Abstract
Leukemia is a lethal cancer in which white blood cells undergo proliferation and immature white blood cells are seen in the bloodstream. Without diagnosis and management in early stages, this type of cancer can be fatal. Changes in protooncogenic genes and microRNA genes are the most important factors involved in development of leukemia. At present, leukemia risk factors are not accurately identified, but some studies have pointed out factors that predispose to leukemia. Studies show that in the absence of genetic risk factors, leukemia can be prevented by reducing the exposure to risk factors of leukemia, including smoking, exposure to benzene compounds and high-dose radioactive or ionizing radiation. One of the most important treatments for leukemia is chemotherapy which has devastating side effects. Chemotherapy and medications used during treatment do not have a specific effect and destroy healthy cells besides leukemia cells. Despite the suppressing effect of chemotherapy against leukemia, patients undergoing chemotherapy have poor quality of life. So today, researchers are focusing on finding more safe and effective natural compounds and treatments for cancer, especially leukemia. Chitosan is a valuable natural compound that is biocompatible and non-toxic to healthy cells. Anticancer, antibacterial, antifungal and antioxidant effects are examples of chitosan biopolymer properties. The US Food and Drug Administration has approved the use of this compound in medical treatments and the pharmaceutical industry. In this article, we take a look at the latest advances in the use of chitosan in the treatment and improvement of leukemia.
Similar content being viewed by others
Introduction
Leukemia is a type of blood cancer that is caused by abnormal function of blood tissue [1, 2]. Leukemia manifests itself in the form of abnormal growth of immature white blood cells. This disease can kill a person if it is not diagnosed and controlled in its early stages [3, 4]. Leukemia usually manifests as high, rapid, and uncontrolled proliferation of leukocytes and their precursor cells, leading to the accumulation of immature leukocytes in the bloodstream [5, 6]. Excessive production of immature white blood cells and their entry into the bloodstream can cause anemia in patients. In 2016, statistics showed that 34,090 men and 26,050 women were suffering from leukemia and 24,400 out of these 60,140 individuals died [7]. Changes in protooncogenic genes and microRNA genes are among the most important factors involved in the development of leukemia [3, 8,9,10]. Leukemia can generally be divided into four types: (a) chronic lymphocytic leukemia (CLL), (b) acute lymphocytic leukemia (ALL), (c) chronic myelogenous leukemia (CML), and (d) acute myeloid leukemia (AML). While in CLL, B lymphocytes proliferate irreguralely, immature B or T lymphocytes are involved in the pathogensis of ALL. Furthermore, granulocyte precursors and immature myeloid cells are involved cells in CML and in AML, respectively [1, 11, 12]. The incidence and survival rate of different types of this heterogenous cancer are not similar: “Among adults (20 years of age and older), the most common types of leukemia are CLL (38%) and AML (31%), whereas ALL is most common in children and adolescents (ages 0 to 19 years), accounting for 74% of cases” [13]. “AML is the most common form of acute leukemia in adults and has the shortest survival (5-year survival = 24%)”. CML is also more common in adults and “It accounts for approximately 15% of newly diagnosed cases of leukemia in adults” [14,15,16].
The incidence of leukemia in developing countries is much higher than in other countries where urbanization problems, efforts to control infections, and high tobacco use are among the predisposing factors for leukemia in these countries [5, 17]. Patients with leukemia generally have low hemoglobin levels and the number of leukocytes in their bloodstream is high. Also, the number of platelets in these patients decreases to less than normal. Weakness, persistent paleness and yellowing of the skin, anemia, purpura, retinal hemorrhage, and lymphadenopathy are among the main symptoms that leukemia patients suffer from [5, 18].
Studies show that mutations and the lack of regulated cell death (RCD) proteins cause resistance to conventional therapies in patients suffering from leukemia. Chemotherapy and the use of corticosteroid drugs, P13K/mTOR inhibitors, tyrosine kinase inhibitors and stem cell transplantation are examples of first-line leukemia treatment methods. These treatments are invasive therapies and destroy the immune system [3, 19,20,21]. After chemotherapy, which is common in the treatment of almost all types of leukemia, the survival rate is less than 70% in children and less than 20% in adults [22]. In addition to chemo- and radio-therapy, allogeneic bone marrow transplantation is another option on the table for leukemia treatment, especially in adults. It seems that using stem cell transplantation is more common in AML and CML compared to ALL [23]. Thus, in recent years, efforts have been made to find treatments that show less side effects on other parts of the body and provide better quality of life similar to studies about other types of cancer [24].
Since ancient times, the use of natural ingredients to treat diseases such as cancer has been common and has helped scientists to discover and develop more effective drugs in this field [25, 26]. Epigallocatechin gallate, curcumin, quercetin, silymarin and stilbene resveratrol are examples of plant compounds that have anti-cancer potential by regulating the cell cycle and controlling the apoptosis pathway associated with p53, or have similar performance to potent chemotherapy drugs [25, 27,28,29,30,31,32,33,34].
Chitosan is also a natural compound that is non-toxic and biocompatible [35, 36]. Chitosan is a biological and cationic polysaccharide found in the skeleton of arthropods, the skin of a variety of crabs, the cell wall of fungi and the skin of insects [37,38,39,40,41,42]. O-carboxymethyl chitosan, Ncarboxymethyl chitosan and N, O-carboxymethyl chitosan are three types of chitosan derivatives that are produced by its carboxymethylation [38, 41]. Studies show that O-carboxymethyl chitosan is more compatible with blood, so it is widely used in medicine [38, 43]. Various studies have shown that chitosan has antibacterial, anti-tumor, and antioxidant effects and has significant pharmacological effects [1, 44,45,46,47]. The inhibitory effects of chitosan on degranulation and cytokine production in rats’ basophilic leukemia cells have been confirmed [1, 48]. Antioxidant activity against superoxide anion is one of the most important properties of chitosan. The antioxidant activity of chitosan is important for the production of broad-spectrum drugs with high antioxidant activity [38, 49]. Reports indicate that chitosan induces apoptosis, but chitosan carboxymethyl has the property of inhibiting apoptosis [50, 51]. Chitosan is also used in the treatment of cancer as a low-toxicity chemotherapeutic drug [38, 41]. The US Food and Drug Administration (FDA) has approved the use of chitosan in medicine and the manufacture of drugs [35, 52].
Overall, the advantages of chitosan as a drug or a delivery system has guided us through precisely looking into the studies using chitosan on one of the most lethal cancers: leukemia.
Leukemia: risk factors and primary prevention
So far, the risk factors of leukemia have been identified in several studies. In general, the risk factors involved in developing leukemia can be divided into four categories: (a) genetic, (b) environmental, (c) familial, and (d) lifestyle factors. Studies show that in the absence of genetic risk factors, leukemia can be prevented by reducing the exposure to some risk factors, including smoking, exposure to benzene compounds and high-dose radioactive or ionizing radiation [53].
Here is a summary of some of the important factors that contribute to leukemia.
Genetic and familial risk factors
Genetic factors only play a role in some cases of leukemia [53,54,55]. So far, several examples of chromosomal abnormalities leading to leukemia have been identified. Philadelphia chromosomal abnormalities play a role in the development of CML, which usually involves gene displacement between chromosomes 9 and 22 [53, 55]. Some genetic syndromes are caused by chromosomal mutations. Diseases such as Down syndrome, Bloom syndrome, ataxia telangiectasia, and Fanconi anemia can be predisposing factors for leukemia [53, 56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. Gene transfer between chromosomes 8 and 21 or between 12 and 21 chromosomes usually supports AML [74, 75].
Large-scale genome-wide association studies have revealed that various genomic loci with common polymorphisms are correlated with the susceptibility to ALL. Most of these polymorphisms are located in genes related to hematopoietic transcription factors, such as ERG, ARID5B, IKZF3, IKZF1, CEBPE, and GATA3. Individually, these risk alleles contribute to limited significance in clinic. However, their aggregation causes a ninefold rise in the risk of leukemia in cases with multiple risk alleles compared with cases with no risk alleles [76]. Based on the investigations done on pediatric populations, it is found that some genetic syndromes are associated with the increased risk of ALL, including Bloom syndrome, Nijmegen breakdown syndrome, Fanconi anemia, Down syndrome, and ataxia telangiectasia. Although chromosomal changes are not enough for the development of leukemia, some aberrations are characteristic of ALL, such as MLL rearrangement, t(9;22) [BCR-ABL1], t(12;21) [ETV6-RUNX1], and t(1;19) [TCF3-PBX1] [77].
Environmental risk factors
Benzene and benzene compounds
Benzene is widely used as an important solvent in various industries for the production of materials and compounds, including printing, leather and petrochemical industries [53, 78]. Benzene compounds can affect people by smoking or in the workplace [53, 79,80,81,82]. Statistics show that mortality has increased in patients with leukemia, especially AML [53, 81, 83, 84]. From time immemorial, there is strong evidence that people with leukemia are affected by benzene compounds, which can be considered a high-risk carcinogen [81, 82, 85,86,87,88,89,90,91,92,93].
