Abstract
Long noncoding RNAs (lncRNAs), are transcripts longer than 200 nucleotides that are considered to be vital regulators of many cellular processes, particularly in tumorigenesis and cancer progression. long intergenic non-protein coding RNA 261 (LINC00261), a recently discovered lncRNA, is abnormally expressed in a variety of human malignancies, including pancreatic cancer, gastric cancer, colorectal cancer, lung cancer, hepatocellular carcinoma, breast cancer, laryngeal carcinoma, endometrial carcinoma, esophageal cancer, prostate cancer, choriocarcinoma, and cholangiocarcinoma. LINC00261 mainly functions as a tumor suppressor that regulates a variety of biological processes in the above-mentioned cancers, such as cell proliferation, apoptosis, motility, chemoresistance, and tumorigenesis. In addition, the up-regulation of LINC00261 is closely correlated with both favorable prognoses and many clinical characteristics. In the present review, we summarize recent research documenting the expression and biological mechanisms of LINC00261 in tumor development. These findings suggest that LINC00261, as a tumor suppressor, has bright prospects both as a biomarker and a therapeutic target.
Similar content being viewed by others
Background
It is well-known that cancer is the leading cause of death worldwide [1,2,3]. Although many clinical therapies exist, the death rate for cancer remains high [4, 5] and prognoses remain poor due to a lack of effective biological markers for early diagnosis. Therefore, the identification of novel biomarkers is very important for improved diagnosis and treatment [6, 7], and lncRNAs have recently been shown to be a group of such novel biomarkers [8,9,10].
Noncoding RNAs (ncRNAs), are functional RNAs which don’t encode proteins, including microRNAs (miRNAs), circular RNAs (circRNAs), and lncRNAs functions. Most ncRNAs operate as RNA-protein complexes and exert many cellular functions, including miRNAs and lncRNAs [11]. ncRNAs regulate specific gene expression at the transcriptional level, through regulating transcriptional, post-transcriptional, and post-translational processes [12]. As a result, through their profound effect on protein expression and function, ncRNAs have participated in many cellular events such as proliferation, migration, invasion, differentiation, apoptosis, immune responses, etc. [12]. Considered important regulators of tumor progression, lncRNAs are noncoding RNAs of more than 200 nucleotides [13], that are transcribed by RNA polymerases II and III without the ability to code for proteins [14, 15]. Accumulating evidence indicates that many lncRNAs are abnormally expressed in many tumor types [16,17,18], and play crucial roles in a variety of cellular events, including the regulation of gene transcription [19, 20], protein translation [18, 21], post-transcriptional modification [22, 23], and messenger RNA (mRNA) processing [24, 25]. Most usually, lncRNAs mainly sponge miRNAs and decrease the level of special miRNAs, which accelerate the degradation of mRNAs. In addition, lncRNA expression has been related to the occurrence [26, 27], progression [28, 29], metastases [30, 31], and prognoses of a variety of tumors [32, 33].
The transcription site for LINC00261 is located on the 20th chromosome from site 22,560,552 to 22,578,642 [24, 34]. LINC00261 has been widely reported to be a tumor inhibitor in a variety of cancers, involving in many cellular processes [35, 36]. For example, one study found that LINC00261 was a potential biomarker for endometrial carcinoma prognosis [37]. LINC00261 inhibits tumor-cell growth primarily by inhibiting cell proliferation and promoting cell apoptosis [34, 36]. Additionally, LINC00261 has been shown to reduce tumor-cell invasiveness by inhibiting the epithelial-mesenchymal transition (EMT) [38, 39]. LINC00261 expression has also been reported to reduce the proliferation and migration of breast cancer cells [40]. These studies mainly focused on the anti-tumor functions of LINC00261 and its different roles in the pathogenesis of different tumor types.
The present review has two main objectives. The first is to survey of the most recent LINC00261 studies related to cancer, enlarging the map of its influence and highlighting new research insights. The second is to discuss how these recent studies have guided innovation. It will be greatly helpful for researchers to learn about the general research status.
The upstream mechanism underlying the dysregulation of LINC00261
Many studies have revealed that lncRNAs can be regulated in both gene level and transcription level. At the genome level, both the increased copy number of special oncogene and the chromosome deletion can influence lncRNA level [41, 42]. Additionally, lncRNAs also can be regulated by transcription factor [43], histone modification [44], and the methylation level of promoter region [45]. The lncRNAs stability even can be mediated through post-transcriptional mechanism [46]. In terms of LINC00261, Liu et al. [47] demonstrated that LINC00261, as a tumor suppressor, can be inactivated by methylation of promoter region, while the demethylation could upregulate LINC00261 and inhibit the progress of pancreatic cancer. Therefore, in pan-carcinoma, we summarize the expression and clinical features of LINC00261 in the following.
The expression and clinical characters of LINC00261 in multiple human cancers
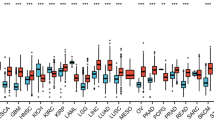
An increasing number of studies have indicated that LINC00261 plays vital roles in a variety of cancers, as discussed below. This overview of the many types of cancer related to LINC00261 expression expands the map of LINC00261 and influences further researches. The expression of LINC00261 and its clinical-characteristic associations in multiple tumors are shown in Table 1.
