Abstract
Background
The aims of this study are to evaluate the effect of Speckle-type POZ protein (SPOP) in colorectal cancer (CRC) patients and explore its significance in the prognosis.
Methods
We used immunohistochemistry to detect the expression of SPOP in CRC. Moreover, this result was further confirmed at the protein and messenger RNA (mRNA) level in paired CRC specimens and matched adjacent noncancerous colon tissues by Western blotting and real-time quantitative PCR (qRT-PCR), respectively. Furthermore, we evaluate the effects of SPOP on CRC cell proliferation and migration in vitro. The Kaplan–Meier method and log-rank test were employed to compare the overall survival between SPOP low expression group and SPOP high expression group. Correlation of survival with clinicopathologic parameters, including SPOP level, was investigated with multivariate analyses.
Results
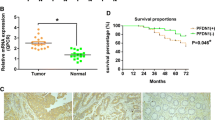
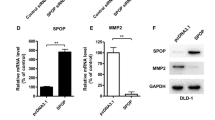
We confirmed frequent SPOP downregulation in both mRNA (P = 0.0286) and protein (P = 0.004) levels in CRC tissues as compared to matched adjacent nontumorous tissues. Besides, the downregulated SPOP expression in CRC tissues was significantly correlated to poor differentiation (P = 0.013), distant metastasis (P = 0.003), gross type (P < 0.001), and high TNM stage (P = 0.002). Kaplan–Meier survival analysis showed that low SPOP expression exhibited a significant correlation with poor prognosis for CRC patients. Overexpression of SPOP in CRC cell lines significantly suppressed cell proliferation, migration, and clone formation. In contrast, SPOP knockdown dramatically promoted cell proliferation, migration, and clone formation in vitro. In addition, overexpression of SPOP increased E-cadherin and suppressed vimentin in HCT116 cells and silencing of SPOP reversed all these biomarkers. Furthermore, SPOP significantly downregulated MMP2 and MMP7 protein levels in HCT116 cell lines.
Conclusion
Our results suggest that SPOP plays a pivotal role in colorectal cancer (CRC) through mesenchymal–epithelial transition and MMPs, and it may be a potential therapeutic target in colorectal cancer.







Similar content being viewed by others
References
Huang, C. J.; Chen, C. Y.; Chen, H. H.; Tsai, S. F.; Choo, K. B., TDPOZ, a family of bipartite animal and plant proteins that contain the TRAF (TD) and POZ/BTB domains. Gene 2004, 324, 117–27.
Berger, M. F.; Lawrence, M. S.; Demichelis, F.; Drier, Y.; Cibulskis, K.; Sivachenko, A. Y.; Sboner, A.; Esgueva, R.; Pflueger, D.; Sougnez, C.; Onofrio, R.; Carter, S. L.; Park, K.; Habegger, L.; Ambrogio, L.; Fennell, T.; Parkin, M.; Saksena, G.; Voet, D.; Ramos, A. H.; Pugh, T. J.; Wilkinson, J.; Fisher, S.; Winckler, W.; Mahan, S.; Ardlie, K.; Baldwin, J.; Simons, J. W.; Kitabayashi, N.; MacDonald, T. Y.; Kantoff, P. W.; Chin, L.; Gabriel, S. B.; Gerstein, M. B.; Golub, T. R.; Meyerson, M.; Tewari, A.; Lander, E. S.; Getz, G.; Rubin, M. A.; Garraway, L. A., The genomic complexity of primary human prostate cancer. Nature 2011, 470, (7333), 214–20.
Kwon, J. E.; La, M.; Oh, K. H.; Oh, Y. M.; Kim, G. R.; Seol, J. H.; Baek, S. H.; Chiba, T.; Tanaka, K.; Bang, O. S.; Joe, C. O.; Chung, C. H., BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. J Biol Chem 2006, 281, (18), 12664–72.
Errington, W. J.; Khan, M. Q.; Bueler, S. A.; Rubinstein, J. L.; Chakrabartty, A.; Prive, G. G., Adaptor protein self-assembly drives the control of a cullin-RING ubiquitin ligase. Structure 2012, 20, (7), 1141–53.
Li, C.; Ao, J.; Fu, J.; Lee, D. F.; Xu, J.; Lonard, D.; O'Malley, B. W., Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1. Oncogene 2011, 30, (42), 4350–64.