Ionizing radiation
Reports indicate that ionizing radiation is a major risk factor for AML, ALL, and CML [53]. Studies show that these rays do not play a role in CLL. Pierce et al. studied the lifespan of atomic bomb survivors from 1950 to 1990. Statistics from their study show that out of 86,572 people studied, 249 died of leukemia due to exposure to ionizing radiation [53]. A study by Preston et al. Found that about 50% of leukemia patients were exposed to ionizing radiation in 1945 in Hiroshima and Nagasaki [94].
Another source of radiation which can lead to secondary leukemia is using radiotherapy for treating other cancers. A study on patients with gynecologic malignancies treated in the past 20 years found that there is only 0.38% chance of leukemia development in these patients. However, this percent is not specific to radiotherapy and it involves leukemias developed secondary to chemotherapy, as well [95]. Another study examined patients with invasive tumors of the vulva, cervix, uterus, anus, and rectosigmoid treated with radiotherapy and found that not only the risk of developing leukemia is 72% higher in these patients, but also “the risk of secondary leukemia peaks at 5 to 10 years after primary treatment and remains elevated even 10 to 15 years after initial treatment” [96]. After all, it can be concluded that radiotherapy might have a role in initiating leukemia in patients who suffer from other types of cancer.
Lifestyle risk factors
Obesity is a lifestyle-related risk factor for leukemia. Impaired immune response and decreased leptin levels in the blood may be functional mediators of obesity-induced leukemia [97,98,99,100,101]. Leptin increases the proliferation of CD4+ T cells and stimulates the proliferation of myelocytic and primary progenitor cells [102,103,104]. Some studies have shown that a daily diet rich in vegetables can reduce the risk of AML [105, 106].
Another factor in the lifestyle-related risk factors is smoking. Various epidemiological studies have confirmed the risk of leukemia, especially myeloid leukemia, in smokers [107,108,109,110,111,112,113,114,115]. Sandler et al. [116] showed that there was a link between smoking and acute leukemia [116]. In 2004, the International Agency for Research and Cancer and General Surgeon Carmona and colleagues found that smoking is a major risk factor for AML [53, 117,118,119]. Kasim et al. [54] in their case study in Canada, confirmed the significant effect of lifestyle factors on leukemia [54]. Some studies have also cited smoking as a risk factor for ALL and CLL [53, 120, 121].
Chitosan: biochemical structure and medicinal, therapeutic properties
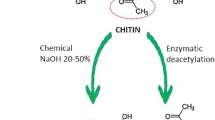
Chitosan is a natural polysaccharide biopolymer composed of N-acetyl glucosamine and glucosamine components and is obtained in industry by hydrolysing amino acetyl groups in chitin (β-(1-4)-poly-N-acetyl-d-glucosamine which is shown in (Fig. 1) [25, 122,123,124,125,126,127]. After cellulose, chitosan is the second most abundant substance in nature. Crabs and shrimp are the most important sources of chitosan. Chitosan is also found in the cell walls of fungi and insect scales [25, 122, 125,126,127,128,129]. It has many valuable properties in addition to being natural such as non-toxicity, degradability, biocompatibility, low immunogenicity, and high affinity for metals, proteins, and dyes as well as high water resistance and ductility in various forms such as gel, nanoparticles and grains. All of these valuable properties have led to chitosan being regarded as a promising effective biopolymer in the pharmaceutical industry, especially anticancer drugs. It is also used in drug delivery systems to control the process of drug release in the body [25, 123, 130,131,132,133,134,135,136,137]. The mentioned effect of chitosan is mostly relying on its effects on paracellular and transcellular transportation. Through facilitating these ways of transport and opening the tight junctions of epithelial cells, chitosan is able to ferry drugs [138]. However, how it delivers drugs to specific sites is not completely understood. It seems that its pH-dependent drug release, protein adsorption onto nanoparticles following the serum exposure, getting involved in phagocytic pathways, and the enhanced permeation and retention (EPR) effect might be the reasons why chitosan acts specifically on cancer cells [138,139,140].
Low chitosan solubility in physiological pH (> 6.0) is one of the factors that limit its use. Due to the carboxymethylation of chitosan, a compound called carboxymethyl chitosan is produced, which, in addition to having some of chitosan’s properties, it also has better flexibility and good solubility in water [123, 141,142,143,144,145]. On the other hand, the end-carboxymethyl groups in its structure are the arm that binds to drugs such as 6-mercaptopurine [123, 146, 147]. The hydrolysed compounds derived from chitosan are soluble in water. d-Glucosamine oligomer, chitosan oligosaccharide, is one of these derivatives that has antibacterial, antioxidant and anti-tumor properties and can be used in drug delivery systems [1, 44,45,46,47, 148]. Chitosan oligosaccharide can suppress degranulation and cytokine production in live mice’s basophilic leukemia cells [48]. Reports indicate that the secretion and expression of tumor necrosis factor (TNF)-α, inflammatory cytokines, and interleukin (IL)-6 are inhibited in human astrocytoma cells treated with soluble chitosan [149].
The latest methods of using chitosan as suppressive compounds and delivery systems in leukemia patients
A combination of chemotherapy and radiotherapy is the most common treatment for various types of cancer, such as leukemia. These therapies and the drugs used in them are considered dangerous because they do not have sufficient specificity to affect the target or the desired tissue. Despite the possible cure for cancer, quality of life is deteriorating in patients who have received these treatments. Therefore, the discovery and presentation of treatment options that are selective and at the same time have fewer side effects is a necessity. Studies show that chitosan, as a small transfer biomolecule, is very valuable in the production of nano-anti-cancer drugs. In addition to its anti-cancer properties, chitosan in these drugs selectively transports small particles and drug molecules into target cellular organelles. The transfer of small drug molecules into cellular organelles increases the toxicity of the drug to cancer cells and reduces it in healthy cells. Chitosan also enhances the effects of other chemotherapy drugs combined with it [150,151,152]. In the following, we will discuss the latest leukemia treatment strategies that have used chitosan (Table 1).
Folic acid modified carboxymethyl chitosan (fa-cmcs) self-assemble nanoparticles: toxicity to leukemia cells
Carboxymethyl chitosan (CMCS) is one of the water-soluble derivatives of chitosan that is used in different pharmaceutical industries. Combining CMCS with folic acid (FA) forms a hydrophobic compound consisting of pteroyl, in which case in the aquatic environment the amphiphilic groups easily and spontaneously form nanoparticles. In this case, CMCS combined with folic acid (FA) is an effective nanocarrier for long-term drug release. The desired hydrophobic drug accumulates in the hydrophobic microdomains of these nanoparticles and the drug is gradually released through the polysaccharide skeleton into the target tissue [153,154,155,156,157]. HU and colleagues show that folic acid modified carboxymethyl chitosan nanoparticles can act as a highly efficient drug carrier and gradually release drug particles into the target tissue or cell [153]. In their study, HU and colleagues placed Methotrexate in Folic Acid Modified Carboxymethyl Chitosan nanoparticles and tested its effect on human promyelocytic leukemia cells (HL60) in vitro. They showed that these nanoparticles could specifically transport the drug to target cells without affecting healthy cells and gradually release it by changing the pH in the target environment [153]. Direct administration of anticancer drugs, due to the lack of adequate specificity for tumor cells, also affects other healthy cells and causes side effects. Therefore, the design of particles that can direct these drugs specifically to tumor cells will lead to a safer and more effective treatment because in this case, the toxicity of the drug to normal cells is reduced and its toxicity to cancer cells is increased.
Fe3O4-PEG-LAC-chitosan-PEI nanoparticles: survivin siRNA delivery
In recent years, the use of RNA interference in gene therapy methods has been widely welcomed by researchers. However, one of the major problems with this treatment is the transfer of small interfering RNAs (siRNAs) to target tumor cells [158,159,160,161,162,163,164]. Various pathways are involved in the inhibition or progression of cancer in the body, the most important of which are cell death and survival. Anti-apoptotic genes that are directly linked to caspases encode factors that suppress apoptotic processes by suppressing the apoptotic proteins. The Survivin gene is involved in a variety of biological processes, including cell cycle regulation, cell protection, and cell death suppression, and through these processes, it maintains the survival of cancer cells. This gene is highly expressed in cancer cells. Reports indicate that if survivin expression is suppressed, cancer cells become sensitive to anticancer compounds and drugs [158, 165, 166]. From these findings, it can be concluded that inhibiting Survivin expression is a potentially valuable target for cancer treatment and suppression of its progression.
Polyethyleneglycol (PEG) and chitosan are two compounds which are commonly used in the synthesis of oligonuleotide cationic particles [167,168,169,170,171,172]. Polyethyleneimine (PEI) is one of the most effective carriers of the gene, with its “proton-sponge effect” in laboratory and in vivo. Polyethyleneimine acts as a buffer around the endosome, releasing compounds into the cytoplasm [163]. Chitosan polysaccharide effectively coats the nanoparticles and thereby, stabilizes them and prevents particles from accumulating. In the synthesis of these carrier nanoparticles, PEG reduces PEI toxicity and ensures the stability of colloidal particles. On the other hand, PEG, in addition to its high biocompatibility, prevents the deposition of nanoparticles [158, 173]. Arami et al. [158] designed the “Fe3O4-PEG-LAC-chitosan-PEI” nanoparticle carrier, which due to the properties of the compounds used in it, has a sufficient positive charge to react with siRNAs [158]. In their study, they used the nanoparticle to transfer survivin siRNA to human breast cancer cells (MCF-7) and human chronic myelogenous leukemia cells (K562) in vitro. Their findings show that Fe3O4-PEG-LAC-chitosan-PEI nanoparticles combine well with survivin siRNA, and their nanoscale-size makes them a good carrier for gene delivery in the treatment of various cancers such as breast and leukemia. Survivin siRNA therapy using Fe3O4-PEG-LAC-chitosan-PEI nanoparticle is a safe and specific treatment that does not affect healthy cells [158]. Therefore, this nanoparticle is a valuable case study for the treatment of cancers based on siRNA delivery.
