Abstract
Background
Programmed death-ligand 1 (PD-L1) was the first identified ligand of programmed death-1 (PD-1). PD-1/PD-L1 interactions inhibit T cell-mediated immune responses, limit cytokine production, and promote tumor immune escape. Recently, many studies have investigated the prognostic value of PD-L1 expression in patients with melanoma. However, the results of these analyses remain a subject of debate. We have therefore carried out a meta-analysis to identify the prognostic role of PD-L1 in melanoma.
Methods
A thorough medical literature search was performed in the databases PubMed, Web of Science, and Embase until October 2019. The pooled hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated to evaluate the correlation between PD-L1 overexpression and prognosis. Publication bias was evaluated using Begg’s test and Egger’s test.
Results
Thirteen articles with 1062 enrolled patients were included in this meta-analysis. High PD-L1 expression did not correlate with overall survival (OS) (HR = 0.93, 95% CI 0.57–1.52, P = 0.781) or progression-free survival (PFS) (HR = 0.82, 95% CI 0.43–1.54, P = 0.535). However, PD-L1 overexpression correlated with the absence of lymph node (LN) metastasis (OR = 0.46, 95% CI 0.22–0.95, P = 0.036). Further, there was no significant relationship between PD-L1 expression and sex (OR = 1.29, 95% CI 0.90–1.84, P = 0.159), age (OR = 0.90, 95% CI 0.51–1.57, P = 0.708), or Eastern Cooperative Oncology Group Performance Status (OR = 0.55, 95% CI 0.06–4.83, P = 0.592).
Conclusions
This meta-analysis suggested that PD-L1 expression did not predict an inferior prognosis in patients with melanoma. However, high PD-L1 expression was associated with absence of LN metastasis in such patients.
Similar content being viewed by others
Background
Melanoma is the most fatal form of skin cancer, and the incidence rates continue to increase dramatically [1]. Worldwide, approximately 232,100 new cases of cutaneous melanoma are diagnosed each year, and 55,500 patients die annually [2]. Ultraviolet exposure, skin type, indoor tanning, and a personal history of prior melanoma are risk factors of melanoma [3,4,5]. The most important prognostic factor of melanoma is the BRAF mutational status [6]. The other prognostic factors are American Joint Committee on Cancer (AJCC) melanoma TNM (tumor, node, metastasis) staging [7], Clark level, and Breslow thickness [8], and they are useful for the clinical management of patients with melanoma. In the United States, patients present melanoma at different stages, with 84% of them presenting localized disease, 9% presenting regional disease, and 4% exhibiting distant metastasis [9]. The prognosis for patients with localized disease is promising, with a 5-year survival rate of over 90% [10]. Whereas the prognosis for patients with unresectable stage III–IV tumors is poor, as the 10‐year overall survival (OS) is only 10% to 15% for those patients [1]. In recent years, significant progress has been achieved in the development of targeted therapies and immunotherapy [11, 12]; however, novel prognostic markers are still needed for tailoring personal treatment strategies.
In recent years, immune inhibitory signaling pathways have been recognized to play a pivotal role in the maintenance of an immunosuppressive microenvironment that favors cancer development [13]. One important co-inhibitory pathway is the programmed death-ligand 1 (PD-L1) and programmed death-1 (PD-1) axis [14]. PD-1 is expressed in a wide range of immune cells, and its expression is induced on effector T‐cells in response to inflammatory signals [15]. PD-L1 (also known as B7-H1 or CD274) was the first identified ligand of PD-1 [15, 16]. PD-L1 is also widely expressed in various cell types including lymphocytes, vascular endothelium, mesenchymal stem cells, neuronal cells, and tumor cells [15]. PD-1/PD-L1 interactions inhibit T-cell-mediated immune responses, limit cytokine production, and promote tumor immune escape [17]. Recent studies have also demonstrated that tumor-derived extracellular vesicles (EVs) act as messengers of intercellular communication [18]. Exosomal microRNAs (miRNAs), which are transferred by EVs, are promising and reliable tools for cancer diagnosis and clinical application [18]. PD-L1 overexpression has been examined as a prognostic factor in diverse cancers including lung cancer [19], gastric cancer [20], ovarian cancer [21], breast cancer [22], prostate cancer [23], bladder cancer [24], cervical cancer [25], cholangiocarcinoma [26], colorectal cancer [27], nasopharyngeal carcinoma [28], diffuse large B-cell lymphoma [29], pancreatic cancer [30], soft-tissue sarcoma [31], renal cell carcinoma [32], and head and neck squamous cell carcinoma [33]. In addition, in patients with melanoma, exosomal PD-L1 is an indicator of immune activation early after the initiation of treatment with immune checkpoint inhibitors (ICIs) and is associated with clinical response to ICIs [34].
