Abstract
Background
Controversial findings have been reported in the impact of speckle-type POZ protein (SPOP) on clinicopathological features and prognosis in diverse cancers. We conducted this meta-analysis to confirm whether SPOP was an effective biomarker to predict clinical stage, cancer differentiation and survival.
Methods
We searched studies published before June 2021 through Medline, Embase, the Cochrane library register of controlled trials and Wanfang databases. The corrections of SPOP expression with expression disparity, tumor differentiation, clinical stage and survival were analyzed.
Results
Our meta-analysis found that higher expression of SPOP was significantly associated with earlier clinical stage, well differentiation and better overall survival. Subgroup analysis showed that the SPOP expression of adjacent tissue was significantly higher than that in cancer tissues of prostate and liver. However, renal cancer presented improved expression of SPOP in cancer tissue.
Conclusions
SPOP has the potential function to act as a novel and effective biomarker for cancer diagnosis and prognostic stratification.
Similar content being viewed by others
Introduction
Speckle-type POZ domain protein (SPOP), a Cullin3-RING ubiquitin ligase adaptor, is found to be expressed in various human tissues. The relative molecular mass of SPOP protein is about 47,000 including 374-amino acid [1]. SPOP contains a typical POZ/BTB domain in N-terminal and a MATH/TRAF domain in C-terminal. It acts as a substrate adapter that the BTB domain can bind to the ubiquitin ligase cullin3 and MATH domain bind to a specific substrate [2, 3]. In general, SPOP as a cullin3 ubiquitin ligase adaptor can specifically recognize and recruit substrate proteins for ubiquitylation and degradation [4].
SPOP is an important molecule that is paid a large amount of attention by researchers in recent years, which plays critical roles during normal development and cancer progress. Zhang et al. uncovered that SPOP could promote ubiquitination-mediated programmed death ligand 1 (PD-L1) degradation, leading to decreased PD-L1 levels and increased numbers of tumour-infiltrating lymphocytes to regulate cancer immune surveillance [5]. Dai et al. elucidated the tumor suppressor role of SPOP in prostate cancer, in which it promoted the degradation of the bromodomain and extraterminal (BET) proteins to further impact the treatment effectiveness of BET inhibitors [6]. In addition, SPOP might regulate androgen (AR) signaling way and SPOP down-expression led to the activation of AR signaling exerting oncogenic effect in prostate cancer [7]. Thus, SPOP not only can predict cancer prognosis, but also is a novel therapeutic target to affect anti-cancer therapy effectiveness.
As these important roles of SPOP in cancers, increasing evidence pay attention to the relationship between SPOP and tumors in recent years [8]. However, the impact of SPOP on clinicopathological features and prognosis was controversial in these findings. For example, some studies supported that reduced SPOP expression was commonly correlated with a lager tumor size, the present of lymph metastasis and poor differentiation [9]. Nevertheless, several reports had the opposite results. They indicated that SPOP played a tumor-promoting role, and higher SPOP expression was associated with worse clinical stage [10]. Meanwhile, the other studies showed that SPOP expression and tumor size or metastasis of tumor patients were not statistically significant [11, 12]. Due to the small sample size and discrete outcomes, these factors prevented consensus on the role of SPOP. Thus, a meta-analysis to investigate the impact of SPOP on cancers was warranted. Therefore, we performed a meta-analysis to systematically evaluate the value of SPOP in the clinicopathological characteristics and prognosis of patients with cancer using the newest publications.
Methods
Data sources and literature search
The reporting of this meta-analysis was based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We searched studies published before June 2021 through Medline, Embase, the Cochrane library register of controlled trials and Wanfang databases. The following combined keywords were used in the search: (“speckle-type POZ protein” or “SPOP”) and (“Neoplasms” or “Neoplasia” or “Neoplasias” or “Neoplasm” or “Tumors” or “Tumor” or “Cancer” or “Cancers” or “Malignancy” or “Malignancies”). The reference lists were manually searched to find eligibility records.
Eligibility criteria
The studies which reported the correlation between SPOP expression and clinical stage or cancer differentiation or overall survival (OS), or compared SPOP expression in cancer and adjacent tissue were included. Studies that were case reports, letters, reviews, animal trials, meeting abstracts were filtered out.