S-Nitroso-MSA-chitosan nanoparticles: toxicity to leukemia cells
The free radical nitric oxide (NO) produced in the body is involved in regulating important processes such as wound healing, cellular communication, dilation of blood vessels, prevention of platelet aggregation, immune defense, and bronchial dilation [174,175,176,177,178,179,180,181,182,183,184]. Inside the body, nitric oxide is produced by the oxidation of l-arginine to l-citrulline by the activity of nitric oxide synthase enzyme (NOS) [179]. Studies show that NO plays an important role in the defense of the immune system and has anti-tumor properties [174, 184, 185]. Therefore, the use of NO-releasing nanoparticles in cancer treatment is a new treatment strategy that requires several studies to further evaluate its side effects and benefits. Pelegrino et al. [174] used chitosan to design a NO-releasing nanoparticle that has antibacterial, antifungal, and anti-cancer effects [174]. In their study, they used chitosan to produce capsules of low molecular weight mercaptosuccinic acid and examined the effect of its toxicity on human hepatocellular carcinoma (HepG2) and human chronic myeloid leukemia cells (K562). Mercaptosusic acid contains the thiol group (S-nitroso-MSA), which actually acts as an NO donor. Therefore, the nanoparticle composition designed by Pelegrino et al. is “S-nitroso-MSA-CS”. The results obtained after treating HepG2 and K562 cells with this nanoparticle in vitro show that the gradual release of NO from S-nitroso-MSA-CS nanoparticles have toxic effects on HepG2 and K562 cells but no effect on healthy noncancerous cells [174]. Therefore, the use of this nanoparticle can be a promising treatment based on NO therapy in cancers such as leukemia.
FA-CS-PTX-SPION: nanocarrier for paclitaxel drug delivery
Most common cancer treatments and medications have devastating side effects and are not specific enough for tumor cells. Today, there is a lot of research to achieve and provide new methods of treatment that, while effective, have minimal side effects. Tumor drug delivery is one of the new treatment strategies that has been welcomed by researchers in recent years. In this method, a specific drug is placed inside a biocompatible compound. This capsule-like structure targets cancer cells and gradually releases the drug into the environment [186,187,188]. Therefore, in these conditions, healthy non-cancerous cells are protected from destructive effects, and on the other hand, the toxicity of the drug to cancer cells increases. In fact, it is relatively safer and more effective. There have been many studies on the use of chitosan biocompatible biopolymers in drug delivery systems, and very good results have been obtained. This polysaccharide has the ability to absorb proteins and metals, and due to its adhesive properties to the mucosa, it can increase the absorption of the drug in the tissues and control its release [186, 189,190,191,192]. The role of magnetic nanoparticles, such as super-magnetic iron oxide nanoparticles (SPION), in cell isolation processes, cell apoptosis, and enzyme inactivation has been extensively investigated. The results show that SPION performs well in drug delivery systems and genes [52, 186, 193,194,195,196,197].
Paclitaxel (PTX) is a drug used to treat a variety of cancers, such as cervical, breast, pancreatic, lung, ovarian carcinoma, head and neck carcinoma, and acute leukemia. Lack of water solubility, low biocompatibility and resistance of cancer cells to Paclitaxel are some of the problems in the use of this drug [198,199,200]. So far, many efforts have been made to provide appropriate methods for using this drug, one of which is the efficient use of “FA-CS-PTX-SPION” nanoparticles, which Al-Musawi and his colleagues succeeded in designing using chitosan and SPION [186, 201,202,203]. By loading Paclitaxel into these nanoparticles, they were able to provide a more effective treatment for leukemia patients [186]. They concluded that FA-CS-PTX-SPION by targeting leukemia cells induced apoptosis in them and did not have a detrimental effect on normal noncancerous cells [186]. Therefore, the use of FA-CS-PTX-SPION can be considered as a new safe and effective treatment method in leukemia patients.
Chitosan nanoparticles: ROS-dependent cell death
Reports indicate that a sudden and rapid increase in the amount of reactive oxygen species (ROS) in cancer cells makes them irreversibly vulnerable to external factors. Free oxygen radicals act as a second messenger in various signaling pathways that regulate the activity of enzymes involved in cell death and play an important role in regulating apoptosis [150, 204,205,206]. Sarangapani et al. [150] showed that the use of chitosan nanoparticles induces selective induction of apoptosis in leukemia cells [150]. Chitosan nanoparticles stimulate apoptosis by inducing oxidative stress by reducing glutathione and increasing ROS in cancer cells, including leukemia [150]. CH-AuNPs are another chitosan nanoparticles designed by Carolina et al. [20]. They used gold nanoparticles to design these new nanoparticles. Their findings show that treatment T-acute lymphocytic leukemia cells (CEM) and chronic myeloid leukemia cells (K562) with CH-AuNPs greatly increases the production of reactive oxygen species (ROS) and damages mitochondria and cell nuclei. They also found that CH-AuNPs induced apoptotic cell death in T-acute lymphocytic leukemia cells and induced necrotic cell death in chronic myeloid leukemia cells. These nanoparticles do not affect healthy non-cancerous cells [20]. Therefore, these nanoparticles have pro-apoptotic properties and use of them is a promising treatment for cancer cells that has no detrimental effects on healthy cells.
Chitosan-nanoparticles-linked zinc (Zn-CS NPs): apoptosis inducer
Zinc is one of the body’s essential nutrients that has antioxidant properties and is very valuable for participating in the biosynthesis of proteins and DNA. Zinc is involved in the proper regulation of most cellular functions such as erythrocytes, bone cells, DNA replication and RNA transcription, neutrophils, interferon gamma secretion, and genetic division in the cell [3, 207]. Zinc supports the immune system’s response to a variety of chronic diseases such as cardiovascular disease, carcinomas and leukemia. Research has shown that supplements reduce the risk of leukemia. Studies have shown that serum zinc levels are lower in leukemia patients than in healthy individuals. In these patients, the risk of systemic errors in zinc metabolism is higher [3, 4, 208,209,210]. Saravanakumar et al. [3] used zinc and chitosan to design nanoparticles that could serve as new treatments for diseases caused by zinc deficiency and acute leukemia [3]. The results of their study show that after treating leukemia cells with Zn-CS NPs, zinc is gradually released from nanoparticles and activates the apoptosis pathway by targeting cancer cells. Zinc released from ZnCSNPs activates the first apoptotic signal Fas/CD95 and expresses the genes that regulate apoptosis, causing 70% damage to acute T-lymphocyte leukemia and eventually cell death [3].
Chitosan coated anthraquinone nanoparticles: suppressing the cell growth
One of the most widely used anticancer drugs is the anthraquinone (AQ) group, including epirubicin, daunorubicin, mitoxantrone, and doxorubicin [25, 211]. These anti-cancer drugs prevent cancer such as leukemia from progressing by inhibiting cell growth and proliferation [25, 212]. The mechanism of anti-cancer function of anthraquinone is very complex [213, 214]. The DNA interfering agents of these drugs, by placing between two strands of DNA molecules, cause the strands to separate. DNA damage occurs due to production of free radicals especially ROS, in response to inhibition of topoisomerase II and induction of apoptosis by p53 and ROS-induced inhibition of topoisomerase II. Anthraquinone also stimulate apoptosis through mitochondrial pathways, Akt/PKB, and c-Jun N-terminal kinase [25, 215,216,217,218,219].
Redah and colleagues studied acute myeloid leukemia (HL-60) in a research [25]. To increase the effectiveness of anthraquinone and reduce its side effects, they designed chitosan nanoparticles, CS-AQ NPs, and loaded anthraquinone into it. Their findings show that CS-AQ nanoparticles inhibit the proliferation and growth of leukemia cells by stopping the cell cycle in the pre-G0 phase and directing them toward apoptosis [25]. To assess the severity of the nanoparticles toxicity on leukemia cells, they evaluated the amount of released lactate dehydrogenase (LDH) into the cell culture medium. The level of this enzyme increased significantly after 24 h. They found that by increasing the dose of nanoparticles, also the amount of secreted enzymes increased. Therefore, it can be said that these nanoparticles toxicity for leukemia cells depends on the prescribed dose [25]. Cellular studies in the Redah’s research confirm the DNA fragments presence after treatment of leukemia cells with CS-AQ NPs in the cell culture medium. The presence of these fragments supports the apoptosis process in leukemia cells [25]. Therefore, by inhibiting the cell cycle in the pre-G0 phase, these nanoparticles stimulate apoptosis in leukemia cells.