Pancreatic cancer
For cancer-related death rankings, pancreatic cancer (PC) is number seven worldwide [48]. With surgery being its most effective treatment, however, PC prognoses are still extremely poor [49,50,51]. Müller et al. [52] reported that LINC00261 expression was significantly down-regulated in PC, and Dorn et al. [53] also indicated that LINC00261 expression was significantly reduced in the squamous subtype. This last report, using bioinformatics, also showed that LINC00261 expression was negatively correlated with both tumor grade and tumor stage, and significantly related to positive disease outcomes [53]. The up-regulation of LINC00261 has been shown to obviously suppress PC cell proliferation and migration both in vitro and in vivo [47]. In addition, Zhang et al. [54] reported that LINC00261 expression was down-regulated in both PC tissues and in serum, with the level of expression being negatively correlated with clinical stages. LINC00261 expression has also been reported to suppress PC glycolysis and proliferation and to induce both cell-cycle arrest and apoptosis [35]. This study further our understanding of PC pathogenesis and suggests that LINC00261 may be a PC prognostic and therapeutic target [35].
Gastric cancer
With the third highest cancer-related death ranking, the gastric cancer (GC) mortality rate is 75 % [55], and it represents the fourth most-common tumor type in the world [56]. Due to imperceptible symptoms in the early period of GC, the rate of early diagnosis remains low [57,58,59]. Therefore, further studies of effective GC targets are crucial for improving both early diagnosis and the prognoses of GC patients. Compared to expressions in normal gastric tissue and para cancerous tissue, LINC00261 expression was found to be downregulated in GC [60, 61], and low LINC00261 expression was correlated with tumor stage, lymphatic metastases, and tumor invasiveness [61]. In addition, low LINC00261 expression was reported to predict poor prognosis and active cell motility, resulting in the suppression of tumor metastasis both in vitro and in vivo [61]. Moreover, up-regulated LINC00261 expression was reported to suppress cell motility by inhibiting the EMT [62]. These results suggest that LINC00261 can be regarded as a potential target for GC to prevent metastasis and invasiveness.
Colorectal cancer
Colorectal cancer (CRC) is one of the deadliest types of digestive system tumors, caused by interactions between genetic and environmental factors [63,64,65]. Interestingly, LINC00261 expression was reported to be low in both colon-cancer cell lines and tissues and in cisplatin-resistant cells [63]. In addition, LINC00261 over-expression was found to promote cell apoptosis and to inhibit cell viability, migration, invasiveness, and proliferation [63, 66]. Moreover, the up-regulation of LINC00261 was found to suppress cell-colony formation and to accelerate apoptosis [66]. Clinically, the low expression of LINC00261 was reported to be an independent risk factor for CRC patients that influenced recurrence-free survival time after surgery operation [67]. These findings suggest that LINC00261 may also be a potential therapeutic target for CRC.
Lung cancer
The leading cause of cancer deaths globally is lung cancer (LC) [68,69,70], with 80–85 % of cases being non-small-cell lung cancer (NSCLC) [71]. Liu et al. [72] found that, compared to adjacent normal lung tissues, LINC00261 expression was down-regulated in NSCLC tissues and that its expression was correlated with TNM stage, lymph-node status, distant metastases, and poor overall survival. It was also found to inhibit cell proliferation and metastasis by downregulating Snail expression via the EMT [73]. Furthermore, Shi et al. [34] showed that up-regulated LINC00261 expression in NSCLC cell lines suppressed both cell proliferation and invasiveness, while at the same time accelerating apoptosis. Moreover, LINC00261 was reported to be the most common down-regulated lncRNA in tumor metastasis, and low LINC00261 expression was correlated with poor overall patient survival using five independent lung-adenocarcinoma cohorts (n = 881) [74]. In addition to this, low LINC00261 expression was also found to be an independent indicator for poor NSCLC prognoses [72]. These results further our understanding of NSCLC pathogenesis and indicate another potential target to combat this deadly disease.
Hepatocellular carcinoma
As the sixth most common cancer worldwide, hepatocellular carcinoma (HCC) represents one of the top causes for cancer deaths worldwide, with more than 700,000 cases diagnosed annually [75,76,77]. HCC tumorigenesis and progression is closely associated with lncRNAs [78]. Zhang et al. [79] demonstrated that LINC00261 expression was significantly lower in in HCC tissues compared to adjacent noncancerous tissues, and that its expression was significantly correlated with TNM stage, tumor size, and overall HCC patient survival time. In addition, up-regulated LINC00261 expression in HCC cells was shown to suppress cell proliferation, colony formation, invasiveness, and the EMT in vitro. For HCC clinical features, Sun et al. [80] showed that LINC00261 was an independent prognostic marker and that HCC patients with lower LINC00261 expression had significantly shortened survival times for both tumor-free survival and postoperative recurrence-free survival. Using HCC-derived cell lines, low LINC00261 expression was reported to significantly promote cell motility [81]. Chen et al. [82] also reported that LINC00261 over-expression inhibited the EMT in liver cancer cells, thereby suppressing migration, invasiveness, and the formation of lung metastatic lesions. Therefore, based on these analyses, LINC00261 expression may serve as prognostic marker for HCC patients.
Breast cancer
Most prevalent in middle-aged women, breast cancer (BC) is ranked second among common causes for cancer-related deaths in the US [83,84,85]. At present, lncRNAs identified as being associated with BC include ATB and CCAT1. LINC00261 has also been shown to be downregulated in BC tissues compared to adjacent normal tissues. Up-regulation of LINC00261 has been reported to inhibit both BC-cell proliferation and migration, and the low expression of LINC00261 was reported to be adequate to promote BC tumorigenesis [39]. In addition, Li et al. [40] reported that LINC00261 over-expression inhibited both the viability and motility of BC cells, with potential implications for treatments, so LINC00261 may also be a new potential target for BC.