Data extraction and quality assessment
The following information was extracted from each studies: the name of the first author, publication year, country, sample size, sample source, detection method, cutoff standard, antibody and interesting outcomes. The quality assessment was conducted according to the Newcastle–Ottawa quality assessment scale (NOS). The score ranged from 0 to 9. The NOS score ≥ 5 suggested that the study was high or moderate quality and it could be included in this meta-analysis.
Statistical analysis
Risk ratios (RR) were used to assess dichotomous data and hazard ratios (HR) were chosen for survival data with corresponding 95% confidence intervals (95% CI). RR > 1 indicated that SPOP expression level of the former is higher than the later. Both the random-effect and fixed-effect models were conducted. The value of the inconsistency index (I2) was applied to evaluate the heterogeneity among the included studies. If significant heterogeneity was observed (I2 ≥ 50%), the random-effect modes was used; If not, the results of fixed-effect model was used. Subgroup analyses were based on the cancer type. The robustness of pooled results was assessed by the sensitivity analysis [13]. The potential publication bias was detected by the funnel plot and Egger’s test. P value < 0.05 was considered statistically significant. All statistical analyses were performed by the R software (version 4.0.3, Vienna, Austria). The literature search, record selection, data extraction, quality assessment and statistical analysis were conducted independently by two authors and disagreement was resolved by all authors.
Results
Literature search and the study characteristics
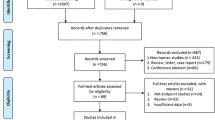
A total of 678 records were found after the initial search of Medline, Embase, Cochrane library register of controlled trials and Wanfang databases. Due to duplication, 238 records were removed. Then, titles and abstracts were scanned, and 411 records were excluded according to exclusion criteria. Next, after the evaluation of the full-text articles, 9 records were excluded for the shortage of clinicopathological or prognostic data and 6 records were reference abstracts [14,15,16,17,18,19]. Finally, as shown in Fig. 1, a total of 14 studies [9–11, 20,21,22,23,24,25,26,27,28,29,30] were eligible for this meta-analysis, which contained 2582 cases from 3 different countries (China, USA, and Korea) and were published from 2009 to 2020. Among them, more than 8 cancer types were investigated, including prostate cancer, ovarian cancer, renal cancer, gastric cancer, liver cancer, lung cancer, glioma and colorectal cancer. Basic characteristics, including first author, publication year, country, sample size, sample source, detection method, cutoff standard, antibody, and NOS score were listed in Table 1.
Meta-analysis
In the term of the correlation of SPOP expression with clinicopathological characteristics, SPOP expression was insignificant between cancer and adjacent tissue in total (RR 1.44, 95% CI 0.90–2.32, I2 = 95%, random effect model, 11 comparisons, 2490 cases). Pathologically well differentiated tumors were associated with higher SPOP expression level (RR 2.36, 95% CI 1.16–4.81, I2 = 77%, random effect model, 7 comparisons, 618 cases) (Fig. 2). Meanwhile, early clinical stage also was statistically associated with higher expression of SPOP (RR 2.41, 95% CI 1.67–3.47, I2 = 0%, fix effect model, 5 comparisons, 476 cases). Similarly, although the difference had no statistical significance, in consideration of RR value, larger tumor size (RR 1.30, 95% CI 0.67–2.54, I2 = 90%, random effect model, 6 comparisons, 480 cases), more positive lymph node metastasis (RR 1.94, 95% CI 0.86–4.36, I2 = 89%, random effect model, 5 comparisons, 480 cases) and distant metastasis (RR 1.30, 95% CI 0.54–3.15, I2 = 92%, random effect model, 5 comparisons, 323 cases) showed reduced expression level of SPOP (Fig. 3). Regarding for the correlation of SPOP expression with prognosis, the patients with increased expression of SPOP was statistically associated with better overall survival (HR 0.56, 95% CI 0.48–0.67, I2 = 29%, fix effect model, 4 comparisons, 464 cases) (Fig. 4).