Fe3O4-CMC-genistein nanoparticles: cell growth deterrence and apoptosis induction
Genistein is a soy isoflavone that has anti-cancer properties and can be used as an herbal chemotherapy drug. Genistein can induce apoptosis by inhibiting topoisomerase II and inhibit cell proliferation [220,221,222,223,224]. Many attempts have been made to discover a suitable method that can increase the effects of genistein. One of these methods is the use of magnetic nanoparticles with carboxymethylated chitosan (CMC) designed by Ghasemi et al. [38]. Magnetic nanoparticles, including SPIONs, are widely used in drug delivery systems due to their ease of synthesis, biocompatibility and their ability to absorb a variety of drugs [225,226,227,228,229]. By designing “Fe3O4-CMC-genistein” nanoparticles, Ghasemi and colleagues were able to amplify the anti-cancer effects of genistein at a lower dose in leukemia cells so that other healthy cells could be protected from its effects [38]. Their results show that the gradual secretion of genistein from these nanoparticles suppresses the growth of leukemia cells and stimulates apoptosis in them for a long time, and SPIONs and carboxymethylated chitosan enhance this genistein function [38].
Ag NPs-chitosan: cytotoxicity effect
Metal nanoparticles are a good choice for drug delivery due to their wide surface area. In recent years, among the various types of metal nanoparticles, silver nanoparticles have received more attention due to their antimicrobial, antioxidant and non-toxic potential for healthy cells. Silver nanoparticles have also shown amazing anti-cancer effects [230,231,232,233,234]. Hemmati and colleagues first designed silver nanoparticles using chitosan, which have anti-cancer properties against mouse leukemia cells. The results of their study show that chitosan enhances the antioxidant and anti-cancer potentials of Ag NPs [230]. Therefore, Ag NPs-chitosan can be used as a chemotherapeutic drug in the treatment of leukemia, although the implementation of this work requires more clinical and human experiments.
Conclusions
Due to the unsuccessful therapeutic and diagnostic procedures, leukemia is considered as a fatal disease. The conventional treatments for leukemia are invasive and toxic to the immune system. Furthermore, due to their nonspecific action, a reduction in the life-quality of patients is common. Since ancient times, the use of natural ingredients to treat diseases such as cancer has been common and has helped scientists to discover and develop more effective drugs in this field [31, 235, 236]. Chitosan is an example of these natural compounds that is non-toxic and biocompatible [31]. Due to the flexibility of chitosan, researchers use it to design and manufacture various nanoparticles for drug delivery purposes [237].
However, chitosan applications are not limited to drug delivery and it has shown a great potential for bone marrow transplant. For instance, an injectable hydrogel based on dextran and chitosan is reported to be effective for growth and differentiation of bone marrow derived mesenchymal stem cells [238]. Another study has also indicated that chitosan and collagen- based scaffolds impregnated with bone marrow mesenchymal stem cells improve neuropathological injury in rats with traumatic brain injury [239]. Currently, there is no evidence showing the effectiveness of chitosan in bone marrow or hematopoietic stem cell transplant in leukemia. However, further studies in this area may reveal new opportunities for treating leukemia patients.
According to the reviewed evidence, chitosan-based nanoparticles are great candidates for being used in the establishment of drug delivery systems for leukemia. Specifically targeting cancerous cells, increasing the efficacy of chemotherapeutic drugs, inducing apoptotic, and suppressing the growth of cancerous cells are some of the properties which makes these nanoparticles suitable as a replacement of conventional therapies. Interestingly, except for drugs, it seems that delivering siRNAs and mercaptosuccinic acid as a NO donor might also be effective for overcoming leukemia; However, these methods are not well-investigated and further evidence are required in these fields.
Overall, nanomedicine is upgrading our therapeutic approaches against cancer and thus, it is expected that in coming years, it would take the place of current risky methods. In this regard, we think that chitosan would be a great aid for facilitating this process, at least in the case of leukemia.
Future directions
In our knowledge, chitosan applications are not limited to drug delivery and it has shown a great potential for bone marrow transplant. For instance, an injectable hydrogel based on dextran and chitosan is reported to be effective for growth and differentiation of bone marrow derived mesenchymal stem cells [238]. Another study has also indicated that chitosan and collagen- based scaffolds impregnated with bone marrow mesenchymal stem cells improve neuropathological injury in rats with traumatic brain injury [239]. Currently, there is no evidence showing the effectiveness of chitosan in bone marrow or hematopoietic stem cell transplant in leukemia. However, further studies in this area may reveal new opportunities for treating leukemia patients. Furthermore, we still have a long way since we can widely use chitosan nanostructures in clinics; however, more human studies would facilitate this way and speed up the process of finding novel therapies for treating leukemia.
Availability of data and materials
Not applicable.
References
Yeh MY, Shih YL, Chung HY, Chou J, Lu HF, Liu CH, et al. Chitosan promotes immune responses, ameliorating total mature white blood cell numbers, but increases glutamic oxaloacetic transaminase and glutamic pyruvic transaminase, and ameliorates lactate dehydrogenase levels in leukemia mice in vivo. Mol Med Rep. 2017;16:2483–90.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias French-American‐British (FAB) co‐operative group. Br J Haematol. 1976;33:451–8.
Saravanakumar K, Jeevithan E, Chelliah R, Kathiresan K, Wen-Hui W, Oh DH, et al. Zinc-chitosan nanoparticles induced apoptosis in human acute T-lymphocyte leukemia through activation of tumor necrosis factor receptor CD95 and apoptosis-related genes. Int J Biol Macromol. 2018;119:1144–53.
Eby GA. Treatment of acute lymphocytic leukemia using zinc adjuvant with chemotherapy and radiation–a case history and hypothesis. Med Hypotheses. 2005;64:1124–6.
Rathee R, Vashist M, Kumar A, Singh S. Incidence of acute and chronic forms of leukemia in Haryana. Int J Pharm Pharm Sci. 2014;6:323–5.
Arora R, Eden T, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer. 2009;46:264.
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30.
Pui C-H, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–48.
Fröhling S, Döhner H. Chromosomal abnormalities in cancer. N Engl J Med. 2008;359:722–34.
Pui C-H, editor. Acute lymphoblastic leukemia: introduction. Seminars in hematology. Bethesda: NIH Public Access; 2009.
Bain B. Acute leukemia cytology, cytochemistry and the FAB classification. Leukemia diagnosis 2a ed. Oxford: Blackwell Science; 1999. pp. 1–52.
Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13:790–801.
Society AC. Available from: Cancer, Facts, Fig. 2021; 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html.
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018;93:442–59.
Kaplan JA. Leukemia in children. Pediatr Rev. 2019;40:319–31.
Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87.
Magrath I, Litvak J. Cancer in developing countries: opportunity and challenge. JNCI J Natl Cancer Inst. 1993;85:862–74.
Kasthuri A, Jaiprakash M, Panicker N, Gupta M, Rajoor G, Basu S, et al. A clinical study of adult leukaemias. J Assoc Phys India. 1990;38:403–7.
Martinez-Torres A-C, Quiney C, Attout T, Boullet H, Herbi L, Vela L, et al. CD47 agonist peptides induce programmed cell death in refractory chronic lymphocytic leukemia B cells via PLCγ1 activation: evidence from mice and humans. PLoS Med. 2015;12:e1001796.
Carolina A, Martínez T, Yarimet H, Lorenzo A, Gerardo M, García J, et al. Chitosan gold nanoparticles induce different ROS-dependent cell death modalities in leukemic cells. Int J Nanomed. 2019;14:7173–90.
Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7:e577-e.
Elgarten CW, Aplenc R. Pediatric acute myeloid leukemia: updates on biology, risk stratification, and therapy. Curr Opin Pediatr. 2020;32:57–66.
Juliusson G, Hough R. Leukemia. Progress Tumor Res. 2016;43:87–100.
Shafabakhsh R, Reiter RJ, Mirzaei H, Teymoordash SN, Asemi Z. Melatonin: a new inhibitor agent for cervical cancer treatment. J Cell Physiol. 2019;234:21670–82.
Redah Alassaif F, Redah Alassaif E, Rani Chavali S, Dhanapal J. Suppressing the growth of HL-60 acute myeloid leukemia cells by chitosan coated anthraquinone nanoparticles in vitro. Int J Polym Mater Polym Biomater. 2019;68:819–26.
Mondal S, Bandyopadhyay S, Ghosh KM, Mukhopadhyay S, Roy S, Mandal C. Natural products: promising resources for cancer drug discovery. Anti Cancer Agents Med Chem Formerly Curr Med Chem Anti Cancer Agents. 2012;12:49–75.
Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352–62.
Lecumberri E, Dupertuis YM, Miralbell R, Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr. 2013;32:894–903.
Kukreja A, Wadhwa N, Tiwari A. Therapeutic role of resveratrol and piceatannol in disease prevention. J Blood Disord Transfus. 2014;5:9.
Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–68.
Eslahi M, Sadoughi F, Asemi Z, Yousefi B, Mansournia MA, Hallajzadeh J. Chitosan and Wnt/2-catenin signaling pathway in different cancers. Comb Chem High Throughput Screen. 2020;24:1323–31.
Shafabakhsh R, Asemi Z. Quercetin: a natural compound for ovarian cancer treatment. J Ovar Res. 2019;12:1–9.
Honari M, Shafabakhsh R, Reiter RJ, Mirzaei H, Asemi Z. Resveratrol is a promising agent for colorectal cancer prevention and treatment: focus on molecular mechanisms. Cancer Cell Int. 2019;19:1–8.