Other cancers
For laryngeal carcinoma tissue, the expression of LINC00261 was found to be significantly lower compared to normal tissues, and its expression in the lymph-node-negative metastasis group was significantly higher compared to that of lymph-node-positive metastasis group [86]. LINC00261 was also found to be downregulated and to suppress both cell proliferation and motility in endometrial carcinoma [37]. Similarly, for esophageal cancer, the anti-tumor influence of LINC00261 was determined using 5-fluorouracil (5-FU) to detect increased drug sensitivity in human esophageal cancer cells [87]. Other studies have also demonstrated an inhibitory effect of LINC00261 expression in prostate cancer, choriocarcinoma, and cholangiocarcinoma [38, 88, 89].
Multiple biological functions of LINC00261 in cancer
LncRNAs generally exert their functions through complex molecular mechanisms, such as the sponging of miRNAs in cancer cells [3, 90, 91]. In addition to discussing LINC00261 expression and its clinicopathological features for the cancers above, the biological functions and molecular mechanisms of LINC00261 are summarized below (see Table 2).
Cell proliferation
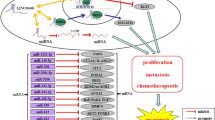
The overexpression of LINC00261 in PC was reported to significantly inhibit cell proliferation both in vitro and in vivo. Mechanistically, LINC00261 was found to bind to the bromo domain of p300/CREB-binding protein (CBP), preventing the recruitment of p300/CBP to promoter region of c-myc and decreasing the expression level of H3K27Ac. In this way, LINC00261 was responsible for reducing downstream c-myc transcription [47]. In addition, LINC00261 inhibition of cell proliferation and promotion of apoptosis were determined to be through sponging of miR-222-3p and reduction of c-myc expression [35]. Similarly, LINC00261 over-expression was shown to promote apoptosis by decreasing miR-23a-3p expression (a member of the mitogen-activated protein kinase [MAPK]-p38 signaling pathway) in PC cells [36]. In LC, the overexpression of LINC00261 was shown to overcome the inhibitory effect of miR-522-3p on corresponding mRNAs, and resulted in suppressed cell proliferation and accelerated apoptosis [34]. Mechanistically, LINC00261 has been shown to inhibit Wnt signaling (Fig. 1) [34], the miR-105/FHL1 axis [92], and the miR-1269a/FOXO1 axis [93]. As for Wnt signaling, LINC00261 participates it by promoting SFRP via ceRNA mechanism, and downregulated SFRP is proved to accelerate the tumorigenesis and metathesis of Choriocarcinoma [94]. SFRP inhibited the combination of Wnt ligand and Wnt receptor [95], and suppressed β-catenin expression. And when SFRP is inhibited, β-catenin expression level will recover [96]. In addition, inhibiting Wnt/β-catenin signaling pathway via other methods like Dickkopf-1 leads to similar effect on biological function compared with SFRP [97]. Thus, we consider that it is SFRP who mediated the degradation of β-catenin. Moreover, after being induced by forkhead box protein A2 (FOXA2), the overexpression of LINC00261 in lung adenocarcinoma (LUAD) cells slowed cell proliferation by inducing G2/M cell-cycle arrest [98].
The downregulation of LINC00261 in non-small cell lung cancer accelerates tumor growth. LINC00261 sponges miR-522-3p, resulting in the modulation of SFRP expression. SFRP inhibits the activation of the Wnt signaling pathway, and accelerating apoptosis of NSCLC. The up arrows indicate upregulated and down arrows indicate downregulated
Similarly, in BC, the reintroduction of LINC00261 was shown to arrest cell proliferation by protecting NME1 (a known tumor suppressor) mRNA from degradation [39]. Moreover, in HCC, Zhang et al. [79] showed that up-regulated LINC00261 significantly suppressed Notch signaling pathway by inhibiting Notch1 and Hes-1 expression. In endometrial carcinoma, LINC00261 was reported to inhibit cell proliferation by promoting the expression of forkhead box protein O1 (FOXO1) through a mechanism of reducing the levels of FOXO1-targeted miRNAs [37]. In addition, LINC00261 overexpression was also shown to promote apoptosis and decrease cell proliferation in choriocarcinoma [89]. In contrast to all other studies, one cholangiocarcinoma study reported that low LINC00261 expression actually increased cell apoptosis and inhibited cell proliferation [38]. Shahabi et al. [98] demonstrated that LINC00261 over-expression in LUAD cells slowed cell proliferation by inducing G2/M cell-cycle arrest. Interestingly, Chen et al. [81] speculated that low LINC00261 expression did not actually influence signaling pathways or gene expression related to cell proliferation and apoptosis, but that its high expression played a significant role in activating apoptosis and inhibiting cell proliferation. These ideas warrant further study.
Cell motility
Cell motility refers to cancer-cell invasiveness into crucial organs. In general, LINC00261 expression may alter tumor-cell motility in many ways. The up-regulation of LINC00261 has been shown to increase cisplatin sensitivity in colon-cancer cells and to inhibit both cell invasion and cell migration [63]. LINC00261 expression has also been reported to sponge miR-550a-3p to regulate Serum deprivation response protein (SDPR), bringing about reduced invasiveness and migration of CD44+/CD24−/low BC stem cells [40]. The overexpression of LINC00261 in endometrial carcinoma has been shown to inhibit both cell invasion and migration [37]. Moreover, down-regulated LINC00261 has been reported to significantly enhance cell migration and invasiveness in HCC cell lines [81]. The low expression of LINC00261 in both RBE and QBC939 cell lines also similarly suppressed cell invasiveness and cell migration [38]. When LINC00261 expression was reintroduced in NSCLC cells [34] and in choriocarcinoma cells [89], cell invasiveness was inhibited. Similarly, the overexpression of LINC00261 in LUAD cells [98], PC cells [47], and BC cells [39] also resulted in the inhibition of cell migration.