Subgroup analysis
Only cancers that were investigated in above 2 studies were used for the subgroup analysis. As shown in Fig. 5, the SPOP expression of adjacent tissue was significantly higher than that in cancer tissue of prostate (RR 1.73, 95% CI 1.19–2.51, I2 = 74%, random effect model, 3 comparisons, 230 cases) and liver cancer (RR 1.48, 95% CI 1.25–1.76, I2 = 45%, fix effect model, 2 comparisons, 184 cases). The following cancers showed insignificant difference in SPOP expression level between cancer and adjacent tissue, including ovarian cancer (RR 1.31, 95% CI 0.00–655.11, I2 = 90%, random effect model, 2 comparisons, 138 cases), renal cancer (RR 0.08, 95% CI 0.00–38.86, I2 = 96%, random effect model, 6 comparisons, 766 cases), gastric cancer (RR 3.31, 95% CI 0.91–12.04, I2 = 45%, random effect model, 2 comparisons, 362 cases), lung cancer (RR 8.08, 95% CI 0.41–158.74, I2 = 79%, random effect model, 2 comparisons, 220 cases) and colorectal cancer (RR 5.63, 95% CI 0.02–1407.30, I2 = 94%, random effect model, 2 comparisons, 160 cases). However, among these cancers, in consideration of RR value or fix effect model, we found that the SPOP expression in adjacent tissue was also higher than that in cancer tissue in gastric cancer (RR 2.40, 95% CI 1.97–2.92, fix effect model), lung cancer (RR 5.29, 95% CI 2.85–9.83, fix effect model) and colorectal cancer (RR 1.55, 95% CI 1.31–1.84, fix effect model). Only renal cancer presented up-regulation of SPOP expression in cancer tissue (RR 0.08, 95% CI 0.05–0.12, fix effect model).
Sensitivity analysis and publication bias
Sensitivity analysis was performed for all pooled results by removing the study one by one. No obvious changes were founded in sensitivity analysis when we removed any studies. It indicated that the results of pooled analysis were stable and reliable. Egger’s test was conducted in the SPOP difference expression between cancer tissue and adjacent tissue, and the result indicated no publication bias (P = 0.98). Similarly, funnel plot showed no publication bias for the meta-analysis of the correction between the expression of SPOP and cancer stage, tissue differential and overall survival (Fig. 6). In conclusion, no obvious publication bias was found in this meta-analysis.
Discussion
This meta-analysis summarized the clinicopathological and prognosis significance of SPOP expression in cancer patients. Pooled results indicated that up-regulation expression of SPOP was associated with early cancer stage, well differentiation and better overall survival. In addition, SPOP expression of adjacent tissue was significantly higher than that in cancer tissue in prostate and liver cancer. To the best of our knowledge, only one meta-analysis which included 9 studies with 928 patients focused on the association between SPOP expression and prognosis [31]. Because it included studies that must reported OS or progression-free survival, so the comparisons were neglected between SPOP expression and clinicopathological characters. In consideration of the potential limitations to extract survival data from Kaplan–Meier curves, we used data directly obtained from articles for analysis to make our result more dependable. Despite the mentioned above, the results of previous study still supported our findings that compared with patients with lower SPOP expression, patients with higher SPOP expression presented longer overall survival (high versus low expression: HR 0.55, 95% CI 0.38–0.79, P = 0.001) [31].
Other studies were also given to support our results. Firstly, a previous study demonstrated that the expression of SPOP was higher in adjacent or normal gastric tissues than that in gastric cancer [23]. It was found that the down-regulated SPOP expression was significantly correlated to poor differentiation (P = 0.013) and advanced clinical stage (P = 0.002) in colorectal cancer [22]. Another study also revealed that down-regulated SPOP occurred early in prostate tumorigenesis, suggesting that SPOP was an oncogene that could be a predictive marker for prostate cancer [32, 33]. Meanwhile, it was also reported that SPOP played a favorable prognostic factor for liver cancer and might act as a novel tumor suppressor for tumor progression [27]. As mentioned above and based on our research results, down-regulated SPOP expression could predict clinicopathological characters and poor prognosis, suggesting that SPOP protein had the potential to function as prognostic biomarker in cancer patients.
The functions of SPOP in cancer is predominantly dependent on the function of its substrate proteins and the related signaling pathways. It was reported that SPOP expression levels were frequently down-regulated in multiple human tumors and were involved in several signaling pathways [23, 34, 35]. In detail, the loss-of-function mutations of SPOP could prevent ubiquitination-mediated PD-L1 degradation, demonstrating that patients with prostate cancer had a worse prognosis and therapy effect through increasing PD-L1 levels and decreasing tumor-infiltrating lymphocytes [5]. Meanwhile, except for the loss-of-function mutations of SPOP, the hypermethylation of SPOP also led to a decrease in SPOP mRNA and protein levels, suggesting that SPOP was regulated by epigenetic pathways [36, 37]. Notably, SPOP not only can be functionalized as a tumor suppressor by targeting androgen receptor for degradation, but also as an oncoprotein in renal cancer, resulting in activation of androgen receptor driven pathways [38]. Therefore, the potential mechanisms of SPOP in cancers were not fully understood. Understanding the clinical and biological characters of SPOP will lay the foundation and provide a novel view to screen potential targets for precise cancer therapy [38, 39]. Further research on the utility of SPOP as a therapeutic target is also advised. Although early work on the subject reported promising results, there are still few published studies.
