Abstract
Background
Cocoa butter (CB) extracted from cocoa beans (Theobroma cacao) is the main raw material for chocolate production, but CB supply is insufficient due to the increased chocolate demand and limited CB production. CB is mainly composed of three different kinds of triacylglycerols (TAGs), 1,3-dipalmitoyl-2-oleoyl-glycerol (POP, C16:0-C18:1-C16:0), 1-palmitoyl-3-stearoyl-2-oleoyl-glycerol (POS, C16:0-C18:1-C18:0) and 1,3-distearoyl-2-oleoyl-glycerol (SOS, C18:0-C18:1-C18:0). In general, Saccharomyces cerevisiae produces TAGs as storage lipids, which consist of C16 and C18 fatty acids. However, cocoa butter-like lipids (CBL, which are composed of POP, POS and SOS) are not among the major TAG forms in yeast. TAG biosynthesis is mainly catalyzed by three enzymes: glycerol-3-phosphate acyltransferase (GPAT), lysophospholipid acyltransferase (LPAT) and diacylglycerol acyltransferase (DGAT), and it is essential to modulate the yeast TAG biosynthetic pathway for higher CBL production.
Results
We cloned seven GPAT genes and three LPAT genes from cocoa cDNA, in order to screen for CBL biosynthetic gene candidates. By expressing these cloned cocoa genes and two synthesized cocoa DGAT genes in S. cerevisiae, we successfully increased total fatty acid production, TAG production and CBL production in some of the strains. In the best producer, the potential CBL content was eightfold higher than the control strain, suggesting the cocoa genes expressed in this strain were functional and might be responsible for CBL biosynthesis. Moreover, the potential CBL content increased 134-fold over the control Y29-TcD1 (IMX581 sct1Δ ale1Δ lro1Δ dga1Δ with TcDGAT1 expression) in strain Y29-441 (IMX581 sct1Δ ale1Δ lro1Δ dga1Δ with TcGPAT4, TcLPAT4 and TcDGAT1 expression) further suggesting cocoa GPAT and LPAT genes functioned in yeast.
Conclusions
We demonstrated that cocoa TAG biosynthetic genes functioned in S. cerevisiae and identified cocoa genes that may be involved in CBL production. Moreover, we found that expression of some cocoa CBL biosynthetic genes improved potential CBL production in S. cerevisiae, showing that metabolic engineering of yeast for cocoa butter production can be realized by manipulating the key enzymes GPAT, LPAT and DGAT in the TAG biosynthetic pathway.
Similar content being viewed by others
Background
Theobroma cacao, also known as cacao tree or cocoa tree, is an evergreen tree distributed in tropical areas [1, 2]. Its seeds, cocoa beans, can be used for extraction of cocoa butter (CB), which is a raw material for chocolate production. With chocolate demand increasing, more CB is needed [2]. Considering that cocoa trees grow only in the tropics and that replacing tropical forest with cocoa trees is not acceptable, planting more cocoa trees is not the choice for increasing CB production [3]. Moreover, CB production is easily affected by climate change, pest harm and microbial disease [4, 5]. Therefore, CB supply is limited and insufficient, and developing other stable and sustainable sources of CB supply is of interest [1].
Triacylglycerols (TAGs) of 1,3-dipalmitoyl-2-oleoyl-glycerol (POP, C16:0-C18:1-C16:0), 1-palmitoyl-3-stearoyl-2-oleoyl-glycerol (POS, C16:0-C18:1-C18:0) and 1,3-distearoyl-2-oleoyl-glycerol (SOS, C18:0-C18:1-C18:0) composed of C16 and C18 fatty acids are the three main components in CB [6]. Plant-derived CB-like lipids (CBL, mainly composed of POP, POS and SOS), such as illipe butter, shea butter and kokum butter, can be used as CB equivalents, but they are extracted from tropical plants as well and their lipid production is also limited [6]. In general, yeasts produce TAGs which consist of C16 and C18 fatty acids as storage lipids, making them good candidates for CBL production [7]. However, in the model yeast Saccharomyces cerevisiae, the naturally occurring CBL content is quite low [8]. Even though several oleaginous yeasts contain high amounts of lipids and their lipids have been considered as potential CB substitutes before, the small amount of naturally occurring CBL hindered their application in chocolate production [8, 9].