Tumor angiogenesis
In prostate cancer, the overexpression of LINC00261 was shown to inhibit the transcription of dickkopf-related protein 3 (DKK3) by recruiting GATA binding protein 6 (GATA6), resulting in the inhibition of tumor angiogenesis; this action was reversed by silencing DKK3. Interestingly, in prostate cancer cells, DKK3 expression induced cellular quiescence through the activation of p38 MAPK signaling pathway [88]. These findings demonstrate that LINC00261 expression may suppress prostate cancer progression, and suggest that it may be a new biomarker for early diagnosis of prostate cancer.
Chemoresistance
LINC00261 expression has been shown to increase the anti-tumor effect of cisplatin in colon cancer via the down-regulation of nuclear β-catenin. In addition, LINC00261 expression has been reported to inhibit the activation of Wnt signaling pathway, thereby promoting β-catenin degradation and blocking β-catenin translocation from the cytoplasm to the nucleus. Moreover, it is possible that the overexpression of LINC00261 may alleviate cisplatin resistance in colon-cancer cells by increasing the apoptosis rate [63]. Similarly, in human esophageal cancer, LINC00261 overexpression was observed to increase 5-FU drug sensitivity in tumor cells by modulating the methylation-dependent suppression of dihydropyrimidine dehydrogenase [87].
Tumorigenesis
During both tumorigenesis and the EMT, LINC00261 has been shown to be co-regulated with FOXA2, so LINC00261/FOXA2 expression levels could potentially be used to predict both LUAD cell invasiveness and progression [99]. This study established the foundation for future research on the role of the LINC00261/FOXA2 axis in LC tumorigenesis by describing its capacity for tumor suppression. Moreover, in BC, only the down-regulation of LINC00261 expression was required to cause BC tumorigenesis. Mechanistically, LINC00261 may interact with NME1 (a known tumor suppressor) mRNA as protection against degradation, resulting in higher NME1 levels and increased tumor suppression [39].
Molecular mechanisms underlying the functions of LINC00261
Many kinds of RNAs can bind to special miRNAs and regulate the gene expression, because they process miRNA recognition elements (MREs). These RNAs include lncRNAs, circRNAs, and pseudogenic RNAs, which are called competitive endogenous RNA (ceRNA) [100]. In this review, we found that LINC00261, acting as a ceRNA, sponge the miRNAs and downregulate the miRNAs level, further influencing the mRNAs level of target genes [101]. Many target genes of LINC00261 participate in multiple signaling pathway, such as Wnt/ β-catenin signaling pathway, p38 MAPK signaling pathway, and Notch signaling pathway. These signaling pathways regulated by LINC00261 are all related to tumor occurrence and progression.
Wnt/β-catenin signaling pathway
Cooperating with other pathways, Wnt/β-catenin signaling pathway is highly conserved and plays a critical role in embryonic development and tumorigenesis of multiple cancers [102]. β-catenin is part of a complex of proteins that constitute adherens junctions (AJs). β-catenin also anchors the actin cytoskeleton and may be responsible for transmitting the contact inhibition signal that causes cells to stop dividing once the epithelial sheet is complete. Therefore, when Wnt/β-catenin signaling pathway is dysregulated, cell proliferation will be no longer restrained even if the epithelial sheet is complete [103]. Wang et al. [63] indicated that LINC00261 could inactivate Wnt pathway through regulating β-catenin at the transcriptional level. They also found LINC00261 can promote degradation of β-catenin and inhibit it in nuclei. Moreover, LINC00261 could recruit GATA6 and suppress DKK3 expression. And DKK3 can interact with miRNA and regulate Wnt/β-catenin signaling pathway [88]. Shi et al. [34] also revealed that LINC00261 bound to miR-522‐3p and inhibited Wnt signaling pathway, alleviating progression of NSCLC cells.
Notch signaling pathway
Notch signaling pathway can directly connect events which happen on the cell membrane and transcriptional regulation. And the function of Notch signaling in different environments is based on the ability to influence the development and selection between adjacent cells [104]. Notch signaling pathway is related to tumor cell proliferation, differentiation, and metastasis. NOTCH was also conversed in multiple mammals, especially in mice, encoding a series of cell membrane receptors. Zhang et al. [79] recently demonstrated that upregulating LINC00261 prominently inhibited Notch1 and Hes-1 expression in HCC cells, which are vital members of Notch signaling pathway. And Hes-1 is a key downstream target of Notch signaling pathway and can be activated by the release and transport of the Notch intercellular domain, which interacts with the transcriptional complex [105, 106]. Therefore, increased expression of LINC00261 suppressed Notch signaling pathway to exert tumor suppression function in HCC.
P38 MAPK signaling pathway
P38 MAPK signaling pathway plays a critical role in the regulation of many cellular functions, such as cell growth, differentiation, and respond to stress and inflammatory reaction. For prostate cancer, Li et al. [88] indicated that LINC00261 promoted DKK3 transcription expression by recruiting GATA6, and DKK3 could induce cellular quiescence and inhibit tumor progression through activating the p38 MAPK signaling pathway. DKK3 promoted p-p38 nuclear translocation [107] and mediated the activation of Wnt/β-catenin signaling pathway, which is closely interacted with many other signaling pathways, causing cell proliferation, migration and invasion of PCa [108].