Hoseini A, Namazi G, Farrokhian A, Reiner Ž, Aghadavod E, Bahmani F, et al. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019;10:6042–51.
Li X, Dong W, Nalin AP, Wang Y, Pan P, Xu B, et al. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. OncoImmunology. 2018;7:e1431085.
Toan NV, Ng CH, Aye KN, Trang TS, Stevens WF. Production of high-quality chitin and chitosan from preconditioned shrimp shells. J Chem Technol Biotechnol Int Res Process Environ Clean Technol. 2006;81:1113–8.
Li J, Cai C, Li J, Li J, Li J, Sun T, et al. Chitosan-based nanomaterials for drug delivery. Molecules. 2018;23:2661.
Ghasemi Goorbandi R, Mohammadi MR, Malekzadeh K. Synthesizing efficacious genistein in conjugation with superparamagnetic Fe3O4 decorated with bio-compatible carboxymethylated chitosan against acute leukemia lymphoma. Biomater Res. 2020;24:9.
Sheng J, Han L, Qin J, Ru G, Li R, Wu L, et al. N-trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl Mater Interfaces. 2015;7:15430–41.
Şenel S, McClure SJ. Potential applications of chitosan in veterinary medicine. Adv Drug Deliv Rev. 2004;56:1467–80.
Mourya V, Inamdar NN, Tiwari A. Carboxymethyl chitosan and its applications. Adv Mater Lett. 2010;1:11–33.
Motiei M, Kashanian S, Lucia LA, Khazaei M. Intrinsic parameters for the synthesis and tuned properties of amphiphilic chitosan drug delivery nanocarriers. J Control Release. 2017;260:213–25.
Zhu A, Chan-Park MB, Dai S, Li L. The aggregation behavior of O-carboxymethylchitosan in dilute aqueous solution. Colloids Surf B. 2005;43:143–9.
Lee E, Kim H, Lee I-H, Jon S. In vivo antitumor effects of chitosan-conjugated docetaxel after oral administration. J Control Release. 2009;140:79–85.
No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74:65–72.
Yuan W-P, Liu B, Liu C-H, Wang X-J, Zhang M-S, Meng X-M, et al. Antioxidant activity of chito-oligosaccharides on pancreatic islet cells in streptozotocin-induced diabetes in rats. World J Gastroenterol WJG. 2009;15:1339.
Francesko A, Tzanov T. Chitin, chitosan and derivatives for wound healing and tissue engineering. Biofunctionalization of polymers and their applications. Berlin: Springer; 2010. p. 1–27.
Yeh M-Y, Wu M-F, Shang H-S, Chang J-B, Shih Y-L, Chen Y-L, et al. Effects of chitosan on xenograft models of melanoma in C57BL/6 mice and hepatoma formation in SCID mice. Anticancer Res. 2013;33:4867–73.
Xie W, Xu P, Liu Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett. 2001;11:1699–701.
Hasegawa M, Yagi K, Iwakawa S, Hirai M. Chitosan induces apoptosis via caspase-3 activation in bladder tumor cells. Jpn J Cancer Res. 2001;92:459–66.
Chen Q, Liu S-Q, Du Y-M, Peng H, Sun L-P. Carboxymethyl-chitosan protects rabbit chondrocytes from interleukin-1β-induced apoptosis. Eur J Pharmacol. 2006;541:1–8.
Nagpal K, Singh SK, Mishra DN. Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull. 2010;58:1423–30.
Ilhan G, Karakus S, Andic N. Risk factors and primary prevention of acute leukemia. Asian Pac J Cancer Prev. 2006;7:515.
Kasim K, Levallois P, Abdous B, Auger P, Johnson KC. Lifestyle factors and the risk of adult leukemia in Canada. Cancer Causes Control. 2005;16:489–500.
Greaves M. Aetiology of acute leukaemia. Lancet. 1997;349:344–9.
Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355:165–9.
Lange B. The management of neoplastic disorders of haematopoeisis in children with Down’s syndrome. Br J Haematol. 2000;110:512–24.
Nakashima K, Hasegawa D, Tomizawa D, Miyamura T, Hama A, Iwamoto S, et al. Characteristics and outcomes of children with acute myeloid leukemia and Down syndrome who are ineligible for clinical trials due to severe comorbidities. Pediatr Blood Cancer. 2019;66:e27942.
Stiller C, Chessells J, Fitchett M. Neurofibromatosis and childhood leukaemia/lymphoma: a population-based UKCCSG study. Br J Cancer. 1994;70:969–72.
Altieri A, Bermejo JL, Hemminki K. Familial risk for non-Hodgkin lymphoma and other lymphoproliferative malignancies by histopathologic subtype: the Swedish Family-Cancer Database. Blood. 2005;106:668–72.
Altieri A, Chen B, Bermejo JL, Castro F, Hemminki K. Familial risks and temporal incidence trends of multiple myeloma. Eur J Cancer. 2006;42:1661–70.
Shaw M, Eden O, Grace E, Ellis P. Acute lymphoblastic leukemia and Klinefelter’s syndrome. Pediatr Hematol Oncol. 1992;9:81–5.
Horsman DE, Pantzar JT, Dill FJ, Kalousek DK. Klinefelter’s syndrome and acute leukemia. Cancer Genet Cytogenet. 1987;26:375–6.
Özyörük D, Kocayozgat A, Yaman-Bajin İ, Çetindağ F, Oğuz-Erdoğan AS, Güneş A. A synchronous occurrence of bifocal intracranial germinoma and bilateral testicular epidermoid cyst in an adolescent patient with Klinefelters syndrome. Turk J Pediatr. 2019;61:456–9.
Poppe B, Van Limbergen H, Van Roy N, Vandecruys E, De Paepe A, Benoit Y, et al. Chromosomal aberrations in Bloom syndrome patients with myeloid malignancies. Cancer Genet Cytogenet. 2001;128:39–42.
Schoen EJ, Shearn MA. Immunoglobulin deficiency in Bloom’s syndrome. Am J Dis Child. 1967;113:594–6.
LANDAU JW, Sasaki M, NEWCOMER VD. Bloom’s syndrome: the syndrome of telangiectatic erythema and growth retardation. Arch Dermatol. 1966;94:687–94.
Cunniff C, Djavid AR, Carrubba S, Cohen B, Ellis NA, Levy CF, et al. Health supervision for people with Bloom syndrome. Am J Med Genet Part A. 2018;176:1872–81.
Ratnaparkhe M, Hlevnjak M, Kolb T, Jauch A, Maass K, Devens F, et al. Genomic profiling of acute lymphoblastic leukemia in ataxia telangiectasia patients reveals tight link between ATM mutations and chromothripsis. Leukemia. 2017;31:2048–56.
Schoenaker M, Suarez F, Szczepanski T, Mahlaoui N, Loeffen J. Treatment of acute leukemia in children with ataxia telangiectasia (AT). Eur J Med Genet. 2016;59:641–6.
Du W, Li X, Wilson AF, Pang Q. A small molecule p53 activator attenuates Fanconi anemia leukemic stem cell proliferation. Stem Cell Res Ther. 2018;9:145.
Maung KZY, Leo PJ, Bassal M, Casolari DA, Gray JX, Bray SC, et al. Rare variants in Fanconi anemia genes are enriched in acute myeloid leukemia. Blood Cancer J. 2018;8:1–5.
Chatla S, Wilson AF, Pang Q. Inactivation of the NHEJ Activity of DNA-PKcs Prevents Fanconi Anemia Pre-Leukemic HSC Expansion. Int J Stem Cells. 2019;12:457.
Gilliland DG, editor. Molecular genetics of human leukemias: new insights into therapy. Seminars in hematology. Amsterdam: Elsevier; 2002.
Alcalay M, Orleth A, Sebastiani C, Meani N, Chiaradonna F, Casciari C, et al. Common themes in the pathogenesis of acute myeloid leukemia. Oncogene. 2001;20:5680–94.
Gocho Y, Yang JJ. Genetic defects in hematopoietic transcription factors and predisposition to acute lymphoblastic leukemia. Blood. 2019;134:793–7.
Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7:e577.
Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36.
Rinsky RA, Smith AB, Hornung R, Filloon TG, Young RJ, Okun AH, et al. Benzene and leukemia. N Engl J Med. 1987;316:1044–50.
McHale CM, Zhang L, Smith MT. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis. 2012;33:240–52.
Rinsky RA, Young RJ, Smith AB. Leukemia in benzene workers. Am J Ind Med. 1981;2:217–45.
Fiebelkorn S, Meredith C. Estimation of the leukemia risk in human populations exposed to benzene from tobacco smoke using epidemiological data. Risk Anal. 2018;38:1490–501.
Cancer IAfRo. Evaluation of the carcinogenic risk of chemicals to humans. Chemicals, industrial processes and industries associated with cancer in humans. Lyon: International Agency for Research on Cancer location; 1982.
Brandt L. Exposure to organic solvents and risk of haematological malignancies. Leuk Res. 1992;16:67–70.
Yaris F, Dikici M, Akbulut T, Yaris E, Sabuncu H. Story of benzene and leukemia: epidemiologic approach of Muzaffer Aksoy. J Occup Health. 2004;46:244–7.
Linos A, Kyle RA, O’Fallon WM, Kurland LT. A case-control study of occupational exposures and leukaemia. Int J Epidemiol. 1980;9:131–6.
