Abstract
Background
The immune escape or tolerance of cancer cells is considered to be closely involved in cancer progression. Programmed death-1 (PD-1) is an inhibitory receptor expressed on activating T cells, and several types of cancer cells were found to express PD-1 ligand 1 (PD-L1) and ligand 2 (PD-L2).
Methods
In the present study, we investigated PD-L1/2 expression in papillary renal cell carcinoma (pRCC).
Result
We found PD-L1 expression in 29 of 102 cases, but no PD-L2 expression was seen. PD-L1 expression was not significantly correlated with any clinicopathological factor, including progression-free survival and overall survival. The frequency of PD-L1-positive cases was higher in type 2 (36%) than in type 1 (22%) pRCC; however, there was no significant difference in the percentages of score 0 cases (p value = 0.084 in Chi-square test). The frequency of high PD-L1 expression cases was higher in type 2 (23%) than in type 1 (11%), and the frequency of high PD-L1 expression cases was higher in grade 3/4 (21%) than in grade 1/2 (13%). However, no significant association was found between PD-L1 expression and all clinicopathological factors in pRCC.
Conclusion
High expression of PD-L1 in cancer cells was potentially associated to highly histological grade of malignancy in pRCC. The evaluation of the PD-L1 protein might still be useful for predicting the efficacy of anti-cancer immunotherapy using immuno-checkpoint inhibitors, however, not be useful for predicting the clinical prognosis.
Similar content being viewed by others
Background
Renal cell carcinoma (RCC) is a common cancer of the kidney, and the three most frequent histological subtypes are clear cell RCC (ccRCC, 70 to 80%), papillary RCC (pRCCc, 10 to 20%), and chromophobe RCC (5%) [1]. Although patients with sporadic RCC of any histological subtype usually show a good clinical outcome, patients with metastatic pRCC show a significantly worse clinical course than patients with ccRCC or chromophobe RCC [2–4]. Recent findings have indicated that MET gene activation, which is known to promote proliferative activity and cell survival, is frequently observed in pRCC, and MET inhibitors have become a new type of therapeutic agent for patients with advanced pRCC [5, 6].
Anti-cancer immune responses were considered to play important roles in preventing cancer progression in RCC [7]; however, immunotherapy against advanced RCC such as interferon therapy and vaccine therapy has shown limited anti-cancer effects over the past fewdecades [8]. In recent years, immuno-checkpoint inhibitors have attracted much attention. Anti-CTLA-4 antibodies, which are used to treat advanced melanoma patients, have been reported to have an excellent therapeutic effect [9]. Subsequently, anti-PD-1 antibody was discovered and used to treat kidney cancer, non-small cell lung cancer and malignant melanoma patients, and superior therapeutic effects have been reported [10]. Some clinical trials demonstrated that combination therapy using anti-CTLA-4 antibody and anti-PD-1 antibody produced significant anti-cancer effects [11]. However, although the antibodies produced excellent therapeutic effects in most patients, no therapeutic effect was observed in some patients. PD-1 ligand 1 (PD-L1) expression in cancer tissues is considered to be a biomarker for predicting the therapeutic effect of immuno-checkpoint inhibitors [12]. Although some studies have demonstrated that a high expression of PD-L1 was associated with poor clinical outcomes [13–17], few studies have investigated PD-L1 expression in pRCC. Therefore, we analyzed the correlation between PD-L1 expression and clinicopathological factors in pRCC.
Methods
Patients and Samples
We reviewed 102 cases of pRCC that were excised at Kumamoto University, the University of Occupational and Environmental Health, and Kyushu University between 2001 and 2014. All samples were obtained with informed consent from patients in accordance with the study protocols that were approved by the review board of each university (Kumamoto University Hospital Review Board, Kyushu University Review Board, Review Board of University of Occupational and Environmental Health). Tissue samples of primary site were fixed in 10% neutral buffered formalin and were embedded in paraffin as per a routine method. Nuclear grade and T classification were assessed according to the World Health Organization classification. Patient characteristics, such as age, gender, Fuhrman grade, pathological TNM stage and follow-up data were retrospectively collected.