Conclusions
Considerable researches have shown that lncRNAs play vital roles in both tumorigenesis and tumor development via the regulation of gene expression [109,110,111]. In view of the fact that LINC00261 can be promoted by the demethylation of promoter region, we summarized the roles of LINC00261, a tumor suppressor gaining increasingly important status, in cancer research. Although LINC00261 was first identified approximately eight years ago [112], its roles as a novel lncRNA involved in a variety of diseases and its molecular mechanisms have only been reported recently. The present LINC00261 review indicates that its expression is widely low expressed in many cancer types (e.g., PC, GC, LC, CRC, BC, and HCC). At the same time, LINC00261 expression has been found to be correlated with clinicopathological characteristics (e.g., tumor stage, lymph-node metastasis, patient survival, and tumor prognosis). As a tumor suppressor, LINC00261 contributes to modulating cancer-cell biology via multiple molecular mechanisms (e.g., cell proliferation, apoptosis, invasiveness, migration, chemoresistance, and tumorigenesis). Mechanistically, the sponging and binding to miRNAs indicates that LINC00261 acts as a ceRNA to inhibit the expression of cancer-related genes (Fig. 2). The evidence that LINC00261 plays an antineoplastic role in a variety of human cancers indicates that it has the potential to be a target for both cancer diagnosis and treatment and could become a biomarker for many types of cancer.
Availability of data and materials
All data are included in the article.
Abbreviations
- lncRNAs:
-
Long noncoding RNAs
- LINC00261:
-
Long intergenic non-protein coding RNA 261
- ncRNAs:
-
Noncoding RNAs
- miRNAs:
-
microRNAs
- circRNAs:
-
Circular RNAs
- mRNA:
-
Messenger RNA
- EMT:
-
Epithelial-mesenchymal transition
- PC:
-
Pancreatic cancer
- GC:
-
Gastric cancer
- CRC:
-
Colorectal cancer
- LC:
-
Lung cancer
- NSCLC:
-
Non-small-cell lung cancer
- HCC:
-
Hepatocellular carcinoma
- BC:
-
Breast cancer
- 5-FU:
-
5-fluorouracil
- CBP:
-
CREB-binding protein
- FOXA2:
-
Forkhead box protein A2
- LUAD:
-
Lung adenocarcinoma
- FOXO1:
-
Forkhead box protein O1
- SDPR:
-
Serum deprivation response protein
- DKK3:
-
Dickkopf-related protein 3
- GATA6:
-
GATA binding protein 6
- MREs:
-
miRNA recognition elements
- ceRNA:
-
Competitive endogenous RNA
- AJs:
-
Adherens junctions
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27.
Wang J-J, Han K-FL,F. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci. 2018;22(12):3855–64.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Hulvat MC. Cancer incidence and trends. Surg Clin North Am. 2020;100(3):469–81.
Wu L, Qu X. Cancer biomarker detection: recent achievements and challenges. Chem Soc Rev. 2015;44(10):2963–97.
Zou J, Wang E. Cancer biomarker discovery for precision medicine: new progress. Curr Med Chem. 2019;26(42):7655–71.
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407.
Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–109.
Chan JJ, Tay Y. Noncoding RNA:RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):23–32.
Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94.
Panda AC, Abdelmohsen K, Gorospe M. SASP regulation by noncoding RNA. Mech Ageing Dev. 2017;168:37–43.
Hauptman N, Glavac D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14(3):4655–69.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–14.
Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–63.
Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y, Jiang X. LncRNA-ATB: an indispensable cancer-related long noncoding RNA. Cell Prolif. 2017;50(6):12–20.
Wang J, Su Z, Lu S, Fu W, Liu Z, Jiang X, Tai S. LncRNA HOXA-AS2 and its molecular mechanisms in human cancer. Clin Chim Acta. 2018;485:229–33.
Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–7.
Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24(3):257–77.
McHugh CA, Chen CK, Chow A, Surka CF, Tran C, McDonel P, Pandya-Jones A, Blanco M, Burghard C, Moradian A, et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature. 2015;521(7551):232–6.
Charles Richard JL, Eichhorn PJA. Platforms for Investigating LncRNA Functions. SLAS Technol. 2018;23(6):493–506.
Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z, Shen F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int J Mol Sci. 2019;20(22):235–43.
Xiao Y, Xiao T, Ou W, Wu Z, Wu J, Tang J, Tian B, Zhou Y, Su M, Wang W. LncRNA SNHG16 as a potential biomarker and therapeutic target in human cancers. Biomark Res. 2020;8:41.
Li X, Li J, Lu P, Li M. LINC00261 relieves the progression of sepsis-induced acute kidney injury by inhibiting NF-kappaB activation through targeting the miR-654-5p/SOCS3 axis. J Bioenerg Biomembr 2021:43–49.
Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer. 2021;21(1):22–36.
Li YH, Hu YQ, Wang SC, Li Y, Chen DM. LncRNA SNHG5: a new budding star in human cancers. Gene. 2020;749:144724.
Gong CY, Tang R, Nan W, Zhou KS, Zhang HH. Role of SNHG16 in human cancer. Clin Chim Acta. 2020;503:175–80.
Cao Z-JL H-L, Huang P-L. lncRNA-RMRP promotes proliferation, migration and invasion of bladder cancer via miR-206. Eur Rev Med Pharmacol Sci. 2019;23(3):1012–21.
Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang Y, Jia L, Li S, Cancer Genome Atlas Research N, Xie W, et al. lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell. 2018;33(4):706-20 e709.
Chen Z, Yu W, Zhou Q, Zhang J, Jiang H, Hao D, Wang J, Zhou Z, He C, Xiao Z. A Novel lncRNA IHS promotes tumor proliferation and metastasis in HCC by regulating the ERK- and AKT/GSK-3beta-signaling pathways. Mol Ther Nucleic Acids. 2019;16:707–20.
Wu H, Hu Y, Liu X, Song W, Gong P, Zhang K, Chen Z, Zhou M, Shen X, Qian Y, et al. LncRNA TRERNA1 function as an enhancer of SNAI1 promotes gastric cancer metastasis by regulating epithelial-mesenchymal transition. Mol Ther Nucleic Acids. 2017;8:291–9.