Girard R, Revol L. Incidence of expose to benzene in severe hemopathies. Nouvelle revue francaise d’hematologie. 1970;10:477–83.
Ott MG, Townsend JC, Fishbeck WA, Langner RA. Mortality among individuals occupationally exposed to benzene. Arch Environ Health Int J. 1978;33:3–10.
Infante P, Wagoner J, Rinsky R, Young R. Leukaemia in benzene workers. Lancet. 1977;310:76–8.
Adegoke OJ, Blair A, Shu XO, Sanderson M, Jin F, Dosemeci M, et al. Occupational history and exposure and the risk of adult leukemia in Shanghai. Ann Epidemiol. 2003;13:485–94.
Janitz AE, Campbell JE, Magzamen S, Pate A, Stoner JA, Peck JD. Benzene and childhood acute leukemia in Oklahoma. Environ Res. 2017;158:167–73.
Kerzic PJ, Irons RD. Distribution of chromosome breakpoints in benzene-exposed and unexposed AML patients. Environ Toxicol Pharmacol. 2017;55:212–6.
Austin H, Delzell E, Cole P. Benzene and leukemia. A review of the literature and a risk assessment. Am J Epidemiol. 1988;127:419–39.
Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, et al. Cancer incidence in atomic bomb survivors. Part III: leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat Res. 1994;137:68–97.
Shimada T, Saito T, Okadome M, Shimamoto K, Ariyoshi K, Eto T, et al. Secondary leukemia after chemotherapy and/or radiotherapy for gynecologic neoplasia. Int J Gynecol Cancer. 2014;24:178–83.
Wright JD, St Clair CM, Deutsch I, Burke WM, Gorrochurn P, Sun X, et al. Pelvic radiotherapy and the risk of secondary leukemia and multiple myeloma. Cancer. 2010;116:2486–92.
Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr. 1997;66:460S-3S.
Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obes Rev. 2001;2:131–40.
Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist. 2010;15:1083.
Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122:1418–21.
Butturini AM, Dorey FJ, Lange BJ, Henry DW, Gaynon PS, Fu C, et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25:2063–9.
Umemoto Y, Tsuji K, Yang F-C, Ebihara Y, Kaneko A, Furukawa S, et al. Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood J Am Soc Hematol. 1997;90:3438–43.
Bruserud O, Huang T-S, Glenjen N, Gjertsen BT, Foss B. Leptin in human acute myelogenous leukemia: studies of in vivo levels and in vitro effects on native functional leukemia blasts. Haematologica. 2002;87:584–95.
Konopleva M, Mikhail A, Estrov Z, Zhao S, Harris D, Sanchez-Williams G, et al. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: proliferative and anti-apoptotic activities. Blood J Am Soc Hematol. 1999;93:1668–76.
Ross JA, Kasum CM, Davies SM, Jacobs DR, Folsom AR, Potter JD. Diet and risk of leukemia in the Iowa Women’s Health Study. Cancer Epidemiol Prev Biomark. 2002;11:777–81.
Kwiatkowski A. Dietary and other environmental risk factors in acute leukaemias: a case-control study of 119 patients. Eur J Cancer Prev. 1993;2:139–46.
Friedman GD. Cigarette smoking, leukemia, and multiple myeloma. Ann Epidemiol. 1993;3:425–8.
Kane E, Roman E, Cartwright R, Parker J, Morgan G. Tobacco and the risk of acute leukaemia in adults. Br J Cancer. 1999;81:1228–33.
Siegel M. Smoking and leukemia: evaluation of a causal hypothesis. Am J Epidemiol. 1993;138:1–9.
Adami J, Nyrén O, Bergström R, Ekbom A, Engholm G, Englund A, et al. Smoking and the risk of leukemia, lymphoma, and multiple myeloma (Sweden). Cancer Causes Control. 1998;9:49–56.
Freedman DS, Tolbert PE, Coates R, Brann EA, Kjeldsberg CR. Relation of cigarette smoking to non-Hodgkin’s lymphoma among middle-aged men. Am J Epidemiol. 1998;148:833–41.
Schnatter AR, Armstrong TW, Thompson LS, Nicolich MJ, Katz AM, Huebner WW, et al. The relationship between low-level benzene exposure and leukemia in Canadian petroleum distribution workers. Environ Health Perspect. 1996;104:1375–9.
Pasqualetti P, Festuccia V, Acitelli P, Collacciani A, Giusti A, Casale R. Tobacco smoking and risk of haematological malignancies in adults: a case–control study. Br J Haematol. 1997;97:659–62.
Mejía Aranguré JM, Álvarez MCO, Gutiérrez AF. Epidemiología de las leucemias agudas en niños. Parte 1. Revista Médica del Instituto Mexicano del Seguro Social. 2005;43:323–33.
Engeland A, Bjørge T, Haldorsen T, Tretli S. Use of multiple primary cancers to indicate associations between smoking and cancer incidence: an analysis of 500,000 cancer cases diagnosed in Norway during 1953–93. Int J Cancer. 1997;70:401–7.
Sandler DP, Shore DL, Anderson JR, Davey FR, Arthur D, Mayer RJ, et al. Cigarette smoking and risk of acute leukemia: associations with morphology and cytogenetic abnormalities in bone marrow. JNCI J Natl Cancer Inst. 1993;85:1994–2003.
Humans IWGotEoCRt, Organization WH. Cancer IAfRo. Tobacco smoke and involuntary smoking. Lyon: Iarc; 2004.
Smoke T, Smoking I. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC; 2004. pp. 1–1452.
Health UDo, Services H. The health consequences of smoking. Nicotine addiction: a report of the Surgeon General; 1988.
Stagnaro E, Ramazzotti V, Crosignani P, Fontana A, Masala G, Miligi L, et al. Smoking and hematolymphopoietic malignancies. Cancer Causes Control. 2001;12:325–34.
Clavel J, Mandereau L, Cordier S, Goaster CL, Heamon D, Conso F, et al. Hairy cell leukaemia, occupation, and smoking. Br J Haematol. 1995;91:154–61.
Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31:603–32.
Wei X, Liao J, Davoudi Z, Zheng H, Chen J, Li D, et al. Folate receptor-targeted and gsh-responsive carboxymethyl chitosan nanoparticles containing covalently entrapped 6-mercaptopurine for enhanced intracellular drug delivery in leukemia. Mar Drugs. 2018;16:439.
Kas HS. Chitosan: properties, preparations and application to microparticulate systems. J Microencapsul. 1997;14:689–711.
Pillai C, Paul W, Sharma CP. Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci. 2009;34:641–78.
Azuma K, Ifuku S, Osaki T, Okamoto Y, Minami S. Preparation and biomedical applications of chitin and chitosan nanofibers. J Biomed Nanotechnol. 2014;10:2891–920.
Azuma K, Osaki T, Minami S, Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J Funct Biomater. 2015;6:33–49.
Shariatinia Z. Pharmaceutical applications of chitosan. Adv Coll Interface Sci. 2019;263:131–94.
Khoushab F, Yamabhai M. Chitin research revisited. Mar Drugs. 2010;8:1988–2012.
Coviello T, Matricardi P, Marianecci C, Alhaique F. Polysaccharide hydrogels for modified release formulations. J Control Release. 2007;119:5–24.
Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60:1650–62.
Li P, Dai Y-N, Zhang J-P, Wang A-Q, Wei Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int J Biomed Sci IJBS. 2008;4:221.
Zhao M, Hu B, Gu Z, Joo K-I, Wang P, Tang Y. Degradable polymeric nanocapsule for efficient intracellular delivery of a high molecular weight tumor-selective protein complex. Nano Today. 2013;8:11–20.
Zeng T, Zhang Y, Yan Q, Huang Z, Zhang L, Yi X, et al. Construction and in vitro evaluation of enzyme nanoreactors based on carboxymethyl chitosan for arginine deprivation in cancer therapy. Carbohydr Polym. 2017;162:35–41.
Davoudi Z, Rabiee M, Houshmand B, Eslahi N, Khoshroo K, Rasoulianboroujeni M, et al. Development of chitosan/gelatin/keratin composite containing hydrocortisone sodium succinate as a buccal mucoadhesive patch to treat desquamative gingivitis. Drug Dev Ind Pharm. 2018;44:40–55.
Nogueira DR, Tavano L, Mitjans M, Pérez L, Infante MR, Vinardell MP. In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials. 2013;34:2758–72.
Rekha M, Sharma CP. Simultaneous effect of thiolation and carboxylation of chitosan particles towards mucoadhesive oral insulin delivery applications: an in vitro and in vivo evaluation. J Biomed Nanotechnol. 2015;11:165–76.
Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9:53.
Syed A, Chan WC. How nanoparticles interact with cancer cells. Nanotechnol Based Precis Tools Detect Treat Cancer. 2015;166:227–44.
Adhikari HS, Yadav PN. Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int J Biomater. 2018. https://doi.org/10.1155/2018/2952085.
Du H, Yang X, Zhai G. Design of chitosan-based nanoformulations for efficient intracellular release of active compounds. Nanomedicine. 2014;9:723–40.
Fonseca-Santos B, Chorilli M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater Sci Eng C. 2017;77:1349–62.