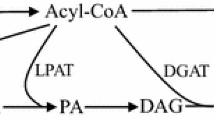
TAG biosynthesis is mainly catalyzed by glycerol-3-phosphate acyltransferase (GPAT), lysophospholipid acyltransferase (LPAT) and diacylglycerol acyltransferase (DGAT), which add acyl chains from acyl-coenzyme A (acyl-CoA) to the sn-1, sn-2 and sn-3 position of the backbone glycerol, respectively [10]. Therefore, besides sufficient C16 and C18 supply, modulation of yeast GPAT, LPAT and DGAT activity is essential for higher CBL production. There are two GPATs (Gpt2p and Sct1p), two LPATs (Slc1p and Ale1p), one DGAT (Dga1p) and one phospholipid:diacylglycerol acyltransferase, PDAT (Lro1p) that participate in TAG synthesis in S. cerevisiae (Fig. 1) [11,12,13,14]. The simultaneous deletion of either the two GPAT genes or the two LPAT genes (SLC1 and ALE1) is lethal to S. cerevisiae, but double deletion of DGA1 and LRO1 genes does not affect yeast growth [11, 15]. Single deletion of one GPAT gene or one LPAT gene alters yeast fatty acid profiles. For example, Sct1p has a preference towards C16 fatty acids and sct1∆ cells showed a different distribution of fatty acids in the phospholipids with less C16 fatty acids [11, 15]. Thus, disruption of some genes of the yeast TAG biosynthetic pathway and introducing corresponding heterologous genes would likely alter yeast CBL production. Overexpression of several synthesized cocoa TAG biosynthetic genes increased the CBL content in S. cerevisiae, but CBL production of these S. cerevisiae strains was still low [9]. As there are thirteen GPAT and nine LPAT genes in the cocoa genome [16, 17], deep and global characterization of cocoa GPAT and LPAT genes might reveal optimal cocoa genes responsible for CB biosynthesis, and expressing them in S. cerevisiae could improve CBL production [9].
Three Enzymes, GPAT, LPAT and DGAT, determine the TAG structure in the TAG biosynthetic pathway. G3P glycerol-3-phosphate, LPA lysophosphatidic acid, PA phosphatidic acid, DAG diacylglycerol, TAG triacylglycerol, GPAT glycerol-3-phosphate acyltransferase, LPAT lysophosphatidic acid acyltransferase, DGAT acyl-CoA:diacylglycerol acyltransferase, PDAT phospholipid:diacylglycerol acyltransferase. SCT1 and GPT2 are GPAT genes, SLC1 and ALE1 are LPAT genes, DGA1 is the DGAT gene, LRO1 is the PDAT gene. The genes in red were deleted in some of the yeast strains used in this study
Here, we cloned and expressed ten cocoa CB biosynthetic genes individually in S. cerevisiae, and compared the lipid production of the engineered yeasts. We also expressed some of the cloned cocoa genes together with two previously characterized cocoa DGAT genes in order to increase yeast CBL production. Moreover, we improved CBL production by metabolic engineering of the TAG biosynthetic pathway of S. cerevisiae.
Methods
Strains, plasmids, and media
The cloning host in this study was Escherichia coli strain DH5α. The Lipomyces starkeyi strain DSM 70296 was purchased from the culture collection of the DSMZ (Braunschweig, Germany). The S. cerevisiae strain CEN.PK 113-11C (MATa MAL2-8c SUC2 ura3-52 his3-Δ1), which was kindly provided by P. Kötter [18] and S. cerevisiae strain IMX581 (MATa ura3-52 can1∆::cas9-natNT2 TRP1 LEU2 HIS3) [19] were used in this study. S. cerevisiae CEN.PK 113-11C was used to express cocoa genes and homologous recombination was used to construct S. cerevisiae CEN.PK 113-11C-derived strains (Additional file 1: Figure S1). The plasmids pMEL10 and pMEL13 [19] were used for construction of strains derived from IMX581. All primers used to construct the yeast strains are listed in Additional file 1: Table S1, and all yeast strains constructed and used in this study are listed in Table 1. The strains YJ0 and SYJ0 were the same, but they were constructed and tested at different time as the control strain (Table 1).