Previous studies have also assessed the prognostic value of PD-L1 expression in patients with melanoma [35,36,37,38,39,40,41,42,43,44,45,46,47]; however, the results remain controversial. We have therefore performed a meta-analysis to assess whether PD-L1 expression was associated with prognosis and clinicopathological factors in patients with melanoma.
Materials and methods
Search strategy
We carried out the meta-analysis in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [48]. We comprehensively searched the databases PubMed, Web of Science, and Embase using the following keywords: (PD-L1 OR B7-H1 OR programmed cell death 1 ligand 1 OR CD274) AND (melanoma OR malignant melanoma) AND (survival OR prognostic OR prognosis OR outcome). We searched articles until October 2019. The reference lists were also carefully checked to identify additional eligible studies. All analyses were performed using the data of previously published studies. Therefore, no ethical approval or patient consent was required for this study.
Selection criteria
Studies were included if they met the following criteria: (1) inclusion of patients diagnosed with histologically confirmed melanoma; (2) detection of PD-L1 expression in the melanoma tissue using immunohistochemistry (IHC) studies; (3) identification of a definite cut-off value to determine PD-L1 overexpression; (4) reporting a correlation between PD-L1 and survival including OS and/or progression-free survival (PFS), or providing sufficient information to compute the hazard ratio (HR) and 95% confidence interval (95% CI); (5) published in English language. The exclusion criteria were as follows: (1) case reports, reviews, letters, and correspondences; (2) studies without available or usable information; (3) studies lacking survival data; (4) animal studies; (5) non-English articles; (6) duplicate studies.
Data extraction and quality assessment
Two researchers (JY and MD) independently extracted basic information from the included studies, and any disagreements were resolved by discussion with a third researcher (XG). The following data were extracted from eligible studies: the first author’s name, publication year, ethnicity of patients, sample size, age, tumor stage, study period, sampling specimen, detection method, treatment, cut-off values, methods of survival analysis, follow-up time, and clinicopathologic parameters. When both univariate and multivariate analyses of OS and/or PFS were conducted in the included studies, we extracted the data of multivariate analysis. The results of univariate analysis were adopted when only the univariate analysis was performed. The quality of each eligible study was evaluated using the Newcastle–Ottawa Scale (NOS) [49]. The NOS scale consists of three items describing the study quality: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). The maximum NOS score is 9 points, and studies with a score of 6 points or higher are considered high-quality studies.
Statistical analysis
The pooled HRs and 95% CIs were calculated to evaluate the correlation between PD-L1 overexpression and prognosis (OS and PFS). The association between PD-L1 expression and clinicopathological parameters were assessed by combining the odds ratios (ORs) and their 95% CIs. Cochrane’s Q test and I2 metric were used to evaluate the statistical heterogeneity of the pooled data. A P value of less than 0.1 or an I2 value of more than 50% indicated significant heterogeneity, and a random effects model was employed for calculation. Otherwise, a fixed effects model was applied. Subgroup analyses were performed to detect sources of heterogeneity. Sensitivity analysis was carried out by omitting each individual study to examine the robustness of the results. The potential publication bias was assessed with Begg’s test and Egger’s test. All statistical analyses were performed using Stata version 12.0 (STATA Corp., College Station, TX). P < 0.05 was considered to indicate statistical significance.
