–dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–8.
Sridaran S, McClintock SK, Syphard LM, Herman KM, Barnwell JW, Udhayakumar V. Anti-folate drug resistance in Africa: meta-analysis of reported dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutant genotype frequencies in African Plasmodium falciparum parasite populations. Malar J. 2010;9:247.
Gesase S, Gosling RD, Hashim R, Ord R, Naidoo I, Madebe R, et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS One. 2009;4:e4569.
WHO. WHO Evidence Review Group on Intermittent Preventive Treatment (IPT) of malaria in pregnancy. Geneva: World Health Organization; 2013. http://www.who.int/malaria/mpac/mpac_sep13_erg_ipt_malaria_pregnancy_report.pdf. Accessed 16 July 2015.
Chico RM, Cano J, Ariti C, Collier TJ, Chandramohan D, Roper C, Greenwood B. Influence of malaria transmission intensity and the 581G mutation on the efficacy of intermittent preventive treatment in pregnancy: systematic review and meta-analysis. Trop Med Int Health. 2015. doi:10.1111/tmi.12595. [Epub ahead of print].
Gutman J, Kalilani L, Taylor S, Zhou Z, Wiegand RE, Thwai KL, et al. The A581G mutation in the gene encoding Plasmodium falciparum dihydropteroaten synthetase reduces the effectiveness of sulfadoxine–pyrimethamine preventive therapy in Malawian pregnant women. J Infect Dis. 2015;211:1997–2005.
Ministry of Health Kampala, Uganda: Uganda Clinical Guidelines. Box 7272, 405 Kampala, Uganda 2010. http://apps.who.int/medicinedocs/documents/s21741en/s21741en.pdf. Accessed 23 Sept 2015.
Mbonye AK, Birungi J, Yanow SK, Shokoples S, Malamba S, Alifrangis M, et al. The prevalence of P. falciparum resistance markers to sulphadoxine–pyrimethamine among pregnant women receiving intermittent preventive treatment of malaria in Uganda. Antimicrob Agents Chemother. 2015;59:5475–82.
Nankabirwa J, Cundill B, Clarke S, Kabatereine N, Rosenthal PJ, Dorsey G, et al. Efficacy, safety, and tolerability of three regimens for prevention of malaria: a randomized, placebo-controlled trial in Ugandan schoolchildren. PLoS One. 2010;5:e13438.
Cox J, Craig M, Le Sueur D, Sharp B. Mapping malaria risk in the highlands of Africa. MARA/HIMAL. Technical report. 1999. https://idl-bnc.idrc.ca/dspace/bitstream/10625/31899/1/117291.pdf. Accessed 16 July 2015.
Uganda Bureau of Statistics (UBOS) and ICF International. Uganda Malaria Indicator Survey 2014–15: key indicators. 2015. Kampala, Uganda, and Rockville, Maryland, USA: UBOS and ICF International. http://dhsprogram.com/pubs/pdf/PR64/PR64.pdf. Accessed 16 July 2015.
Sullivan KM, Mei Z, Grummer-Strawn L, Parvanta I. Haemoglobin adjustments to define anaemia. Trop Med Int Health. 2008;13:1267–71.
Finnstrom O. Studies on maturity in newborn infants. IX. Further observations on the use of external characteristics in estimating gestational age. Acta Paediatr Scand. 1977;66:601–4.
Rubio JM, Post RJ, van Leeuwen WM, Henry MC, Lindergard G, Hommel M. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: the semi-nested multiplex malaria PCR (SnM-PCR). Trans R Soc Trop Med Hyg. 2002;96(Suppl 1):S199–204.
Duraisingh MT, Curtis J, Warhurst DC. Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp Parasitol. 1998;89:1–8.
ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine–pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–16.
Cottrell G, Moussiliou A, Luty AJ, Cot M, Fievet N, Massougbodji A, et al. Submicroscopic Plasmodium falciparum infections are associated with maternal anemia, premature births, and low birth weight. Clin Infect Dis. 2015;60:1481–8.
Kapito-Tembo A, Meshnick SR, van Hensbroek MB, Phiri K, Fitzgerald M, Mwapasa V. Marked reduction in prevalence of malaria parasitemia and anemia in HIV-infected pregnant women taking cotrimoxazole with or without sulfadoxine–pyrimethamine intermittent preventive therapy during pregnancy in Malawi. J Infect Dis. 2011;203:464–72.