Yeast strains were selected on synthetic complete (SC) dropout medium (Formedium Ltd) or YPD medium (10 g l−1. Bacto yeast extract, 20 g l−1 Bacto peptone and 20 g l−1 glucose) (Merck Millipore or Difco) containing 200 mg ml−1 G418. L. starkeyi was cultivated on YPD medium. Minimal medium (7.5 g l−1 (NH4)2SO4, 14.4 g l−1 KH2PO4, 0.5 g l−1 MgSO4·7H2O, 20 g l−1 glucose, trace metal solution and vitamin solution), supplemented with 100 mg l−1 histidine or 200 mg l−1 G418 (Formedium), was used for 20 ml shake flask batch cultivations [20, 21]. A nitrogen-limited medium (named NLM medium in the text) [22] was used for large-scale (1 l) shake flask batch cultivations.
Cocoa sample collection
The cocoa fruit samples were collected from the same tree in the Gothenburg Botanical Garden in a nearly ripe (May 26, 2015) and unripe (October 12, 2015) state, respectively (Additional file 1: Figure S2). One ripe cocoa fruit and several unripe cocoa fruits including small and big fruits were collected, respectively. After collection, the cocoa samples were cut into small pieces and immediately put into liquid nitrogen. The samples were kept at − 80 °C before use.
RNA preparation and cDNA synthesis
Cocoa fruits were grinded to fine powder in liquid N2 using mortar and pestle, and the fine powder was divided into 100 mg aliquots in 1.5 ml Eppendorf tubes. Subsequently, 0.5 ml cold (4 °C) PureLink plant RNA reagent (Life Technologies) was added to each tube, mixed briefly by vortexing until the sample was thoroughly resuspended, and then the tubes were incubated for 5 min at room temperature. The resulting solution was cleared by centrifuging at 12,000g in a microcentrifuge for 2 min at room temperature. Next, the lysate was transferred to a QIAshredder spin column placed in a 2 ml collection tube and the instructions of the RNeasy plant mini kit (Qiagen) were followed to obtain total RNA. For extraction of RNA from L. starkeyi, the yeast was cultivated in YPD medium for 48 h at 30 °C and 200 rpm, and the biomass was collected by centrifugation at 12,000 rpm for 1 min. The yeast RNA was extracted using the Qiagen RNeasy mini kit. The cocoa and yeast RNA was transcribed into cDNA by reverse transcriptase using the Qiagen QuantiTect Reverse Transcription Kit.
Phylogenetic analysis of cocoa GPAT, LPAT and DGAT genes
The GPAT and LPAT gene sequences of T. cacao were downloaded from the Genbank database. Reference GPAT and LPAT genes sequences of Arabidopsis thaliana, Homo sapiens and S. cerevisiae were downloaded from the KEGG database [23]. Amino acid sequences of GPATs, LPATs or DGATs were aligned using the MAFFT online version [24], and the multiple alignment results were used to create phylogenetic trees using the MEGA 7.0.21 software [25]. The Neighbor-Joining method with Poisson correction was used to create the tree and the bootstrap confidence values were based on 1000 replicates. Moreover, gaps in the alignment of GPAT and LPAT sequences were treated with the pairwise deletion option.
Plasmid and yeast strain construction
Cocoa genes encoding GPATs and LPATs were amplified using primers described in Additional file 1: Table S1 from cocoa fruit cDNA using the PrimeSTAR HS DNA polymerase (Takara) according to the manufacturer’s instruction. The one GPAT and one LPAT sequences amplified from cocoa cDNA, which were different from the available annotated genes, were deposited at the GenBank database under the accession numbers MF352000-MF352001. The primers used to amplify cocoa genes, promoters and terminators are listed in Additional file 1: Table S1 and some primers used for cocoa gene combination expression were the same as described before [9]. The LsGPAT gene was cloned from L. starkeyi cDNA using PrimeSTAR HS DNA polymerase (Takara) and the sequence was the same as described before [26].
The cocoa gene expression cassettes were verified by sequencing and illustrated in Fig. 2. Gibson assembly (NEB) was used to construct cocoa gene expression plasmids by ligation of the gene expression cassettes and the amplified linear backbone fragment of plasmid pBS01A, and were further verified by PCR and Sanger sequencing (Additional file 1: Table S2). The constructed plasmids were used to transform S. cerevisiae to construct the strains listed in Table 1.