Several limitations exist in this meta-analysis. Firstly, the sample sizes of included studies were usually small, which could potentially explain the non-significant findings in subgroup analysis. Secondly, the most of included cases were from Asian, and the results from other populations were required to confirm these corrections. Finally, although we adopted conservative results and explored the sources of heterogeneity, the possible impact on the results due to heterogeneity was not avoid. Therefore, our results need to be further identified in the future.
Conclusions
This meta-analysis revealed that up-regulation expression of SPOP was associated with early cancer stage, well differentiation and better overall survival. SPOP expression level was insignificant between cancer and adjacent tissue in total. In addition, SPOP expression in adjacent tissue was significantly higher than that in cancer tissue in prostate and liver cancer. The differential expression of SPOP have the potential function to act as a novel and effective biomarker for cancer diagnosis and prognosis.
Availability of data and materials
Not applicable.
Abbreviations
- SPOP:
-
Speckle-type POZ protein
- PD-L1:
-
Programmed death ligand 1
- BET:
-
The bromodomain and extraterminal
- AR:
-
Androgen
- OS:
-
Overall survival
- NOS:
-
The Newcastle–Ottawa quality assessment scale
- RR:
-
Risk ratios
- HR:
-
Hazard ratios
References
Nagai Y, Kojima T, Muro Y, Hachiya T, Nishizawa Y, Wakabayashi T, et al. Identification of a novel nuclear speckle-type protein, SPOP. FEBS Lett. 1997. https://doi.org/10.1016/S0014-5793(97)01340-9.
Wang L, Lin M, Chu M, Liu Y, Ma J, He Y, et al. SPOP promotes ubiquitination and degradation of LATS1 to enhance kidney cancer progression. EBioMedicine. 2020;56:102795.
Singh A, Fatima K, Srivastava A, Khwaja S, Priya D, Singh A, et al. Anticancer activity of gallic acid template-based benzylidene indanone derivative as microtubule destabilizer. Chem Biol Drug Des. 2016;88(5):625–34. https://doi.org/10.1111/cbdd.12805.
Yuan D, Yang Z, Chen Y, Li S, Tan B, Yu Q. Hypoxia-induced SPOP attenuates the mobility of trophoblast cells through inhibition of the PI3K/AKT/GSK3β pathway. Cell Biol Int. 2021. https://doi.org/10.1002/cbin.11501.
Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3–SPOP to control cancer immune surveillance. Nature. 2018;553(7686):91–5.
Dai X, Gan W, Li X, Wang S, Zhang W, Huang L, et al. Prostate cancer–associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4. Nat Med. 2017;23(9):1063–71.
Geng C, Rajapakshe K, Shah SS, Shou J, Eedunuri VK, Foley C, et al. Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer. Cancer Res. 2014;74(19):5631–43. https://doi.org/10.1158/0008-5472.CAN-14-0476.
Khan OM, Almagro J, Nelson JK, Horswell S, Encheva V, Keyan KS, et al. Proteasomal degradation of the tumour suppressor FBW7 requires branched ubiquitylation by TRIP12. Nat Commun. 2021;12(1):2043.
Li J-J, Zhang J-F, Yao S-M, Huang H, Zhang S, Zhao M, et al. Decreased expression of speckle-type POZ protein for the prediction of poor prognosis in patients with non-small cell lung cancer. Oncol Lett. 2017;14(3):2743–8. https://doi.org/10.3892/ol.2017.6567.
Zhao W, Zhou J, Deng Z, Gao Y, Cheng Y. SPOP promotes tumor progression via activation of β-catenin/TCF4 complex in clear cell renal cell carcinoma. Int J Oncol. 2016;49(3):1001–8. https://doi.org/10.3892/ijo.2016.3609.