Hu R, Zheng H, Cao J, Davoudi Z, Wang Q. Synthesis and in vitro characterization of carboxymethyl chitosan-CBA-doxorubicin conjugate nanoparticles as pH-sensitive drug delivery systems. J Biomed Nanotechnol. 2017;13:1097–105.
Upadhyaya L, Singh J, Agarwal V, Tewari RP. Biomedical applications of carboxymethyl chitosans. Carbohydr Polym. 2013;91:452–66.
Chakraborty SP, Sahu SK, Pramanik P, Roy S. Biocompatibility of folate—modified chitosan nanoparticles. Asian Pac J Trop Biomed. 2012;2:215–9.
Gåserød O, Jolliffe IG, Hampson FC, Dettmar PW, Skjåk-Bræk G. The enhancement of the bioadhesive properties of calcium alginate gel beads by coating with chitosan. Int J Pharm. 1998;175:237–46.
George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Control Release. 2006;114:1–14.
Aam BB, Heggset EB, Norberg AL, Sørlie M, Vårum KM, Eijsink VG. Production of chitooligosaccharides and their potential applications in medicine. Mar Drugs. 2010;8:1482–517.
Kim M-S, Sung M-J, Seo S-B, Yoo S-J, Lim W-K, Kim H-M. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid β peptide and interleukin-1β. Neurosci Lett. 2002;321:105–9.
Sarangapani S, Patil A, Ngeow YK, Elsa Mohan R, Asundi A, Lang MJ. Chitosan nanoparticles’ functionality as redox active drugs through cytotoxicity, radical scavenging and cellular behaviour. Integr Biol (United Kingdom). 2018;10:313–24.
Qi L, Xu Z, Jiang X, Li Y, Wang M. Cytotoxic activities of chitosan nanoparticles and copper-loaded nanoparticles. Bioorg Med Chem Lett. 2005;15:1397–9.
Zargar V, Asghari M, Dashti A. A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015;2:204–26.
Hu Z, Zheng H, Li D, Xiong X, Tan M, Huang D, et al. Self-assembled nanoparticles based on folic acid modified carboxymethyl chitosan conjugated with targeting antibody. J Wuhan Univ Technol Mater Sci Ed. 2016;31:446–53.
Saboktakin MR, Tabatabaie RM, Maharramov A, Ramazanov MA. Synthesis and in vitro evaluation of carboxymethyl starch–chitosan nanoparticles as drug delivery system to the colon. Int J Biol Macromol. 2011;48:381–5.
Mathew ME, Mohan JC, Manzoor K, Nair S, Tamura H, Jayakumar R. Folate conjugated carboxymethyl chitosan–manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohydr Polym. 2010;80:442–8.
Wang F, Zhang D, Duan C, Jia L, Feng F, Liu Y, et al. Preparation and characterizations of a novel deoxycholic acid–O-carboxymethylated chitosan–folic acid conjugates and self-aggregates. Carbohydr Polym. 2011;84:1192–200.
Yang S-J, Lin F-H, Tsai K-C, Wei M-F, Tsai H-M, Wong J-M, et al. Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX accumulation in colorectal cancer cells. Bioconjug Chem. 2010;21:679–89.
Arami S, Rashidi MR, Mahdavi M, Fathi M, Entezami AA. Synthesis and characterization of Fe3O4-PEG-LAC-chitosan-PEI nanoparticle as a survivin siRNA delivery system. Hum Exp Toxicol. 2017;36:227–37.
Plianwong S, Opanasopit P, Ngawhirunpat T, Rojanarata T. Fast, facile and ethidium bromide-free assay based on the use of adsorption indicator for the estimation of polyethylenimine to nucleic acid ratio of complete polyplex assembly for gene delivery. Talanta. 2013;115:241–5.
Debus H, Baumhof P, Probst J, Kissel T. Delivery of messenger RNA using poly (ethylene imine)–poly (ethylene glycol)-copolymer blends for polyplex formation: Biophysical characterization and in vitro transfection properties. J Control Release. 2010;148:334–43.
Aigner A. Applications of RNA interference: current state and prospects for siRNA-based strategies in vivo. Appl Microbiol Biotechnol. 2007;76:9–21.
Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–502.
Wang J, Dou B, Bao Y. Efficient targeted pDNA/siRNA delivery with folate–low-molecular-weight polyethyleneimine–modified pullulan as non-viral carrier. Mater Sci Eng C. 2014;34:98–109.
Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86.
Ghanbari P, Mohseni M, Tabasinezhad M, Yousefi B, Saei AA, Sharifi S, et al. Inhibition of survivin restores the sensitivity of breast cancer cells to docetaxel and vinblastine. Appl Biochem Biotechnol. 2014;174:667–81.
Zhang J, Li X, Huang L. Non-viral nanocarriers for siRNA delivery in breast cancer. J Control Release. 2014;190:440–50.
Jere D, Jiang H-L, Kim Y-K, Arote R, Choi Y-J, Yun C-H, et al. Chitosan-graft-polyethylenimine for Akt1 siRNA delivery to lung cancer cells. Int J Pharm. 2009;378:194–200.
Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Targets. 2008;12:463–76.
Liu J, Wang L, Wang J, Zhang L. Simple solvothermal synthesis of hydrophobic magnetic monodispersed Fe3O4 nanoparticles. Mater Res Bull. 2013;48:416–21.
Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Can Res. 2007;67:5999–6002.
Song HP, Yang JY, Lo SL, Wang Y, Fan WM, Tang XS, et al. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials. 2010;31:769–78.
Wang C, Ravi S, Martinez GV, Chinnasamy V, Raulji P, Howell M, et al. Dual-purpose magnetic micelles for MRI and gene delivery. J Control Release. 2012;163:82–92.
Noh SM, Park MO, Shim G, Han SE, Lee HY, Huh JH, et al. Pegylated poly-l-arginine derivatives of chitosan for effective delivery of siRNA. J Control Release. 2010;145:159–64.
Pelegrino MT, Silva LC, Watashi CM, Haddad PS, Rodrigues T, Seabra AB. Nitric oxide-releasing nanoparticles: synthesis, characterization, and cytotoxicity to tumorigenic cells. J Nanopart Res. 2017;19:57.
Basudhar D, Cheng RC, Bharadwaj G, Ridnour LA, Wink DA, Miranda KM. Chemotherapeutic potential of diazeniumdiolate-based aspirin prodrugs in breast cancer. Free Radic Biol Med. 2015;83:101–14.
Kim J, Saravanakumar G, Choi HW, Park D, Kim WJ. A platform for nitric oxide delivery. J Mater Chem B. 2014;2:341–56.
Howard MD, Hood ED, Zern B, Shuvaev VV, Grosser T, Muzykantov VR. Nanocarriers for vascular delivery of anti-inflammatory agents. Annu Rev Pharmacol Toxicol. 2014;54:205–26.
Lasker GF, Pankey EA, Kadowitz PJ. Modulation of soluble guanylate cyclase for the treatment of erectile dysfunction. Physiology. 2013;28:262–9.
Ignarro LJ. Nitric oxide: biology and pathobiology. Cambridge: Academic press; 2000.
Bonavida B, Garban H. Nitric oxide-mediated sensitization of resistant tumor cells to apoptosis by chemo-immunotherapeutics. Redox Biol. 2015;6:486–94.
Scicinski J, Oronsky B, Ning S, Knox S, Peehl D, Kim MM, et al. NO to cancer: the complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox Biol. 2015;6:1–8.
Seabra B, de Lima A, Calderón R. M. Nitric oxide releasing nanomaterials for cancer treatment: current status and perspectives. Curr Top Med Chem. 2015;15:298–308.
Seabra AB, Justo GZ, Haddad PS. State of the art, challenges and perspectives in the design of nitric oxide-releasing polymeric nanomaterials for biomedical applications. Biotechnol Adv. 2015;33:1370–9.
Seabra A, Kitice N, Pelegrino M, Lancheros C, Yamauchi L, Pinge-Filho P et al, editors. Nitric oxide-releasing polymeric nanoparticles against Trypanosoma cruzi. Journal of Physics: Conference Series; 2015. IOP Publishing.
Sharma K, Chakrapani H. Site-directed delivery of nitric oxide to cancers. Nitric Oxide. 2014;43:8–16.
Al-Musawi S, Kadhim MJ, Hindi NKK. Folated-nanocarrier for paclitaxel drug delivery in leukemia cancer therapy. J Pharm Sci Res. 2018;10:749–54.
Bharali DJ, Siddiqui IA, Adhami VM, Chamcheu JC, Aldahmash AM, Mukhtar H, et al. Nanoparticle delivery of natural products in the prevention and treatment of cancers: current status and future prospects. Cancers. 2011;3:4024–45.
Mackness MI, Mackness B, Durrington PN, Fogelman AM, Berliner J, Lusis AJ, et al. Paraoxonase and coronary heart disease. Curr Opin Lipidol. 1998;9:319–24.
Saloustros E, Mavroudis D, Georgoulias V. Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother. 2008;9:2603–16.
Ahmad M, Manzoor K, Singh S, Ikram S. Chitosan centered bionanocomposites for medical specialty and curative applications: a review. Int J Pharm. 2017;529:200–17.
Patel MP, Patel RR, Patel JK. Chitosan mediated targeted drug delivery system: a review. J Pharm Pharm Sci. 2010;13:536–57.