Klement E, Pitché P, Kendjo E, Singo A, D’Almeida S, Akouete F, et al. Effectiveness of co-trimoxazole to prevent Plasmodium falciparum malaria in HIV-positive pregnant women in sub-Saharan Africa: an open-label, randomized controlled trial. Clin Infect Dis. 2014;58:651–9.
Newman PM, Wanzira H, Tumwine G, Arinaitwe E, Waldman S, Achan J, et al. Placental malaria among HIV-infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malar J. 2009;8:254.
Manyando C, Njunju EM, Mwakazanga D, Chongwe G, Mkandawire R, Champo D, et al. Safety of daily co-trimoxazole in pregnancy in an area of changing malaria epidemiology: a phase 3b randomized controlled clinical trial. PLoS One. 2014;9:e96017.
WHO. Guidelines for the treatment of malaria. 2nd ed. Geneva: World Health Organization; 2010.
Mockenhaupt FP, Bedu-Addo G, Junge C, Hommerich L, Eggelte TA, Bienzle U. Markers of sulfadoxine–pyrimethamine-resistant Plasmodium falciparum in placenta and circulation of pregnant women. Antimicrob Agents Chemother. 2007;51:332–4.
Alifrangis M, Nag S, Schousboe ML, Ishengoma D, Lusingu J, Pota H, et al. Independent origin of Plasmodium falciparum antifolate super-resistance, Uganda, Tanzania, and Ethiopia. Emerg Infect Dis. 2014;20:1280–6.
Iriemenam NC, Shah M, Gatei W, van Eijk AM, Ayisi J, Kariuki S, et al. Temporal trends of sulphadoxine–pyrimethamine (SP) drug-resistance molecular markers in Plasmodium falciparum parasites from pregnant women in western Kenya. Malar J. 2012;11:134.
Alifrangis M, Lusingu JP, Mmbando B, Dalgaard MB, Vestergaard LS, Ishengoma D, et al. Five-year surveillance of molecular markers of Plasmodium falciparum antimalarial drug resistance in Korogwe District, Tanzania: accumulation of the 581G mutation in the P. falciparum dihydropteroate synthase gene. Am J Trop Med Hyg. 2009;80:523–7.
Lynch C, Pearce R, Pota H, Cox J, Abeku TA, Rwakimari J, et al. Emergence of a dhfr mutation conferring high-level drug resistance in Plasmodium falciparum populations from southwest Uganda. J Infect Dis. 2008;197:1598–604.
Zeile I, Gahutu JB, Shyirambere C, Steininger C, Musemakweri A, Sebahungu F, et al. Molecular markers of Plasmodium falciparum drug resistance in southern highland Rwanda. Acta Trop. 2012;121:50–4.
Spalding MD, Eyase FL, Akala HM, Bedno SA, Prigge ST, Coldren RL, et al. Increased prevalence of the pfdhfr/phdhps quintuple mutant and rapid emergence of pfdhps resistance mutations at codons 581 and 613 in Kisumu, Kenya. Malar J. 2010;9:338.
González R, Mombo-Ngoma G, Ouédraogo S, Kakolwa MA, Abdulla S, Accrombessi M, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-negative women: a multicentre randomized controlled trial. PLoS Med. 2014;11:e1001733.
Tagbor H, Bruce J, Agbo M, Greenwood B, Chandramohan D. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: a randomised controlled non-inferiority trial. PLoS One. 2010;5:e14425.
Authors’ contributions
JR, NMT, GH, PB, and FPM designed the study. VB, ER, AS, SD, ST, and PB were responsible for patient recruitment, clinical and laboratory examinations. VB and FPM did the PCR analyses and the statistical analyses. VB and FPM wrote the paper with major contributions of the other authors. All authors read and approved the final manuscript.
Acknowledgements
We thank the participating women and the midwives, laboratory staff and administration at Virika Hospital. This study was supported by the German Federal Ministry for Economic Cooperation and Development via the ESTHER programme (Ensemble pour une Solidarité Thérapeutique Hospitalière En Réseau). The sponsor had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. This work forms part of the doctoral theses of VB, ER, AS, and SD.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Braun, V., Rempis, E., Schnack, A. et al. Lack of effect of intermittent preventive treatment for malaria in pregnancy and intense drug resistance in western Uganda. Malar J 14, 372 (2015). https://doi.org/10.1186/s12936-015-0909-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-015-0909-7