Shake flask cultivation and lipid analysis
Shake flask fermentations were carried out in 20 ml minimal medium and cultivated at 30 °C and 200 rpm for 24 h. [9, 27]. Each strain was cultivated in three replicates, and the fatty acid methyl ester (FAME) and lipid profiles were analyzed using a microwave-assisted method [28, 29]. In order to obtain sufficient lipids for TAG analyses, 5 l shake flasks containing 1 l NLM medium were used for yeast biomass collection [9, 27]. The lipids extracted from each strain were used to analyze yeast TAG profiles by UPLC using RI detection [30]. The TAG compositions of each strain were expressed in relative area percentages [27, 30]. CB standards and TAG composition sequences were completed by AAK, and TAG standards were purchased from Larodan by AAK.
Results
Seven cocoa GPAT and three cocoa LPAT were cloned from cocoa cDNA
Usually, there are many GPAT and LPAT genes in one plant species, and 13 genes were annotated as GPAT genes and nine genes were annotated as LPAT genes (TcLPAT10 was removed as a result of standard genome annotation processing) in T. cacao [16, 17]. To characterize potential GPAT and LPAT genes, we designed primers to clone all the possible GPAT and LPAT genes except two GPAT (TcGPAT1 and TcGPAT2) and two LPAT (TcLPAT1 and TcLPAT2) genes, which had been synthesized and characterized in yeast before [9]. A total of seven GPAT and three LPAT genes were cloned from cocoa fruit cDNA. Most of the gene sequences were consistent with the genes described in the genomic data, except two genes, TcGPAT12-4 and TcLPAT3-2, which were different from the published corresponding gene sequences (Fig. 3). TcGPAT12-4 and TcLPAT3-2 were 231 and 114 bp shorter than TcGPAT12 and TcLPAT3, respectively, this might be due to the alternative splicing in plants [31]. Though we tried several different PCR conditions to clone more cocoa GPAT and LPAT genes, no other genes were further cloned from cocoa cDNA samples.
Phylogenetic analysis of GPAT (a) and LPAT (b) amino acid sequences with an unrooted tree. All neighbor-joining trees were constructed using the MEGA 7.0.21 software (bootstrap values: 1000) with the peptide sequences. Cocoa genes are marked with rhombuses, genes marked with red rhombuses are genes cloned from cDNA of T. cacao in this study, genes marked with green rhombuses are genes synthesized in a previous study, genes marked with black rhombuses are genes which were not cloned or synthesized in this study; yeast genes are marked with purple triangles; genes of A. thaliana and H. sapiens are not marked. The bootstrap values are marked above the nodes and the scale bar is indicated under each tree
All cocoa GPAT sequences and their reference sequences can be divided into five clades in a phylogenetic tree, and the cocoa GPAT sequences were distributed in four clades except Clade 4 which contained yeast GPAT genes (Fig. 3a). The highest level of identity between the amino acids sequence of any cocoa GPAT gene and the yeast GPAT genes is less than 15%. At the same time, the identities between yeast GPAT genes and the Lipomyces starkeyi GPAT gene are 45% (Gpt2p and LsGPAT) and 41% (Sct1p and LsGPAT), respectively (Fig. 3a). We successfully cloned TcGPAT3, TcGPAT4, TcGPAT5, TcGPAT9, TcGPAT10, and TcGPAT12. TcGPAT2 had been characterized before, so that each clade had at least one representative cloned or synthesized cocoa GPAT sequence (Fig. 3a). The LPAT sequences can be divided into four clades, and the cocoa genes TcLPAT3, TcLPAT4 and TcLPAT5 all formed part of LPAT clade 1 (Fig. 3b). The two cocoa DGAT genes, TcDGAT1 and TcDGAT2, have been characterized before. Additional annotated DGATs (TcDGAT3-TcDGAT11) were similar with wax ester synthase genes of A. thaliana, hinting they would not participate in TAG biosynthesis [9].