Chao Y, Zhou D. lncRNA-D16366 is a potential biomarker for diagnosis and prognosis of hepatocellular carcinoma. Med Sci Monit. 2019;25:6581–6.
Ma Y, Zhang J, Wen L, Lin A. Membrane-lipid associated lncRNA: a new regulator in cancer signaling. Cancer Lett. 2018;419:27–9.
Shi J, Ma H, Wang H, Zhu W, Jiang S, Dou R, Yan B. Overexpression of LINC00261 inhibits non-small cell lung cancer cells progression by interacting with miR-522-3p and suppressing Wnt signaling. J Cell Biochem. 2019;120(10):18378–87.
Zhai S, Xu Z, Xie J, Zhang J, Wang X, Peng C, Li H, Chen H, Shen B, Deng X. Epigenetic silencing of LncRNA LINC00261 promotes c-myc-mediated aerobic glycolysis by regulating miR-222-3p/HIPK2/ERK axis and sequestering IGF2BP1. Oncogene. 2021;40(2):277–91.
Wang X, Gao X, Tian J, Zhang R, Qiao Y, Hua X, Shi G. LINC00261 inhibits progression of pancreatic cancer by down-regulating miR-23a-3p. Arch Biochem Biophys 2020, 689:108469.
Fang Q, Sang L, Du S. Long noncoding RNA LINC00261 regulates endometrial carcinoma progression by modulating miRNA/FOXO1 expression. Cell Biochem Funct. 2018;36(6):323–30.
Gao J, Qin W, Kang P, Xu Y, Leng K, Li Z, Huang L, Cui Y, Zhong X. Up-regulated LINC00261 predicts a poor prognosis and promotes a metastasis by EMT process in cholangiocarcinoma. Pathol Res Pract. 2020;216(1):152733.
Guo G, Dai S, Chen Q. Long noncoding RNA LINC00261 reduces proliferation and migration of breast cancer cells via the NME1-EMT pathway. Cancer Manag Res. 2020;12:3081–9.
Li Y, Wu C. LINC00261/microRNA-550a-3p/SDPR axis affects the biological characteristics of breast cancer stem cells. IUBMB Life 2020:120–129.
Zhang J, Li Z, Liu L, Wang Q, Li S, Chen D, Hu Z, Yu T, Ding J, Li J, et al. Long noncoding RNA TSLNC8 is a tumor suppressor that inactivates the interleukin-6/STAT3 signaling pathway. Hepatology. 2018;67(1):171–87.
Hu X, Feng Y, Zhang D, Zhao SD, Hu Z, Greshock J, Zhang Y, Yang L, Zhong X, Wang LP, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26(3):344–57.
Xie JJ, Jiang YY, Jiang Y, Li CQ, Lim MC, An O, Mayakonda A, Ding LW, Long L, Sun C, et al: Super-enhancer-driven long non-coding RNA LINC01503, regulated by TP63, is over-expressed and oncogenic in squamous cell carcinoma. Gastroenterology 2018, 154(8):2137–2151 e2131.
Zhang E, Han L, Yin D, He X, Hong L, Si X, Qiu M, Xu T, De W, Xu L, et al. H3K27 acetylation activated-long non-coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma. Nucleic Acids Res. 2017;45(6):3086–101.
Fayez Hadji M-CB, Simon-Pierre Guay. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation. 2016;134(23):1848–62.
Hammerle M, Gutschner T, Uckelmann H, Ozgur S, Fiskin E, Gross M, Skawran B, Geffers R, Longerich T, Breuhahn K, et al. Posttranscriptional destabilization of the liver-specific long noncoding RNA HULC by the IGF2 mRNA-binding protein 1 (IGF2BP1). Hepatology. 2013;58(5):1703–12.
Liu S, Zheng Y, Zhang Y, Zhang J, Xie F, Guo S, Gu J, Yang J, Zheng P, Lai J, et al. Methylation-mediated LINC00261 suppresses pancreatic cancer progression by epigenetically inhibiting c-Myc transcription. Theranostics. 2020;10(23):10634–51.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–705.
Ke MJ, Ji LD, Li YX. Bioinformatics analysis combined with experiments to explore potential prognostic factors for pancreatic cancer. Cancer Cell Int. 2020;20:382.
Zhang X, Ma N, Yao W, Li S, Ren Z. RAD51 is a potential marker for prognosis and regulates cell proliferation in pancreatic cancer. Cancer Cell Int. 2019;19:356.
Sören Müller SR. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer. 2015;14:94.
Dorn A, Glass M, Neu CT, Heydel B, Huttelmaier S, Gutschner T, Haemmerle M. LINC00261 is differentially expressed in pancreatic cancer subtypes and regulates a pro-epithelial cell identity. Cancers (Basel). 2020;12(5):543–51.
Baogang Zhang CL, Zhixia S. Long non-coding RNA LINC00346, LINC00578, LINC00673, LINC00671, LINC00261, and SNHG9 are novel prognostic markers for pancreatic cancer. Am J Transl Res. 2018;10(8):2648–58.
Thin KZ, Liu X, Feng X, Raveendran S, Tu JC. LncRNA-DANCR: A valuable cancer related long non-coding RNA for human cancers. Pathol Res Pract. 2018;214(6):801–5.
Chengyu Hu KL, Wang B. DLX6-AS1: an indispensable cancer-related long non-coding RNA. Curr Pharm Des 2020:78–93.
Feng W, Ding Y, Zong W, Ju S. Non-coding RNAs in regulating gastric cancer metastasis. Clin Chim Acta. 2019;496:125–33.
Maconi G, Manes G, Porro GB. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol. 2008;14(8):1149–55.