Le Gallo, M.; O'Hara, A. J.; Rudd, M. L.; Urick, M. E.; Hansen, N. F.; O'Neil, N. J.; Price, J. C.; Zhang, S.; England, B. M.; Godwin, A. K.; Sgroi, D. C.; Hieter, P.; Mullikin, J. C.; Merino, M. J.; Bell, D. W., Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet 2012, 44, (12), 1310–5.
Kan, Z.; Jaiswal, B. S.; Stinson, J.; Janakiraman, V.; Bhatt, D.; Stern, H. M.; Yue, P.; Haverty, P. M.; Bourgon, R.; Zheng, J.; Moorhead, M.; Chaudhuri, S.; Tomsho, L. P.; Peters, B. A.; Pujara, K.; Cordes, S.; Davis, D. P.; Carlton, V. E.; Yuan, W.; Li, L.; Wang, W.; Eigenbrot, C.; Kaminker, J. S.; Eberhard, D. A.; Waring, P.; Schuster, S. C.; Modrusan, Z.; Zhang, Z.; Stokoe, D.; de Sauvage, F. J.; Faham, M.; Seshagiri, S., Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 2010, 466, (7308), 869–73.
Kim, M. S.; Je, E. M.; Oh, J. E.; Yoo, N. J.; Lee, S. H., Mutational and expressional analyses of SPOP, a candidate tumor suppressor gene, in prostate, gastric and colorectal cancers. APMIS 2013, 121, (7), 626–33.
Nakayama, K. I.; Nakayama, K., Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 2006, 6, (5), 369–81.
Bode, A. M.; Dong, Z., Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 2004, 4, (10), 793–805.
Liu, J.; Ghanim, M.; Xue, L.; Brown, C. D.; Iossifov, I.; Angeletti, C.; Hua, S.; Negre, N.; Ludwig, M.; Stricker, T.; Al-Ahmadie, H. A.; Tretiakova, M.; Camp, R. L.; Perera-Alberto, M.; Rimm, D. L.; Xu, T.; Rzhetsky, A.; White, K. P., Analysis of Drosophila segmentation network identifies a JNK pathway factor overexpressed in kidney cancer. Science 2009, 323, (5918), 1218–22.
Larue, L.; Bellacosa, A., Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3ʹ kinase/AKT pathways. Oncogene 2005, 24, (50), 7443–54.
Thiery, J. P.; Sleeman, J. P., Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006, 7, (2), 131–42.
Vu, T. H.; Werb, Z., Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 2000, 14, (17), 2123–33.
Zucker, S.; Vacirca, J., Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev 2004, 23, (1–2), 101–17.
Said, A. H.; Raufman, J. P.; Xie, G., The role of matrix metalloproteinases in colorectal cancer. Cancers (Basel) 2014, 6, (1), 366–75.
Jemal, A.; Siegel, R.; Xu, J.; Ward, E., Cancer statistics, 2010. CA Cancer J Clin 2010, 60, (5), 277–300.
Ishizuka, M.; Kita, J.; Shimoda, M.; Kato, M.; Sawada, T.; Kubota, K., Impact of grading of liver metastasis on postoperative outcome in patients with liver metastases from colorectal cancer. Hepatogastroenterology 2012, 59, (113), 54–8.
Harpaz, N.; Polydorides, A. D., Colorectal dysplasia in chronic inflammatory bowel disease: pathology, clinical implications, and pathogenesis. Arch Pathol Lab Med 2010, 134, (6), 876–95.
Campos, F. G.; Logullo Waitzberg, A. G.; Kiss, D. R.; Waitzberg, D. L.; Habr-Gama, A.; Gama-Rodrigues, J., Diet and colorectal cancer: current evidence for etiology and prevention. Nutr Hosp 2005, 20, (1), 18–25.
Roessler, S.; Jia, H. L.; Budhu, A.; Forgues, M.; Ye, Q. H.; Lee, J. S.; Thorgeirsson, S. S.; Sun, Z.; Tang, Z. Y.; Qin, L. X.; Wang, X. W., A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res 2010, 70, (24), 10202–12.
Kim, J.; Huynh, R.; Abraham, I.; Kim, E.; Kumar, R. R., Number of lymph nodes examined and its impact on colorectal cancer staging. Am Surg 2006, 72, (10), 902–5.
Nagai, Y.; Kojima, T.; Muro, Y.; Hachiya, T.; Nishizawa, Y.; Wakabayashi, T.; Hagiwara, M., Identification of a novel nuclear speckle-type protein, SPOP. FEBS Lett 1997, 418, (1–2), 23–6.