Expression of single cocoa genes in S. cerevisiae changed its total fatty acid production
The cloned cocoa genes were assembled in expression cassettes with strong constitutive promoters and ligated into plasmid pBS01A as described in Fig. 2 and Additional file 1: Table S2. The empty plasmid pBS01A and 10 other plasmids harboring cocoa genes were introduced into S. cerevisiae CEN.PK 113-11C, resulting in the control strain YJ0 and another 10 yeast strains, respectively (Table 1). Fatty acid analysis showed that the most abundant fatty acids in each strain were C16 and C18 fatty acids and some yeast strains harboring cocoa genes produced more fatty acids than the control strain YJ0 (Fig. 4). Especially, yeast strains YJ-G03, YJ-G04, YJ-L03 and YJ-L04 displayed a significant increase of the total fatty acid amount compared to YJ0, indicating that TcGPAT3, TcGPAT4, TcLPAT3-2 and TcLPAT4 were active in the yeast and might be engaged in cocoa CB biosynthesis.
Total fatty acid production in different S. cerevisiae strains expressing single cocoa genes. Others represents the summed content of C12:0, C14:0, C14:1, C20:0, C20:1, C22:0, C24:0 and C26:0 fatty acids. The error bars represent the standard deviation of three biological replicates. Asterisks (*) indicate significant difference between the yeast strains harboring cocoa genes and control strain YJ0. *p < 0.05; **p < 0.01. The p values are calculated based on paired t tests corrected for multiple comparisons
Expression of several cocoa gene combinations in S. cerevisiae altered lipid production and compositions
Six cocoa genes (including the four cDNA-derived cocoa genes TcGPAT3, TcGPAT4, TcLPAT3-2, TcLPAT4, and the two cocoa DGAT genes TcDGAT1 and TcDGAT2) were assembled in eight different combinations to generate 8 additional yeast strains (Table 1). Among all these strains, four (SYJ-331, SYJ-341, SYJ-441 and SYJ-442) displayed a significant increase of total fatty acids (19–84% increase) over the control strain SYJ0 (Fig. 5a). For C16 and C18 fatty acids of the eight strains, C16:0, C16:1 and C18:0 contents of SYJ-331, C18:0 and C18:1 contents of SYJ-432, C16:0, C16:1, C18:0 and C18:1 contents of SYJ-441, and C16:0, C16:1 and C18:1 contents of SYJ-442 increased and showed significant difference compared with SYJ0.
Total fatty acid (a) and lipid (b) production of S. cerevisiae strains harboring combinations of cocoa genes. SE steryl esters. Asterisks (*) of indicate significant differences of fatty acids (a) and lipids (b) between strains harboring the empty plasmid and strains harboring cocoa genes; *p < 0.05; **p < 0.01. The p values are calculated based on paired t tests corrected for multiple comparisons
The neutral lipids steryl esters (SE) and TAG are the two main storage lipids in S. cerevisiae, whose production is affected by the expression of GPAT, LPAT and DGAT genes [14, 32, 33]. The SE content analysis showed that SYJ-341, SYJ-342 and SYJ-441 displayed a significant increase over the control strain SYJ0, while the TAG content analysis showed that SYJ-331, SYJ-341, SYJ-342 and SYJ-441 displayed a significant increase over the control (Fig. 5b), suggesting expression of some cocoa gene combinations were beneficial for yeast storage lipid production. Among the eight strains, SYJ-341 and SYJ-441 produced 150 and 320% more SE than SYJ0, respectively, and they also produced 210% and 290% more TAG than SYJ0, respectively.
Cocoa gene expression partially restored neutral lipid production in a S. cerevisiae mutant with a partial TAG biosynthesis pathway
To further verify if the cloned cocoa GPAT and LPAT genes functioned in S. cerevisiae, we deleted parts of the yeast TAG biosynthetic pathway, including the GPAT gene SCT1, the LPAT gene ALE1, the DGAT gene DGA1 and the PDAT gene LRO1, to construct new yeast strain Y29 and expressed TAG biosynthetic genes in it (Table 1). Compared with the control strain SYJ0, the SE content was reduced, but specifically the TAG content drastically decreased (Figs. 5b, 6b). With regards to total fatty acids, Y29-TcD1 and Y29-441 displayed a significant difference with the control Y29-pBS01A, and the concentration of C16:0 in Y29-TcD2 and Y29-331 increased and showed significant differences with the control (Fig. 6a). Concerning the neutral lipids, SE and TAG in Y29-331 and Y29-441 displayed significant increase over the control strain. Besides, SE in Y29-TcD2 also showed significant increase compared to the control strain (Fig. 6b). Moreover, we also attempted to delete one yeast GPAT gene (SCT1 or GPT2) and replace the other gene with one cocoa GPAT gene, but we failed to obtain viable colonies. However, when we used LsGPAT of L. starkeyi to replace yeast SCT1 in the yeast strain carrying the GPT2 deletion, it succeeded and yeast fatty acid and lipid production changed, indicating that a single of the cloned or previously synthesized cocoa GPAT gene cannot replace the function of yeast GPAT genes (Additional file 1: Figure S3). Unfortunately, the CBL production of yeast strain YJW09 was lower than the wild-type strain, though the CBL content of L starkeyi is higher than that of S. cerevisiae (Additional file 1: Figure S3B) [27].