Results
Literature selection
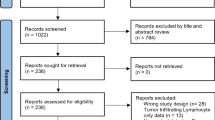
A total of 266 studies were identified by the primary search strategy. After removing duplicates, 134 studies were evaluated by title and abstract screening; 80 studies were then discarded. Thus, 54 articles remained for further full-text estimation. After careful reading of the full text, 41 studies were removed for the following reasons: 24 studies lacked necessary data, 4 studies did not apply the IHC method, 4 studies did not detect PD-L1 expression in tumor cells, 3 studies lacked survival data, 2 studies were non-human studies, 2 studies were letters or correspondences, 1 study was duplicated, and 1 study did not focus on PD-L1. Ultimately, 13 articles [35,36,37,38,39,40,41,42,43,44,45,46,47] were included in this meta-analysis. A flowchart of the literature selection procedure is shown in Fig. 1.
Characteristics of studies
Detailed information of the included studies is given in Table 1. The included studies were published during 2011–2019 and from 7 countries. Three studies were conducted in the United States [37, 41, 42], 3 in China [43, 44, 47], 2 in Italy [38, 40], 2 in Germany [45, 46], 1 in Korea [35], 1 in The Netherlands [36], and 1 in Australia [39]. The total sample size was 1062 patients, ranging from 23 to 147 patients per paper, with a mean value of 81.7. All studies used IHC to detect PD-L1 expression in the tumor tissue. The cutoff values for PD-L1 expression differed by > 5%, > 1%, H-score > 5, and H-score > 1 in the included studies. Two studies had a prospective design [41, 45] and 11 studies were retrospective studies [35,36,37,38,39,40, 42,43,44, 46, 47]. Ten studies [36,37,38, 40,41,42, 44,45,46,47] and 8 studies [35, 37, 39, 40, 43,44,45,46] provided data on OS and PFS, respectively. Eight studies [35,36,37,38, 40,41,42, 45] enrolled patients with metastatic disease and 5 studies [39, 43, 44, 46, 47] recruited patients with the disease at mixed stages. These studies generally had high quality, with NOS scores ranging from 6 to 9.
Correlation between PD-L1 expression and OS
A total of 10 studies enrolling 889 patients [36,37,38, 40,41,42, 44,45,46,47] reported data on PD-L1 for the prognosis of OS. Because of significant heterogeneity among studies (I2 = 77.8%, P < 0.001), a random effects model was used. As shown in Fig. 2 and Table 2, the pooled results indicated a nonsignificant relationship between PD-L1 expression and OS (HR = 0.93, 95% CI 0.57–1.52, P = 0.781). We then performed subgroup analysis for further investigation. As shown in Table 2, PD-L1 overexpression was shown to have no significant prognostic role in OS in the subgroups stratified by ethnicity, stage, sample size, cut-off value, and treatment, with P > 0.05 in all subgroups.
Association between PD-L1 expression and PFS
Eight studies enrolling a total of 565 patients [35, 37, 39, 40, 43,44,45,46] were included in the PFS analysis. Pooled results revealed that elevated PD-L1 expression had no significant effect on PFS in melanoma (HR = 0.82, 95% CI 0.43–1.54, P = 0.535, Fig. 3, Table 2), and a random effects model was used for the significant heterogeneity (I2 = 75.4%, P < 0.001). The subgroup analysis indicated that PD-L1 did not predict PFS in different subgroups (Table 2).
Relationship between PD-L1 and clinicopathological factors
Using the available data of the included studies, the association between PD-L1 expression and 4 clinicopathological features was analyzed, namely, sex (male vs. female), age (≥ 60 vs. < 60, years), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (≥ 1 vs. 0), and lymph node (LN) metastasis (yes vs. no). The data from 2 studies that enrolled 167 patients showed correlation of PD-L1 overexpression with the absence of LN metastasis (OR = 0.46, 95% CI 0.22–0.95, P = 0.036, Table 3, Fig. 4). However, there was no significant relationship between PD-L1 expression and sex (n = 7, OR = 1.29, 95% CI 0.90–1.84, P = 0.159), age (n = 4, OR = 0.90, 95% CI 0.51–1.57, P = 0.708), or ECOG PS (n = 2, OR = 0.55, 95% CI 0.06–4.83, P = 0.592, Table 3, Fig. 4).
Sensitivity analysis
Sensitivity analysis was conducted for OS and PFS (Fig. 5) by sequential omission of each study. As shown in Fig. 5, the overall results of OS and PFS were not substantially changed by deletion of any single study, indicating the credibility of the results.