Muxika A, Etxabide A, Uranga J, Guerrero P, De La Caba K. Chitosan as a bioactive polymer: processing, properties and applications. Int J Biol Macromol. 2017;105:1358–68.
Wahajuddin SA. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomed. 2012;7:3445.
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24–46.
Jin R, Lin B, Li D, Ai H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr Opin Pharmacol. 2014;18:18–27.
Laurent S, Mahmoudi M. Superparamagnetic iron oxide nanoparticles: promises for diagnosis and treatment of cancer. Int J Mol Epidemiol Genet. 2011;2:367.
Zhu L, Ma J, Jia N, Zhao Y, Shen H. Chitosan-coated magnetic nanoparticles as carriers of 5-fluorouracil: preparation, characterization and cytotoxicity studies. Colloids Surf B. 2009;68:1–6.
Aziz K, Nowsheen S, Georgakilas G. A. Nanotechnology in cancer therapy: targeting the inhibition of key DNA repair pathways. Curr Mol Med. 2010;10:626–39.
Khanna C, Rosenberg M, Vail D. A review of paclitaxel and novel formulations including those suitable for use in dogs. J Vet Intern Med. 2015;29:1006–12.
Bergquist PA, Manas D, Hunke WA, Reed RA. Stability and compatibility of tirofiban hydrochloride during simulated Y-site administration with other drugs. Am J Health Syst Pharm. 2001;58:1218–23.
Donyai P, Sewell GJ. Physical and chemical stability of paclitaxel infusions in different container types. J Oncol Pharm Pract. 2006;12:211–22.
Barbuti AM, Chen Z-S. Paclitaxel through the ages of anticancer therapy: exploring its role in chemoresistance and radiation therapy. Cancers. 2015;7:2360–71.
Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Exp Opin Drug Saf. 2007;6:609–21.
Chung EJ, Hwang S-G, Nguyen P, Lee S, Kim J-S, Kim JW, et al. Regulation of leukemic cell adhesion, proliferation, and survival by β-catenin. Blood J Am Soc Hematol. 2002;100:982–90.
Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25:2085–94.
Aranda A, Sequedo L, Tolosa L, Quintas G, Burello E, Castell J, et al. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol in vitro. 2013;27:954–63.
Dhawan D, Chadha VD. Zinc: a promising agent in dietary chemoprevention of cancer. Indian J Med Res. 2010;132:676.
Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S-63S.
Consolo L, Melnikov P, Cônsolo F, Nascimento V, Pontes J. Zinc supplementation in children and adolescents with acute leukemia. Eur J Clin Nutr. 2013;67:1056–9.
Kanter R, Rai K, Muniz F, Michael B, Balkon J, Sawitsky A. Intracellular zinc in chronic lymphocytic leukemia. Clin Immunol Immunopathol. 1982;24:26–32.
Weiss RB (ed). The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19(6):670–86.
Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27:609–30.
Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–41.
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229.
Laurent G, Jaffrézou J-P. Signaling pathways activated by daunorubicin. Blood. 2001;98:913–24.
Xie Ge, Zhu X, Li Q, Gu M, He Z, Wu J, et al. SZ-685 C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. Br J Pharmacol. 2010;159:689–97.
Martín D, Salinas M, Fujita N, Tsuruo T, Cuadrado A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J Biol Chem. 2002;277:42943–52.
Clementi ME, Giardina B, Di Stasio E, Mordente A, Misiti F. Doxorubicin-derived metabolites induce release of cytochrome C and inhibition of respiration on cardiac isolated mitochondria. Anticancer Res. 2003;23:2445.
Perchellet EM, Wang Y, Weber RL, Sperfslage BJ, Lou K, Crossland J, et al. Synthetic 1, 4-anthracenedione analogs induce cytochrome c release, caspase-9,-3, and-8 activities, poly (ADP-ribose) polymerase-1 cleavage and internucleosomal DNA fragmentation in HL-60 cells by a mechanism which involves caspase-2 activation but not Fas signaling. Biochem Pharmacol. 2004;67:523–37.
Mizushina Y, Shiomi K, Kuriyama I, Takahashi Y, Yoshida H. Inhibitory effects of a major soy isoflavone, genistein, on human DNA topoisomerase II activity and cancer cell proliferation. Int J Oncol. 2013;43:1117–24.
Zhang S, Wang Y, Chen Z, Kim S, Iqbal S, Chi A, et al. Genistein enhances the efficacy of cabazitaxel chemotherapy in metastatic castration-resistant prostate cancer cells. Prostate. 2013;73:1681–9.
Nadhanan RR, Skinner J, Chung R, Su Y-W, Howe PR, Xian CJ. Supplementation with fish oil and genistein, individually or in combination, protects bone against the adverse effects of methotrexate chemotherapy in rats. PLoS ONE. 2013;8:e71592.
Yu D, Shin H-S, Lee YS, Lee D, Kim S, Lee YC. Genistein attenuates cancer stem cell characteristics in gastric cancer through the downregulation of Gli1. Oncol Rep. 2014;31:673–8.
CARLO-STELLA C, Regazzi E, Garau D, Mangoni L, Rizzo MT, Bonati A, et al. Effect of the protein tyrosine kinase inhibitor genistein on normal and leukaemic haemopoietic progenitor cells. Br J Haematol. 1996;93:551–7.
Dorniani D, Bin Hussein MZ, Kura AU, Fakurazi S, Shaari AH, Ahmad Z. Preparation and characterization of 6-mercaptopurine-coated magnetite nanoparticles as a drug delivery system. Drug Des Dev Ther. 2013;7:1015.
Hou C-H, Hou S-M, Hsueh Y-S, Lin J, Wu H-C, Lin F-H. The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials. 2009;30:3956–60.
Lin J-J, Chen J-S, Huang S-J, Ko J-H, Wang Y-M, Chen T-L, et al. Folic acid–Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials. 2009;30:5114–24.
Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60:1252–65.
Singh D, McMillan JM, Liu X-M, Vishwasrao HM, Kabanov AV, Sokolsky-Papkov M, et al. Formulation design facilitates magnetic nanoparticle delivery to diseased cells and tissues. Nanomedicine. 2014;9:469–85.
Hemmati S, Zamenian T, Delsooz N, Zangeneh A, Mahdi Zangeneh M. Preparation and synthesis a new chemotherapeutic drug of silver nanoparticle-chitosan composite; chemical characterization and analysis of their antioxidant, cytotoxicity, and anti-acute myeloid leukemia effects in comparison to Daunorubicin in a leukemic mouse model. Appl Organometal Chem. 2020;34:5274.
Haroldsen V, Paulino G, Chi-ham C, Bennett A. Research and adoption of biotechnology strategies could improve California fruit and nut crops. Calif Agric. 2012;66:62–9.
Singh P, Kim YJ, Yang DC. A strategic approach for rapid synthesis of gold and silver nanoparticles by Panax ginseng leaves. Artif Cells Nanomed Biotechnol. 2016;44:1949–57.
Zangeneh MM. Green synthesis and chemical characterization of silver nanoparticles from aqueous extract of Falcaria vulgaris leaves and assessment of their cytotoxicity and antioxidant, antibacterial, antifungal and cutaneous wound healing properties. Appl Organomet Chem. 2019;33:e4963.
Abdel-Fattah W, Ali G. On the anti-cancer activities of silver nanoparticles. J Appl Biotechnol Bioeng. 2018;5:43–6.
Singh A, Fatima K, Srivastava A, Khwaja S, Priya D, Singh A, et al. Anticancer activity of gallic acid template-based benzylidene indanone derivative as microtubule destabilizer. Hoboken: Wiley; 2016.
Kumar BS, Kumar A, Singh J, Hasanain M, Singh A, Fatima K, et al. Synthesis of 2-alkoxy and 2-benzyloxy analogues of estradiol as anti-breast cancer agents through microtubule stabilization. Eur J Med Chem. 2014;86:740–51.
Sadoughi F, Mansournia MA, Mirhashemi SM. The potential role of chitosaŽ based nanoparticles as drug delivery systems in pancreatic cancer. IUBMB Life. 2020;72:872–83.
Nelson VJ, Dinnunhan MFK, Turner PR, Faed JM, Cabral JD. A chitosan/dextran-based hydrogel as a delivery vehicle of human bone-marrow derived mesenchymal stem cells. Biomed Mater. 2017;12:035012.
Yan F, Li M, Zhang HQ, Li GL, Hua Y, Shen Y, et al. Collagen-chitosan scaffold impregnated with bone marrow mesenchymal stem cells for treatment of traumatic brain injury. Neural Regen Res. 2019;14:1780–6.
Sarangapani S, Mohan RE, Patil A, Lang MJ, Asundi A, editors. 3D/4D multiscale imaging in acute lymphoblastic leukemia cells: visualizing dynamics of cell death. In: Proceedings of SPIE—the international society for optical engineering; 2017.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JH, PZ, FS, MS and ZA contributed in conception, design and drafting of the manuscript. All authors approved the final version for submission. JH oversaw the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zivarpour, P., Hallajzadeh, J., Asemi, Z. et al. Chitosan as possible inhibitory agents and delivery systems in leukemia. Cancer Cell Int 21, 544 (2021). https://doi.org/10.1186/s12935-021-02243-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02243-w