Total fatty acid (a) and lipid (b) production of Y29 strains harboring an empty plasmid (Y29-pBS01A) or plasmids with cocoa genes. SE steryl esters. Asterisks (*) indicate significant differences in comparison to the control strain; *p < 0.05; **p < 0.01. The p values are calculated based on paired t tests corrected for multiple comparisons
Expression of cocoa gene combinations led to an increase in potential CBL production
We compared TAG production of four different yeast strains: control strain SYJ0, SYJ-331, which displayed a significantly higher fatty acid and TAG content compared to SYJ0, SYJ-342, which had an increased SE and TAG content, and SYJ-441, which displayed the highest amounts of total fatty acids, SE and TAG of the eight strains. The TAG compositions changed after introducing the cocoa genes in S. cerevisiae (Additional file 1: Figure S4A). Though all the four yeast strains produced more than 20 different kinds of TAGs, most of the TAGs only accounted for less than 5% of the total TAGs (Additional file 1: Figure S4A).
Concerning the relative CBL production, SYJ-331 (4.7%) and SYJ-441 (6.9%) produced 64 and 140% more potential CBL than the control strain SYJ0 (2.87%), respectively, but SYJ-342 produced less CBL than the control (Fig. 7a). The potential POP and POS of SYJ-331 and SYJ-441 had increased by at least 30% and by at least 130%, respectively. The potential SOS (C18:0, C18:1, C18:0) proportion of SYJ-441 displayed a significant difference compared with SYJ0 (Fig. 7a). In fact, the potential POP, POS and SOS proportions of SYJ-441 had increased 143, 130 and 164%, respectively, compared with SYJ0. Considering that SYJ-441 produced 288% more TAGs than SYJ0, its potential CBL production increased more than eightfold compared with SYJ0, showing this cocoa gene combination of TcGPAT4, TcLPAT4 and TcDGAT1 not only increased TAG production, but also helped S. cerevisiae accumulate more potential CBL.
Relative potential CBL content of S. cerevisiae CEN.PK 113-11C-derived strains (a) and Y29-derived strains (b) harboring cocoa genes. Shown is the peak area of the respective TAG in comparison to the summed peak areas of all TAGs. The error bars represent the standard deviation of two biological replicates. Asterisks (*) indicate significant differences in comparison to control strain SYJ0 (a) or Y29-TcD1 (b); *p < 0.05; **p < 0.01. The p values are calculated based on paired t tests corrected for multiple comparisons
As the TAG content of Y29-pBS01A was quite low, only TAG compositions in Y29-TcD1, Y29-331 and Y29-441 were further checked and all of them could produce more than 17 different kinds of TAGs (Additional file 1: Figure S4B). The potential CBL proportion had increased from 0.48% of Y29-TcD1 to 2.02% in Y29-331 and to 6.01% in Y29-441, which means an increase of 321 and 1150%, respectively (Fig. 7b). For details, the potential POP and POS in Y29-331 had increased 334 and 331% compared with Y29-TcD1, respectively; the potential POP, POS and SOS in Y29-441 had increased 898, 1543 and 3012% compared with Y29-TcD1 (Fig. 7b). Considering the TAG content in Y29-331 (0.55 mg g−1) and Y29-441 (0.48 mg g−1) was 11.5-fold and 9.8-fold higher than in Y29-TcD1 (Fig. 6b), the potential CBL content in Y29-331 and Y29-441 was 51-fold and 134-fold higher than in Y29-TcD1. The significant increase of potential CBL proportion in Y29-331 and Y29-441 over in Y29-TcD1 further suggested that cocoa GPAT and LPAT genes functioned in yeast and helped accumulate more TAGs.