Publication bias
Begg’s test and Egger’s test were adopted to determine whether potential publication bias existed in this meta-analysis. The funnel plots were symmetric (Fig. 6), and all of the P values of publication bias were more than 0.05 (Begg’s P = 0.721, Egger’s P = 0.662 for OS and Begg’s P = 0.108, Egger’s P = 0.235 for PFS, Fig. 6). These data suggested that there was no significant publication bias in this meta-analysis.
Discussion
The association between PD-L1 expression and prognosis in melanoma has been explored extensively in previous studies; however, the results were inconsistent. The conflicting data from different studies promoted us to conduct the current meta-analysis by pooling data from 13 included studies, which were all strictly selected according to uniform inclusion and exclusion criteria. Our meta-analysis of studies that enrolled 1062 patients demonstrated that high PD-L1 expression was not associated with poor prognosis in patients with melanoma. In addition, PD-L1 expression remained a non-significant prognostic factor in various subgroups of OS and PFS. PD-L1 overexpression was found to be correlated with the absence of LN metastasis, although the predominant connection was based on the data of 2 studies. The results of this meta-analysis suggest that PD-L1 may be not be predictive of outcomes of melanoma management. To the best of our knowledge, the present study is the first meta-analysis investigating the prognostic significance of PD-L1 expression in melanoma.
Immune escape is essential for cancer development, progression, and resistance to therapy [13]. Lieping Chen et al. identified and cloned the human B7-H1 gene in the year 1999 and found that this molecule could negatively regulate T cell function through the induction of IL-10 [16]. Accumulating evidence shows that PD-L1 plays a central role in the regulation of the immune responses in the tumor microenvironment [50]. PD-L1 binds to PD-1 and inhibits T cell proliferation and its cytokine secretion and leads to apoptosis, anergy, and exhaustion of T cells [51]. Therefore, blockade of the PD-1/PD-L1 interaction is an important therapeutic strategy for cancer. Tumor-intrinsic PD-L1 signals can enhance the ability of melanoma cells to proliferate and metastasize [52]. Melanoma has seen the broadest applications and superior responses to anti-PD-L1/PD-1 therapies [53]. Recent studies have demonstrated that anti-PD-L1 antibody induced durable tumor regression and prolonged stabilization of disease in patients with advanced cancer, including non-small cell lung cancer (NSCLC), melanoma, and colorectal cancer [54]. In addition, the combination of PD-1 and CTLA-4 blockade was more effective than either agent alone in metastatic melanoma [55]. Therefore, there is rationale to identify PD-L1 as a biomarker for assessing cancer therapeutic responses and survival outcomes in patients with melanoma. The findings of our meta-analysis indicate that PD-L1 may not be helpful in prognosis of melanoma, which may be validated in further large-scale prospective clinical trials.
Many previous studies have investigated the impact of PD-L1 on the prognosis of solid tumors through meta-analyses [56]. Iacovelli and colleagues conducted a meta-analysis of 6 studies and showed that increased PD-L1 expression was an independent prognostic factor in renal cell carcinoma [57]. Another meta-analysis also demonstrated that high PD-L1 expression was a poor prognostic biomarker in patients with non-Hodgkin lymphoma [58]. A meta-analysis of studies that enrolled 721 patients also confirmed the prognostic significance of PD-L1 expression in thyroid cancer [59]. However, some meta-analyses failed to identify a significant prognostic effect of PD-L1 in cancer. For example, Fan’s meta-analysis reported a non-significant relationship between PD-L1 expression and OS in NSCLC [60]. Moreover, a more recent study of 1060 patients indicated that PD-L1 overexpression did not correlate with the poor prognosis of patients with oral squamous cell carcinoma (OSCC) [61]. The results of the current meta-analysis in melanoma were in line with the findings of NSCLC and OSCC [60, 61].
Although this is the first meta-analysis of the association between PD-L1 and the prognosis of melanoma, some limitations need to be noted. First, the heterogeneity among studies cannot be ignored. Patient ethnicity, treatment, follow-up, and other factors could influence survival, which may have contributed to this heterogeneity. Second, the included studies used different monoclonal and polyclonal PD-L1 antibodies for IHC, and the cut-off values were not uniform. Third, all included studies were published in the English language, and absence of including studies published in non-English languages may lead to publication bias.