Axon A. Symptoms and diagnosis of gastric cancer at early curable stage. Best Pract Res Clin Gastroenterol. 2006;20(4):697–708.
Cao WJ, Wu HL, He BS, Zhang YS, Zhang ZY. Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol. 2013;19(23):3658–64.
Fan Y, Wang YF, Su HF, Fang N, Zou C, Li WF, Fei ZH. Decreased expression of the long noncoding RNA LINC00261 indicate poor prognosis in gastric cancer and suppress gastric cancer metastasis by affecting the epithelial-mesenchymal transition. J Hematol Oncol. 2016;9(1):57.
Yu Y, Li L, Zheng Z, Chen S, Chen E, Hu Y. Long non-coding RNA linc00261 suppresses gastric cancer progression via promoting Slug degradation. J Cell Mol Med. 2017;21(5):955–67.
Wang ZK, Yang L, Wu LL, Mao H, Zhou YH, Zhang PF, Dai GH. Long non-coding RNA LINC00261 sensitizes human colon cancer cells to cisplatin therapy. Braz J Med Biol Res. 2017;51(2):e6793.
Meng S, Li Y, Zang X, Jiang Z, Ning H, Li J. Effect of TLR2 on the proliferation of inflammation-related colorectal cancer and sporadic colorectal cancer. Cancer Cell Int. 2020;20:95.
Jiao G, Huang Q, Hu M, Liang X, Li F, Lan C, Fu W, An Y, Xu B, Zhou J, et al. Therapeutic suppression of miR-4261 attenuates colorectal cancer by targeting MCC. Mol Ther Nucleic Acids. 2017;8:36–45.
Yan D, Liu W, Liu Y, Luo M. LINC00261 suppresses human colon cancer progression via sponging miR-324-3p and inactivating the Wnt/beta-catenin pathway. J Cell Physiol. 2019;234(12):22648–56.
Zhou Z, Ma J. Expression and clinical significance of long-chain noncoding RNA LINC00261 in colon cancer. Clin Lab. 2019;65(12):13–20.
Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24.
Mao Y, Yang D, He J, Krasna MJ. Epidemiology of lung cancer. Surg Oncol Clin N Am. 2016;25(3):439–45.
Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103(3):463–73.
D’Arcangelo M, Hirsch FR. Clinical and comparative utility of afatinib in non-small cell lung cancer. Biologics. 2014;8:183–92.
Liu Y, Xu NX,S-F. Decreased expression of long non-coding RNA LINC00261 is a prognostic marker for patients with non-small cell lung cancer: a preliminary study. Eur Rev Med Pharmacol Sci. 2017;21(24):5691–5.
Liao J. L-PD: Linc00261 suppresses growth and metastasis of non-small cell lung cancer via repressing epithelial-mesenchymal transition. Eur Rev Med Pharmacol Sci. 2019;23(9):3829–37.
Dang HX, White NM, Rozycki EB, Felsheim BM, Watson MA, Govindan R, Luo J, Maher CA. Long non-coding RNA LCAL62 / LINC00261 is associated with lung adenocarcinoma prognosis. Heliyon. 2020;6(3):e03521.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Yuting He XY, Li J. Role of m 5 C-related regulatory genes in the diagnosis and prognosis of hepatocellular carcinoma. Am J Transl Res. 2020;12(3):912–22.
Li X, Yuan J, Song C, Lei Y, Xu J, Zhang G, Wang W, Song G. Deubiquitinase USP39 and E3 ligase TRIM26 balance the level of ZEB1 ubiquitination and thereby determine the progression of hepatocellular carcinoma. Cell Death Differ 2021:3423–3435.
Chen Xue YZ. Jianwen Jiang: Expression levels of lncRNAs are prognostic for hepatocellular carcinoma overall survival. Am J Transl Res. 2020;12(5):1873–83.
Zhang HF, Li W, Han YD. LINC00261 suppresses cell proliferation, invasion and Notch signaling pathway in hepatocellular carcinoma. Cancer Biomark. 2018;21(3):575–82.
Sui J, Miao Y, Han J, Nan H, Shen B, Zhang X, Zhang Y, Wu Y, Wu W, Liu T, et al. Systematic analyses of a novel lncRNA-associated signature as the prognostic biomarker for hepatocellular carcinoma. Cancer Med 2018:289–302.
Chen Z, Xiang L, Huang Y, Fang Y, Li X, Yang D. Expression of long noncoding RNA linc00261 in hepatocellular carcinoma and its association with postoperative outcomes. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(10):1179–86.
Zhanjun Chen LX, Zhigang Hu. Epigenetically silenced linc00261 contributes to the metastasis of hepatocellular carcinoma via inducing the deficiency of FOXA2 transcription. Am J Cancer Res. 2021;11(1):277–96.
Lou W, Ding B, Wang S, Fu P. Overexpression of GPX3, a potential biomarker for diagnosis and prognosis of breast cancer, inhibits progression of breast cancer cells in vitro. Cancer Cell Int. 2020;20:378.
Lu D, Di S, Zhuo S, Zhou L, Bai R, Ma T, Zou Z, Chen C, Sun M, Tang J, et al. The long noncoding RNA TINCR promotes breast cancer cell proliferation and migration by regulating OAS1. Cell Death Discov. 2021;7(1):41.
Lou W, Liu J, Ding B, Xu L, Fan W. Identification of chemoresistance-associated miRNAs in breast cancer. Cancer Manag Res. 2018;10:4747–57.
Zhang CM, Wu WG,YY. The expression of long non-coding RNA LINC00261 in laryngeal carcinoma tissue and their clinical significance. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017, 31(1):68–71.