Expression of cocoa genes in different yeast strains changed fatty acid profiles and TAG composition
The C16 and C18 fatty acids were the main fatty acids in the TAGs of four yeast strains (SYJ0, SYJ-331, -342 and -441) and their proportions were similar, which is consistent with the total fatty acid composition analyses (Figs. 5a, 6a). Generally, saturated fatty acids in the TAGs of SYJ-331 and -441 increased, but decreased in the TAGs of SYJ-342 (Fig. 8a). Compared with SYJ0, for SYJ-331, the C16:0 portion in the TAGs increased, while other C16 and C18 fatty acids in the TAGs were equal or decreased; for SYJ-342, the C16:1 fraction in the TAGs increased, while other C16 and C18 fatty acids in the TAGs were equal or decreased; for SYJ-441, both C16:0 and C18:0 fatty acids in the TAGs increased with a significant difference, while other C16 and C18 fatty acids in the TAGs were equal or decreased.
Fatty acid composition of the TAGs of S. cerevisiae CEN.PK 113-11C-derived (a) and Y29-derived (b) strains. The error bars represent the standard deviation of two biological replicates. Shown is the peak area of the respective TAG in comparison to the summed peak areas of all TAGs. Asterisks (*) indicate significant differences in comparison to reference strain SYJ0 (a) or Y29-TcD1 (b), respectively; *p < 0.05; **p < 0.01. The p values are calculated based on paired t tests corrected for multiple comparisons
Though C16 and C18 fatty acids were the main fatty acids in the TAGs of three Y29 strains, the C16 fatty acid faction decreased in Y29-331 and Y29-441 and C18 fatty acids showed an increase in the TAGs of Y29-331 and Y29-441 compared with Y29-TcD1 (Fig. 8b). Besides, saturated fatty acids in the TAGs of Y29-331 and Y29-441 decreased compared with Y29-TcD1. In details, C14:0, C16:0, C16:1 fractions in the TAGs of Y29-331 and -441 decreased, while C18:0 and C18:1 increased. Besides, the C18:2 and C20:0 content in the TAGs of Y29-441 increased. However, the content of unknown TAGs was higher in these three Y29 strains (Y29-TcD1, Y29-331 and Y29-441) than in the four yeast strains (SYJ0, SYJ-331, -342 and -441) and the TAG profiles of Y29-derived strains were different from the other four yeast strains, further suggesting that cocoa GPAT and LPAT enzymes were active and altered the TAG profiles in the yeast strains (Fig. 8).
Discussion
CB is mainly extracted from cocoa beans, but the genes of its biosynthetic pathway in cocoa tree are unknown, hampering their application for developing microbial based CBL production [1, 16, 17]. 13 GPAT, 9 LPAT and 11 DGAT genes have been identified in the T. cacao genome [9, 16, 17]. Of the DGAT genes, only TcDGAT1 and TcDGAT2 are similar to the yeast DGAT genes, the others are similar to LRO1 of S. cerevisiae or wax ester synthase genes, and they might not participate in CB production. Two GPAT (TcGPAT1 and TcGPAT2), two LPAT (TcLPAT1 and TcLPAT2) and two DGAT (TcDGAT1 and TcDGAT2) genes of T. cacao have been synthesized and characterized before [9]. In this study, we cloned another seven GPAT genes and three LPAT genes from seeds of T. cacao. Expression of some of these genes (TcGPAT3, TcGPAT4, TcLPAT3-2 and TcLPAT4) in yeast resulted in an increased total fatty acid production, indicating that these cocoa genes might play roles in cocoa CB production. Furthermore, expression of cocoa gene combinations including these genes plus the previously characterized DGAT genes (TcGPAT3, TcGPAT4, TcLPAT3-2, TcLPAT4, TcDGAT1 and TcDGAT2) increased fatty acid, neutral lipid and CBL production significantly, suggesting that cocoa GPATs and LPATs functioned in yeast and contributed to a CBL production increase in this heterologous host.