Conclusions
In summary, this meta-analysis suggested that PD-L1 expression did not predict inferior prognosis in patients with melanoma. However, high PD-L1 expression was associated with absence of LN metastasis. Because of the limitations of our meta-analysis, further large-scale and prospective trials that use a uniform cut-off value of PD-L1 expression are needed to verify our results.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- PD-L1:
-
Programmed death-ligand 1
- PD-1:
-
Programmed death-1
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- LN:
-
Lymph node
- AJCC:
-
American Joint Committee on Cancer
- TNM:
-
Tumor, node, metastasis
- IHC:
-
Immunohistochemistry
- EV:
-
Extracellular vesicle
- ICI:
-
Immune checkpoint inhibitors
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- NSCLC:
-
Non-small cell lung cancer
- OSCC:
-
Oral squamous cell carcinoma
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- NOS:
-
Newcastle–Ottawa Scale
- OR:
-
Odds ratio
References
O’Neill CH, Scoggins CR. Melanoma. J Surg Oncol. 2019;120(5):873–81.
Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A, Ugurel S. Melanoma. Lancet. 2018;392(10151):971–84.
Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107(3):362–6.
Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:24.
Berwick M, Erdei E, Hay J. Melanoma epidemiology and public health. Dermatol Clin. 2009;27(2):205.
Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–9.
Gerami P, Busam K, Cochran A, Cook MG, Duncan LM, Elder DE, Fullen DR, Guitart J, LeBoit PE, Mihm MC, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38(7):934–40.
Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20(11):1366–79.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65(1):5–29.
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding SL, et al. Final Version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206.
Fujimura T, Fujisawa Y, Kambayashi Y, Aiba S. Significance of BRAF kinase inhibitors for melanoma treatment: from bench to bedside. Cancers. 2019;11:9.
Weiss SA, Wolchok JD, Sznol M. Immunotherapy of melanoma: facts and hopes. Clin Cancer Res. 2019;25(17):5191–201.
Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27(3):409–16.
Wei R, Guo LB, Wang QS, Miao J, Kwok HF, Lin Y. Targeting PD-L1 protein: translation, modification and transport. Curr Protein Pept Sci. 2019;20(1):82–91.
Jiang XJ, Wang J, Deng XY, Xiong F, Ge JS, Xiang B, Wu X, Ma J, Zhou M, Li XL, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:1–7.
Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9.
Chen LP, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015;125(9):3384–91.
Rahbarghazi R, Jabbari N, Sani NA, Asghari R, Salimi L, Kalashani SA, Feghhi M, Etemadi T, Akbariazar E, Mahmoudi M, et al. Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. CCS. 2019;17(1):73.
Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol. 2016;11(7):964–75.
Liu YX, Wang XS, Wang YF, Hu XC, Yan JQ, Zhang YL, Wang W, Yang RJ, Feng YY, Gao SG, et al. Prognostic significance of PD-L1 expression in patients with gastric cancer in East Asia: a meta-analysis. Oncotarg Ther. 2016;9:2649–54.
Huang LJ, Deng XF, Chang F, Wu XL, Wu Y, Diao QZ. Prognostic significance of programmed cell death ligand 1 expression in patients with ovarian carcinoma A systematic review and meta-analysis. Medicine. 2018;97:43.
Wu Z, Zhang L, Peng J, Xu S, Zhou L, Lin Y, Wang Y, Lu J, Yin W, Lu J. Predictive and prognostic value of PDL1 protein expression in breast cancer patients in neoadjuvant setting. Cancer Biol Ther. 2019;20(6):941–7.
Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, Majores M, Stein J, Uhl B, Muller S, et al. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res. 2016;22(8):1969–77.
Wang B, Pan W, Yang M, Yang W, He W, Chen X, Bi J, Jiang N, Huang J, Lin T. Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Sci. 2019;110(2):489–98.
Gu X, Dong M, Liu Z, Mi Y, Yang J, Zhang Z, Liu K, Jiang L, Zhang Y, Dong S, et al. Elevated PD-L1 expression predicts poor survival outcomes in patients with cervical cancer. Cancer Cell Int. 2019;19:146.