Junfei X. Expression and prognostic analysis of SPOP protein in gastric carcinoma. Med J Commun. 2017;31(5):409–12.
Sathish Kumar B, Kumar A, Singh J, Hasanain M, Singh A, Fatima K, et al. Synthesis of 2-alkoxy and 2-benzyloxy analogues of estradiol as anti-breast cancer agents through microtubule stabilization. Eur J Med Chem. 2014;86:740–51. https://doi.org/10.1016/j.ejmech.2014.09.033.
Srivastava A, Fatima K, Fatima E, Singh A, Singh A, Shukla A, et al. Fluorinated benzylidene indanone exhibits antiproliferative activity through modulation of microtubule dynamics and antiangiogenic activity. Eur J Pharm Sci. 2020;154:105513. https://doi.org/10.1016/j.ejps.2020.105513.
Sathish Kumar B, Singh A, Kumar A, Singh J, Hasanain M, Singh A, et al. Synthesis of neolignans as microtubule stabilisers. Bioorg Med Chem. 2014;22(4):1342–54.
Hamid AA, Kaushal T, Ashraf R, Singh A, Chand Gupta A, Prakash O, et al. (22β,25R)-3β-Hydroxy-spirost-5-en-7-iminoxy-heptanoic acid exhibits anti-prostate cancer activity through caspase pathway. Steroids. 2017;119:43–52. https://doi.org/10.1016/j.steroids.2017.01.001.
Jain S, Singh A, Khare P, Chanda D, Mishra D, Shanker K, et al. Toxicity assessment of Bacopa monnieri L. grown in biochar amended extremely acidic coal mine spoils. Ecol Eng. 2017;108(October 2016):211–9. https://doi.org/10.1016/j.ecoleng.2017.08.039.
Hamid AA, Hasanain M, Singh A, Bhukya B, Vasudev PG, et al. Synthesis of novel anticancer agents through opening of spiroacetal ring of diosgenin. Steroids. 2014;87:108–18. https://doi.org/10.1016/j.steroids.2014.05.025.
Singh A, Fatima K, Singh A, Behl A, Mintoo MJ, Hasanain M, et al. Anticancer activity and toxicity profiles of 2-benzylidene indanone lead molecule. Eur J Pharm Sci. 2015;76:57–67. https://doi.org/10.1016/j.ejps.2015.04.020.
Khwaja S, Fatima K, Hasanain M, Behera C, Kour A, Singh A, et al. Antiproliferative efficacy of curcumin mimics through microtubule destabilization. Eur J Med Chem. 2018;151:51–61. https://doi.org/10.1016/j.ejmech.2018.03.063.
Li Y, Yu Q, Li R, Luo J, Yuan D, Song J, et al. SPOP regulates the biological mechanism of ovarian cancer cells through the Hh signaling pathway. Onco Targets Ther. 2019;12:9239–48.
Liu J, Ghanim M, Xue L, Brown CD, Iossifov I, Angeletti C, et al. Analysis of Drosophila segmentation network identifies a JNK pathway factor overexpressed in kidney cancer. Science. 2009;323(5918):1218–22.
Xu J, Wang F, Jiang H, Jiang Y, Chen J, Qin J. Properties and clinical relevance of speckle-type POZ protein in human colorectal cancer. J Gastrointest Surg. 2015;19(8):1484–96. https://doi.org/10.1007/s11605-015-2767-6.
Zeng C, Wang Y, Lu Q, Chen J, Zhang J, Liu T, et al. SPOP suppresses tumorigenesis by regulating Hedgehog/Gli2 signaling pathway in gastric cancer. J Exp Clin Cancer Res. 2014;33(1):75. https://doi.org/10.1186/s13046-014-0075-8.
Ding D, Song T, Jun W, Tan Z, Fang J. Decreased expression of the SPOP gene is associated with poor prognosis in glioma. Int J Oncol. 2015;46(1):333–41.
Li R, Yuan D, Yu Q, Luo J, Li Y, Sun Y, et al. Effect of SPOP on biological behavior of ovarian cancer and its molecular mechanism. Genom Appl Biol. 2020;39(4):1878–85.
Liu H, Hou J, Zhao Z, Li T, Du X, Zhao X, et al. Expression of speckle-type poxvirus and zinc finger protein in prostate cancer. Chin J Exp Surg. 2017;34(3):509–11.