Immunohistochemistry
Rabbit monoclonal antibodies against PD-L1 (clone E1L3N) and PD-L2 (clone D7U8C) were purchased from Cell Signaling Technology (Danvers, MA, USA). Briefly, after samples were reacted with primary antibodies, they were incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibodies (Nichirei, Tokyo, Japan). Can Get Signal Solution (TOYOBO, Tokyo, Japan) was used to dilute the antibodies for enhancing the immunoreaction. Reactions were visualized using the diaminobenzidine substrate system (Nichirei). Two pathologists (TM and YK), who were blinded to information about the samples, evaluated the immunostaining of PD-L1/2.
Cell lines
Two lymphoma cell lines (PD-L1/2-positive cell line; ATL-T, PD-L1/2-negative cell line; DAUDI) were obtained from RIKEN Cell Bank (Tsukuba, Japan), and cell block specimens were used for confirmation of specificities of anti-PD-L1/2 antibodies. Cells were fixed in 10% neutral buffered formalin and cell block samples were embedded in paraffin.
Statistics
Statistical analysis was carried out with StatMate III (Atoms, Tokyo, Japan). The simultaneous relationships between multiple prognostic factors for survival were assessed using the Cox proportional hazards model with a stepwise backwards reduction. A value of p < 0.05 was considered to be statistically significant.
Results
PD-L1/B7-H1 expression in pRCC and correlations with clinicopathological factors
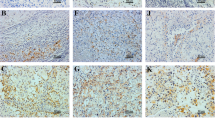
At first, we confirmed the specificities of anti-PD-L1 and PD-L2 monoclonal antibodies used for immunostaining using cell block samples of two cell lines (Fig. 1a). In total, 102 resected specimens of pRCC were used for immunostaining of PD-L1/B7-H1; the characteristics of the patients are shown in Table 1. PD-L1-positive cancer cells were detected in 29 of the 102 cases, and the positive signals were detected on the cell surface membrane and cytoplasm of the cancer cells (Fig. 1b). Macrophages stained positive for PD-L1 in 4 cases (Fig. 1c), but no infiltrated lymphocytes were positive for PD-L1. Notably, neurons were strongly positive for PD-L1 in all cases (Fig. 1d). In contrast, no expression of PD-L2 was observed on any of the cancer cells or infiltrating macrophages and lymphocytes (Fig. 1e).
PD-L1/2 expression in pRCC a Pictures of immunostaining of PD-L1/2 in two cell lines (PD-L1/2-positive cell line; ATL-T, PD-L1/2-negative cell line; DAUDI) b Pictures of H.E. staining and immunostaining of PD-L1 in 2 cases of pRCC. Positive signals are detected most strongly in cancer cells c Positive signals for PD-L1 are also detected in macrophages d Positive signals for PD-L1 are also detected in neuronal fibers e PD-L2 is detected in dendritic cells in the lymph node, whereas no PD-L2 is observed in the cancer cells of any of the pRCC cases
Next, we classified the PD-L1 staining patterns into three scores according to the percentage of PD-L1-positive cancer cells: score 0, negative or less than 2%; score 1, 2 to 30%; and score 2, more than 30% (Fig. 2a, Additional file 1 table S1). Overall, the most frequent pattern was score 0 (71%), while score 1 and score 2 comprised 11 and 18%, respectively, of the cases. Since there are two histological subtypes of pRCC (types 1 and 2), the frequencies of scores 0 1 and 2 were compared between the subtypes. The frequency of score 1/2 (PD-L1-positive) was higher in type 2 (36%) than in type 1 (22%) pRCC; however, there was no significant difference in the percentages of score 0 cases (p value = 0.084 in Chi-square test, Fig. 2). Next, the frequencies of scores 0, 1 and 2 were compared between the nuclear grades. The frequency of score 1/2 (PD-L1-positive) was slightly higher in grade 3/4 (32%) than in grade 1/2 (25%); however, there was no significant difference between grade 1/2 and grade 3/4 (p value = 0.47 in Chi-square test, Fig. 2). The percentage of score 2 seemed to be higher in Type 2 (23%) or grade 3/4 (21%) than that in Type 1 (11%) or grade 1/2 (13%); however, however, there was no significant difference (p value = 0.42 or 0.36 in Chi-square test respectively, Fig. 2). Although score 0 cases seemed to have shorter progression-free survival and longer cancer specific overall survival, there was no significant correlation between PD-L1 scores and clinical course (Fig. 3).