Lin K, Jiang H, Zhuang SS, Qin YS, Qiu GD, She YQ, Zheng JT, Chen C, Fang L, Zhang SY. Long noncoding RNA LINC00261 induces chemosensitization to 5-fluorouracil by mediating methylation-dependent repression of DPYD in human esophageal cancer. FASEB J. 2019;33(2):1972–88.
Li Y, Li H, Wei X. Long noncoding RNA LINC00261 suppresses prostate cancer tumorigenesis through upregulation of GATA6-mediated DKK3. Cancer Cell Int. 2020;20:474.
Wang Y, Xue K, Guan Y, Jin Y, Liu S, Wang Y, Liu S, Wang L, Han L. Long noncoding RNA LINC00261 suppresses cell proliferation and invasion and promotes cell apoptosis in human choriocarcinoma. Oncol Res. 2017;25(5):733–42.
Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci. 2019;20(22):5758.
Li J, Meng H, Bai Y, Wang K. Regulation of lncRNA and its role in cancer metastasis. Oncol Res. 2016;23(5):205–17.
Wang Z, Zhang J, Yang B, Li R, Jin L, Wang Z, Yu H, Liu C, Mao Y, You Q. Long intergenic noncoding RNA 00261 acts as a tumor suppressor in non-small cell lung cancer via regulating miR-105/FHL1 axis. J Cancer. 2019;10(25):6414–21.
Guo C, Shi H, Shang Y, Zhang Y, Cui J, Yu H. LncRNA LINC00261 overexpression suppresses the growth and metastasis of lung cancer via regulating miR-1269a/FOXO1 axis. Cancer Cell Int. 2020;20:275.
Zeng X, Zhang Y, Xu H, Zhang T, Xue Y, An R. Secreted frizzled related protein 2 modulates epithelial-mesenchymal transition and stemness via wnt/beta-catenin signaling in choriocarcinoma. Cell Physiol Biochem. 2018;50(5):1815–31.
Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24(9):811–20.
Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH 3rd, Dang VM, Appleton J, O’Connell MP, Cheng P, et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532(7598):250–4.
Zhou M, Jiao L, Liu Y. sFRP2 promotes airway inflammation and Th17/Treg imbalance in COPD via Wnt/beta-catenin pathway. Respir Physiol Neurobiol. 2019;270:103282.
Shahabi S, Kumaran V, Castillo J, Cong Z, Nandagopal G, Mullen DJ, Alvarado A, Correa MR, Saizan A, Goel R, et al. LINC00261 is an epigenetically regulated tumor suppressor essential for activation of the DNA damage response. Cancer Res. 2019;79(12):3050–62.
Dhamija S, Becker AC, Sharma Y, Myacheva K, Seiler J, Diederichs S. LINC00261 and the adjacent gene FOXA2 are epithelial markers and are suppressed during lung cancer tumorigenesis and progression. Noncoding RNA 2018, 5(1).
Yuheng Yan QS, Xin Yuan. DANCR: an emerging therapeutic target for cancer. Am J Transl Res. 2020;12(7):4031–42.
Gu X, Chu Q, Zheng Q, Wang J, Zhu H. The dual functions of the long noncoding RNA CASC15 in malignancy. Biomed Pharmacother. 2021;135:111212.
Zhong Z, Virshup DM. Wnt signaling and drug resistance in cancer. Mol Pharmacol. 2020;97(2):72–89.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205.
Meurette O, Mehlen P. Notch signaling in the tumor microenvironment. Cancer Cell. 2018;34(4):536–48.
Vanorny DA, Prasasya RD, Chalpe AJ, Kilen SM, Mayo KE. Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol. 2014;28(4):499–511.
Terauchi KJ, Shigeta Y, Iguchi T, Sato T. Role of Notch signaling in granulosa cell proliferation and polyovular follicle induction during folliculogenesis in mouse ovary. Cell Tissue Res. 2016;365(1):197–208.
Yu-Lee LY, Lee YC, Pan J, Lin SC, Pan T, Yu G, Hawke DH, Pan BF, Lin SH. Bone secreted factors induce cellular quiescence in prostate cancer cells. Sci Rep. 2019;9(1):18635.
Liu B, Zhou W, Jiang H, Xiang Z, Wang L. miR-1303 promotes the proliferation, migration and invasion of prostate cancer cells through regulating the Wnt/beta-catenin pathway by targeting DKK3. Exp Ther Med. 2019;18(6):4747–57.
Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018;109(7):2093–100.
Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L, Li J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18(1):143.
Marin-Bejar O, Mas AM, Gonzalez J, Martinez D, Athie A, Morales X, Galduroz M, Raimondi I, Grossi E, Guo S, et al. The human lncRNA LINC-PINT inhibits tumor cell invasion through a highly conserved sequence element. Genome Biol. 2017;18(1):202.
Lin ZY, Chuang WL. Genes responsible for the characteristics of primary cultured invasive phenotype hepatocellular carcinoma cells. Biomed Pharmacother. 2012;66(6):454–8.
Acknowledgements
We sincerely appreciate all the participators in our work.
Funding
This work was supported by the National Natural Science Foundation of China (81902832), the Youth Talent Lifting Project of Henan Province (2021HYTP059), and Key Scientific Research Project of Henan Higher Education Institutions of China (21A320026).
Author information
Authors and Affiliations
Contributions
Zhang MG, Gao F. and Yu X drafted manuscript. Zhang QY and Sun ZZ drew the mechanism diagrams. He YT, and Guo WZ conceived of the study and guided the analysis. He YT, and Guo WZ edited and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethic approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, M., Gao, F., Yu, X. et al. LINC00261: a burgeoning long noncoding RNA related to cancer. Cancer Cell Int 21, 274 (2021). https://doi.org/10.1186/s12935-021-01988-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-01988-8