Xu G, Sun L, Li Y, Xie F, Zhou X, Yang H, Du S, Xu H, Mao Y. The clinicopathological and prognostic value of PD-L1 expression in cholangiocarcinoma: a meta-analysis. Front Oncol. 2019;9:897.
Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, Garcia-Aguilar J, Sadot E, Ntiamoah P, Markowitz AJ, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29(11):1433–42.
Liu X, Shan C, Song Y, Du J. Prognostic value of programmed cell death ligand-1 expression in nasopharyngeal carcinoma: a meta-analysis of 1,315 patients. Front Oncol. 2019;9:1111.
Qiu LP, Zheng HL, Zhao XY. The prognostic and clinicopathological significance of PD-L1 expression in patients with diffuse large B-cell lymphoma: a meta-analysis. BMC Cancer. 2019;19:15.
Liang X, Sun J, Wu H, Luo Y, Wang L, Lu J, Zhang Z, Guo J, Liang Z, Liu T. PD-L1 in pancreatic ductal adenocarcinoma: a retrospective analysis of 373 Chinese patients using an in vitro diagnostic assay. Diagn Pathol. 2018;13(1):5.
Budczies J, Mechtersheimer G, Denkert C, Klauschen F, Mughal SS, Chudasama P, Bockmayr M, Johrens K, Endris V, Lier A, et al. PD-L1 (CD274) copy number gain, expression, and immune cell infiltration as candidate predictors for response to immune checkpoint inhibitors in soft-tissue sarcoma. Oncoimmunology. 2017;6(3):e1279777.
Motoshima T, Komohara Y, Ma C, Dewi AK, Noguchi H, Yamada S, Nakayama T, Kitada S, Kawano Y, Takahashi W, et al. PD-L1 expression in papillary renal cell carcinoma. BMC Urol. 2017;17(1):8.
Yang WF, Wong MCM, Thomson PJ, Li KY, Su YX. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. 2018;86:81–90.
Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;21:7.
Cho J, Ahn S, Yoo KH, Kim JH, Choi SH, Jang KT, Lee J. Treatment outcome of PD-1 immune checkpoint inhibitor in Asian metastatic melanoma patients: correlative analysis with PD-L1 immunohistochemistry. Invest New Drugs. 2016;34(6):677–84.
Gadiot J, Hooijkaas AI, Kaiser ADM, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117(10):2192–201.
Johnson DB, Bordeaux J, Kim JY, Vaupel C, Rimm DL, Ho TH, Joseph RW, Daud AI, Conry RM, Gaughan EM, et al. Quantitative spatial profiling of PD-1/PD-L1 interaction and HLA-DR/IDO-1 predicts improved outcomes of anti-PD-1 therapies in metastatic melanoma. Clin Cancer Res. 2018;24(21):5250–60.
Madonna G, Ballesteros-Merino C, Feng Z, Bifulco C, Capone M, Giannarelli D, Mallardo D, Simeone E, Grimaldi AM, Caraco C, et al. PD-L1 expression with immune-infiltrate evaluation and outcome prediction in melanoma patients treated with ipilimumab. Oncoimmunology. 2018;7(12):e1405206.
Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melan Res. 2015;28(3):245–53.
Massi D, Brusa D, Merelli B, Falcone C, Xue G, Carobbio A, Nassini R, Baroni G, Tamborini E, Cattaneo L, et al. The status of PD-L1 and tumor-infiltrating immune cells predict resistance and poor prognosis in BRAFi-treated melanoma patients harboring mutant BRAFV600. Ann Oncol. 2015;26(9):1980–7.
Morrison C, Pabla S, Conroy JM, Nesline MK, Glenn ST, Dressman D, Papanicolau-Sengos A, Burgher B, Andreas J, Giamo V, et al. Predicting response to checkpoint inhibitors in melanoma beyond PD-L1 and mutational burden. J Immunother Cancer. 2018;6(1):32.
Obeid JM, Erdag G, Smolkin ME, Deacon DH, Patterson JW, Chen L, Bullock TN, Slingluff CL. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology. 2016;5(11):e1235107.