Discussion
Expression of PD-L1 is associated with a poor clinical course in colorectal cancer, lung cancer, ovarian cancer, and ccRCC [17–22]. In this study, we demonstrated that PD-L1 expression on cancer cells is not useful as a biomarker in pRCC. PD-L1 expression is positive in approximately 50% of ccRCC cases [13, 17, 18], which is much higher than in the pRCC cases of the present study. PD-L1 expression is known to be induced by infiltrating T cell-derived interferon γ. It is well known that ccRCC is an immunogenic cancer and many T-cell infiltrations are detected in ccRCC [23]. However, T-cell infiltration was lower in pRCC than in ccRCC in our preliminary observations (data not shown). PD-L1 expression and the density of infiltrating CD8-positive T cells have been shown to be well correlated in ccRCC [24]. The differences in the frequencies of PD-L1-positive cells between ccRCC and pRCC might be due to the immunogenicity of the cancer cells.
PD-L1 expression is also known to be detected in infiltrating leukocytes, including macrophages [25]; however, PD-L1 expression was observed only in macrophages in 4 of the 102 cases. PD-L1 expression in macrophages is induced by cancer-derived factors, and macrophage-derived interleukin 10 is involved in the induction of PD-L1 expression [26], suggesting that cell-cell interaction with cancer cells is necessary for PD-L1 expression in macrophages. The density of macrophages is closely associated with poor clinical prognosis in ccRCC [27], but no report has yet described the relationship between macrophages and clinical course in pRCC. The discrepancy in PD-L1 expression in macrophages between ccRCC and pRCC might be due to the induction of cell-cell interactions between cancer cells and macrophages.
Although PD-L2 expression in cancer cells has been reported in ovarian cancer [16], there are much fewer studies on PD-L2 than on PD-L1 in cancer tissues. This might be due to the fact that no monoclonal antibody suitable for use on paraffin sections had been commercially available. However, a new monoclonal antibody against PD-L2 has recently been made commercially available [28], and as a result, some research articles related to PD-L2 expression have just been published. One such article stated that PD-L2 expression was seen in 49% of pRCC cases; however, no pictures were published [29]. The staining density of PD-L2 expression seemed to be lower than that of PD-L1 expression (Fig. 1a), therefore, appropriate positive or negative controls are required and should be presented in research articles to indicate whether the immunostaining procedure was correctly performed.
Conclusion
In the present study, PD-L1 expression was observed in 28% of the pRCC cases. The frequency of score 2 was high in type 2 or higher nuclear grade cases. Although PD-L1 expression appeared to be related to worse overall survival, there was no significant correlation between PD-L1 expression and clinical prognosis. Further studies are necessary to evaluate if PD-L1 expression might be useful for predicting the efficacy of anti-cancer immunotherapy using immuno-checkpoint inhibitors.
Abbreviations
- PD-1:
-
Programmed death-1
- RCC:
-
Renal cell carcinoma
- ccRCC:
-
Clear cell RCC
- pRCC:
-
Papillary RCC
- CTLA-4:
-
Cytotoxic T lymphocyte antigen 4
- PD-L1/2:
-
Programmed death-1 ligand 1/2
References
Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90.
Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–81.
Beck SD, Patel MI, Snyder ME, Kattan MW, Motzer RJ, Reuter VE, Russo P. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11:71–7.