Huang Y, Tan N, Jia D, Jing Y, Wang Q, Li Z, et al. Speckle-type POZ protein is negatively associated with malignancies and inhibits cell proliferation and migration in liver cancer. Tumor Biol. 2015;36(12):9753–61. https://doi.org/10.1007/s13277-015-3753-z.
Ji P, Liang S, Li P, Xie C, Li J, Zhang K, et al. Speckle-type POZ protein suppresses hepatocellular carcinoma cell migration and invasion via ubiquitin-dependent proteolysis of SUMO1/sentrin specific peptidase 7. Biochem Biophys Res Commun. 2018;502(1):30–42.
Kim MS, Je EM, Oh JE, Yoo NJ, Lee SH. Mutational and expressional analyses of SPOP, a candidate tumor suppressor gene, in prostate, gastric and colorectal cancers. APMIS. 2013;121(7):626–33.
Guodong L, Mingzhen Y, Shulu Z, Shengtian Z. Expression of SPOP in renal clear cell carcinoma. J Shandong Univ. Sci. 2012;50(7):74–7. https://doi.org/10.6040/j.issn.1671-7554.2012.07.016
Cheng F, Zeng C, Zeng L, Wu C, Chen Y. The association of speckle-type POZ protein with lymph node metastasis and prognosis in cancer patients: A meta-analysis. Medicine (United States). 2019.
Stangl A, Willner C, Maahs L, Burmeister C, Hwang C, Pilling A. SPOP mutation as a predictive marker for treatment of metastatic castration-resistant prostate cancer. J Clin Oncol. 2021;39(6_suppl):160–160. https://doi.org/10.1200/JCO.2021.39.6_suppl.160.
Shoag J, Liu D, Ma X, Oromendia C, Christos P, Ballman K, et al. Prognostic value of the SPOP mutant genomic subclass in prostate cancer. Urol Oncol Semin Orig Investig. 2020;38(5):418–22.
Clark A, Burleson M. SPOP and cancer: a systematic review. Am J Cancer Res. 2020;10(3):704–26.
Jin X, Qing S, Li Q, Zhuang H, Shen L, Li J, et al. Prostate cancer-associated SPOP mutations lead to genomic instability through disruption of the SPOP–HIPK2 axis. Nucleic Acids Res. 2021;49(12):6788–803.
Yao S, Chen X, Chen J, Guan Y, Liu Y, Chen J, et al. Speckle-type POZ protein functions as a tumor suppressor in non-small cell lung cancer due to DNA methylation. Cancer Cell Int. 2018;18(1):213. https://doi.org/10.1186/s12935-018-0711-z.
Singh A, Mohanty I, Singh J, Rattan S. BDNF augments rat internal anal sphincter smooth muscle tone via RhoA/ROCK signaling and nonadrenergic noncholinergic relaxation via increased NO release. Am J Physiol Liver Physiol. 2020;318(1):G23-33. https://doi.org/10.1152/ajpgi.00247.2019.
Wang Z, Song Y, Ye M, Dai X, Zhu X, Wei W. The diverse roles of SPOP in prostate cancer and kidney cancer. Nat Rev Urol. 2020;17(6):339–50.
Singh A, Singh J, Rattan S. Evidence for the presence and release of BDNF in the neuronal and non-neuronal structures of the internal anal sphincter. Neurogastroenterol Motil. 2021;December 2020:1–13. https://doi.org/10.1111/nmo.14099.
Acknowledgements
Not applicable.
Funding
The work was supported by National Natural Science Foundation of China (82172842, 81803104 and 81672386), the Sichuan Province Science and Technology Support Program (2021YFSY008 and 2020YFS0276), West China Nursing Discipline Development Special Fund Project (HXHL21008), the Technology Innovation Project of Chengdu Science and Technology Bureau (2019-YF05-00459-SN), Postdoctoral research and Development Fund and Translational medicine fund of West China Hospital (2020HXBH119 and CGZH19002). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Study concepts and design: YH, YX and YS; the literature search, record selection, data extraction, quality assessment and data analysis: YH and CJ; manuscript preparation: YH; manuscript editing: all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, Y., Chen, J., Peng, X. et al. Clinicopathological and prognostic significance of speckle-type POZ protein in cancers: a systematic review and meta-analysis. Cancer Cell Int 21, 626 (2021). https://doi.org/10.1186/s12935-021-02329-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02329-5