Zhuang, M.; Calabrese, M. F.; Liu, J.; Waddell, M. B.; Nourse, A.; Hammel, M.; Miller, D. J.; Walden, H.; Duda, D. M.; Seyedin, S. N.; Hoggard, T.; Harper, J. W.; White, K. P.; Schulman, B. A., Structures of SPOP-substrate complexes: insights into molecular architectures of BTB-Cul3 ubiquitin ligases. Mol Cell 2009, 36, (1), 39–50.
Michaelson, J. S., The Daxx enigma. Apoptosis 2000, 5, (3), 217–20.
Buttice, G.; Duterque-Coquillaud, M.; Basuyaux, J. P.; Carrere, S.; Kurkinen, M.; Stehelin, D., Erg, an Ets-family member, differentially regulates human collagenase1 (MMP1) and stromelysin1 (MMP3) gene expression by physically interacting with the Fos/Jun complex. Oncogene 1996, 13, (11), 2297–306.
Hernandez-Munoz, I.; Lund, A. H.; van der Stoop, P.; Boutsma, E.; Muijrers, I.; Verhoeven, E.; Nusinow, D. A.; Panning, B.; Marahrens, Y.; van Lohuizen, M., Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase. Proc Natl Acad Sci U S A 2005, 102, (21), 7635–40.
Liu, A.; Desai, B. M.; Stoffers, D. A., Identification of PCIF1, a POZ domain protein that inhibits PDX-1 (MODY4) transcriptional activity. Mol Cell Biol 2004, 24, (10), 4372–83.
Bunce, M. W.; Boronenkov, I. V.; Anderson, R. A., Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. J Biol Chem 2008, 283, (13), 8678–86.
Wang, C.; Pan, Y.; Wang, B., Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development 2010, 137, (12), 2001–9.
Miyashita, T.; Reed, J. C., Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995, 80, (2), 293–9.
Ala-aho, R.; Grenman, R.; Seth, P.; Kahari, V. M., Adenoviral delivery of p53 gene suppresses expression of collagenase-3 (MMP-13) in squamous carcinoma cells. Oncogene 2002, 21, (8), 1187–95.
Zhang, Q.; Zhang, L.; Wang, B.; Ou, C. Y.; Chien, C. T.; Jiang, J., A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Dev Cell 2006, 10, (6), 719–29.
Hugo, H.; Ackland, M. L.; Blick, T.; Lawrence, M. G.; Clements, J. A.; Williams, E. D.; Thompson, E. W., Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol 2007, 213, (2), 374–83.
Iwatsuki, M.; Mimori, K.; Yokobori, T.; Ishi, H.; Beppu, T.; Nakamori, S.; Baba, H.; Mori, M., Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 2010, 101, (2), 293–9.
Thiery, J. P.; Acloque, H.; Huang, R. Y.; Nieto, M. A., Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, (5), 871–90.
Brabletz, T.; Jung, A.; Reu, S.; Porzner, M.; Hlubek, F.; Kunz-Schughart, L. A.; Knuechel, R.; Kirchner, T., Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A 2001, 98, (18), 10356–61.
Langenskiold, M.; Holmdahl, L.; Falk, P.; Ivarsson, M. L., Increased plasma MMP-2 protein expression in lymph node-positive patients with colorectal cancer. Int J Colorectal Dis 2005, 20, (3), 245–52.
Brabletz, T.; Jung, A.; Dag, S.; Hlubek, F.; Kirchner, T., beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999, 155, (4), 1033–8.
Maurel, J.; Nadal, C.; Garcia-Albeniz, X.; Gallego, R.; Carcereny, E.; Almendro, V.; Marmol, M.; Gallardo, E.; Maria Auge, J.; Longaron, R.; Martinez-Fernandez, A.; Molina, R.; Castells, A.; Gascon, P., Serum matrix metalloproteinase 7 levels identifies poor prognosis advanced colorectal cancer patients. Int J Cancer 2007, 121, (5), 1066–71.
Acknowledgments
This work is supported by the grants from the China Postdoctoral Science Foundation and the Department of Health of Jiangsu Province of China (no. H201214).
Conflict of Interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Junfei Xu and Feiran Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, J., Wang, F., Jiang, H. et al. Properties and Clinical Relevance of Speckle-Type POZ Protein in Human Colorectal Cancer. J Gastrointest Surg 19, 1484–1496 (2015). https://doi.org/10.1007/s11605-015-2767-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2767-6