Ren M, Dai B, Kong YY, Lv JJ, Cai X. PD-L1 expression in tumour-infiltrating lymphocytes is a poor prognostic factor for primary acral melanoma patients. Histopathology. 2018;73(3):386–96.
Ren Y, Lv Q, Yue W, Liu B, Zou Z. The programmed cell death protein-1/programmed cell death ligand 1 expression, CD3+ T cell infiltration, NY-ESO-1 expression, and microsatellite instability phenotype in primary cutaneous melanoma and mucosal melanoma and their clinical significance and prognostic value: a study of 89 consecutive cases. Melanoma Res. 2019;30:85–101.
Schaper-Gerhardt K, Okoye S, Herbst R, Ulrich J, Terheyden P, Pfohler C, Utikal JS, Kreuter A, Mohr P, Dippel E, et al. PD-L1 status does not predict the outcome of BRAF inhibitor therapy in metastatic melanoma. Eur J Cancer. 2018;88:67–76.
Thierauf J, Veit JA, Affolter A, Bergmann C, Grunow J, Laban S, Lennerz JK, Grunmuller L, Mauch C, Plinkert PK, et al. Identification and clinical relevance of PD-L1 expression in primary mucosal malignant melanoma of the head and neck. Melanoma Res. 2015;25(6):503–9.
Wang HY, Wu XY, Zhang X, Yang XH, Long YK, Feng YF, Wang F. Prevalence of NRAS mutation, PD-L1 expression and amplification, and overall survival analysis in 36 primary vaginal melanomas. Oncologist. 2019;25:2.
Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6:7.
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol. 2019;234(2):1313–25.
Zou WP, Wolchok JD, Chen LP. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328.
Clark CA, Gupta HB, Sareddy G, Pandeswara S, Lao S, Yuan B, Drerup JM, Padron A, Conejo-Garcia J, Murthy K, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 2016;76(23):6964–74.
Frydenlund N, Mahalingam M. PD-L1 and immune escape: insights from melanoma and other lineage-unrelated malignancies. Hum Pathol. 2017;66:13–33.
Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Pyo JS, Kang G, Kim JY. Prognostic role of PD-L1 in malignant solid tumors: a meta-analysis. Int J Biol Markers. 2017;32(1):E68–74.
Iacovelli R, Nole F, Verri E, Renne G, Paglino C, Santoni M, Rocca MC, Giglione P, Aurilio G, Cullura D, et al. Prognostic role of PD-L1 expression in renal cell carcinoma. a systematic review and meta-analysis. Targeted Oncol. 2016;11(2):143–8.
Zhao S, Zhang MH, Zhang Y, Meng HX, Wang Y, Liu YP, Jing J, Huang L, Sun MQ, Zhang Y, et al. The prognostic value of programmed cell death ligand 1 expression in non-Hodgkin lymphoma: a meta-analysis. Cancer Biol Med. 2018;15(3):290–8.
Aghajani M, Graham S, McCafferty C, Shaheed CA, Roberts T, DeSouza P, Yang T, Niles N. Clinicopathologic and prognostic significance of programmed cell death ligand 1 expression in patients with non-medullary thyroid cancer: a systematic review and meta-analysis. Thyroid. 2018;28(3):349–61.
Fan YW, Ma K, Hu Y, Niu WX, Li EX, Wu YY. Prognostic value of PD-L1 expression in non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med. 2017;10(6):8735.
Troiano G, Caponio VCA, Zhurakivska K, Arena C, Pannone G, Mascitti M, Santarelli A, Lo Muzio L. High PD-L1 expression in the tumour cells did not correlate with poor prognosis of patients suffering for oral squamous cells carcinoma: a meta-analysis of the literature. Cell Prolif. 2019;52:2.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
JY, MD, Y Shui, YZ and YM designed the study. ZZ, LJ, KL, JY, MD, and XG performed the literature searches and assessed the quality of included studies. YZ, KL, ZL, and XZ analyzed the data. XG and Y Shi wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, J., Dong, M., Shui, Y. et al. A pooled analysis of the prognostic value of PD-L1 in melanoma: evidence from 1062 patients. Cancer Cell Int 20, 96 (2020). https://doi.org/10.1186/s12935-020-01187-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-020-01187-x