Ronnen EA, Kondagunta GV, Ishill N, Spodek L, Russo P, Reuter V, Bacik J, Motzer RJ. Treatment outcome for metastatic papillary renal cell carcinoma patients. Cancer. 2006;107:2617–21.
Albiges L, Guegan J, Le Formal A, Verkarre V, Rioux-Leclercq N, Sibony M, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20:3411–21.
Schuller AG, Barry ER, Jones RD, Henry RE, Frigault MM, Beran G, et al. The MET inhibitor AZD6094 (savolitinib, HMPL-504) induces regression in papillary renal cell carcinoma patient-derived xenograft models. Clin Cancer Res. 2015;21:2811–9.
Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–561.
Janowitz T, Welsh SJ, Zaki K, Mulders P, Eisen T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin Oncol. 2013;40:482–91.
Dequen P, Lorigan P, Jansen JP, van Baardewijk M, Ouwens MJ, Kotapati S. Systematic review and network meta-analysis of overall survival comparing 3 mg/kg ipilimumab with alternative therapies in the management of pretreated patients with unresectable stage III or IV melanoma. Oncologist. 2012;17:1376–85.
Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–91.
Lussier DM, Johnson JL, Hingorani P, Blattman JN. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. 2015;3:21.
Fusi A, Festino L, Botti G, Masucci G, Melero I, Lorigan P, Ascierto PA. PD-L1 expression as a potential predictive biomarker. Lancet Oncol. 2015;16:1285–7.
Jilaveanu LB, Shuch B, Zito CR, Parisi F, Barr M, Kluger Y, Chen L, Kluger HM. PD-L1 expression in clear cell renal cell carcinoma: an analysis of nephrectomy and sites of metastases. J Cancer. 2014;5:166–72.
Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s.
Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5.
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5.
Xu F, Xu L, Wang Q, An G, Feng G, Liu F. Clinicopathological and prognostic value of programmed death ligand-1 (PD-L1) in renal cell carcinoma: a meta-analysis. Int J Clin Exp Med. 2015;15:14595–603.
Leite KR, Reis ST, Junior JP, Zerati M, Gomes Dde O, Camara-Lopes LH, Srougi M. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn Pathol. 2015;10:189.
Iacovelli R, Nolè F, Verri E, Renne G, Paglino C, Santoni M, Cossu Rocca M, Giglione P, et al. Prognostic Role of PD-L1 Expression in Renal Cell Carcinoma. A Systematic Review and Meta-Analysis. Target Oncol. 2015 In press
Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–40.
Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10:426–30.
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013;8:e76012.
Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8 (+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–6.
Liu XD, Hoang A, Zhou L, Kalra S, Yetil A, Sun M, et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res. 2015;3:1017–29.
Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res. 2015;3:1158–64.
Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165–75.
Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1–8.
Horlad H, Ma C, Yano H, Pan C, Ohnishi K, Fujiwara Y, et al. An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci 2016 in press
Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH, Go H. Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol. 2016;23:694–702.
Acknowledgements
Not applicable
Funding
This work was supported by research grants from KAKENHI (#25,293,089, #25,460,497).
Availability of data and materials
Due to ethical restrictions, the raw data underlying this paper is available upon request from the corresponding author.
Authors’ Contributions
TM, YK, CM, and AKD carried out the immunostaining and evaluate the results. HN, SY, TN, SK, YK, MS, YO and WT participated in the collection of tissue sections and clinicopathological data. TM, TK, MT, NF, and ME participated in the design of the study, performed the statistical analysis, and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All samples were obtained with informed consent from patients in accordance with the study protocols that were approved by the review board of each university (Kumamoto University Hospital Review Board, Kyushu University Review Board, Review Board of University of Occupational and Environmental Health) and written informed consent was obtained from the patients for their data to be used for research purposes.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
The data of PD-L1 and clinicopatholgical factors (DOCX 30 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Motoshima, T., Komohara, Y., Ma, C. et al. PD-L1 expression in papillary renal cell carcinoma. BMC Urol 17, 8 (2017). https://doi.org/10.1186/s12894-016-0195-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-016-0195-x