The esterification of acyl-CoAs to the glycerol backbone during TAG biosynthesis is catalyzed by GPAT, LPAT and DGAT [13]. Our results showed that SE and TAG production of strain Y29 decreased drastically, demonstrating that deletion of yeast TAG biosynthesis genes altered total neutral lipid production [11, 15]. Compared with Y29-derived strains harboring either an empty plasmid or only one of the cocoa DGAT genes (Y29-TcD1 and Y29-TcD2), expression of cocoa gene combinations (Y29-331 and Y29-441) restored part of yeast neutral lipid production and CBL production, suggesting cocoa GPAT and LPAT contributed to the yeast CBL production increase. Furthermore, both GPAT genes of S. cerevisiae were replaced by LsGPAT of L. starkeyi, showing that the GPAT genes of S. cerevisiae can be replaced by homologues from the same clade (Clade 4 in Fig. 3a). However, replacement of both yeast GPAT genes with one cocoa GPAT gene (at least under the conditions used in this study) failed, suggesting that overexpression of one cocoa GPAT gene cannot complement the loss of both GPAT genes in S. cerevisiae. Moreover, neutral lipid production of strain Y29 harboring one cocoa GPAT gene, one cocoa LPAT gene and one cocoa DGAT gene was much lower than the wild-type yeast strains, further demonstrating cocoa TAG biosynthetic gene expression cannot compensate the gene loss in S. cerevisiae. The low identities between cocoa GPAT genes and yeast GPAT genes might be the reason why cocoa GPAT genes cannot replace yeast GPAT genes (Fig. 3a). As the model plant Arabidopsis thaliana contains several genes encoding enzymes with GPAT activities, the situation for the cocoa tree might be similar, pointing to that several genes/enzymes may function simultaneously in vivo [34].
YJ0 and SYJ0 are supposed to have the same genotype, but when we cultivated these two strains in the same conditions (but not in the same experiment), the fatty acid production of YJ0 and SYJ0 was different. This might be due to the fact that YJ0 and SYJ0 were constructed at different time points and/or that they were in a different growth state when sampled. Overexpression of cocoa TAG biosynthetic genes increased the CBL content from 2.87% in the wild-type yeast strain SYJ0 to 6.9% in SYJ-441. In addition, overexpression of cocoa TAG biosynthetic genes in Y29 increased the CBL content, and the highest CBL content of Y29-441 was 6.0%. With regard to the less than 5.4% CBL content in the yeast strain YJ-221 harboring specifically selected cocoa TAG biosynthetic genes [9], this demonstrates that combination of phylogenetic analyses and experimental verification can lead to identification of effective enzymes (TcGPAT4 and TcLPAT4) for CBL production. The relative CBL content in SYJ-441 is still low (only 6.9%) and the relative CBL content in YJW09 harboring LsGPAT is even lower than in the wild-type S. cerevisiae strain, hinting that other metabolic engineering strategies should be attempted to increase yeast CBL production, including an improvement of specific fatty acid supply and the identification of other functional TAG biosynthetic genes from plants [35,36,37,38]. Considering that the C16:1 proportion in the yeast TAGs is more than 40% and that the C16:1 content in CB is very low [1], some strategies should be further implemented to alter the total fatty acid profiles of the yeast, such as screening for heterologous desaturases which can decrease C16:1 production and increase C18:1 production. Besides, the C16:1 content in the TAGs of some of the Y29-derived strains harboring cocoa gene combinations was lower than in the control strain of Y29-TcD1 (Fig. 8b), which is another indication that these cocoa genes are potential CB biosynthetic genes. As several GPAT, LPAT or DGAT genes might be responsible for CB production in vivo simultaneously, overexpression of different cocoa TAG biosynthetic genes in one yeast strain would be a potential strategy to increase CBL production further. In the future, also overexpression of the identified genes in oleaginous yeasts represents a possibility to obtain a high-level of CBL production [27].
Conclusions
Ten different cocoa TAG biosynthetic genes were cloned from cDNA of T. cacao and characterized in yeast. Expression of some cocoa genes in a wild-type S. cerevisiae strain increased CBL production more than eightfold (the relative CBL content is 6.9%) over the wild-type strain, and expression in yeast mutant strain with a reduced TAG pathway could increase CBL production 134-fold (the relative CBL content is 6.0%) over the control strain Y29-TcD1. Besides, our results demonstrate that several cocoa GPAT, LPAT and DGAT genes might function simultaneously in T. cacao for CB production.
Abbreviations
- CB:
-
cocoa butter
- CBL:
-
CB-like lipids
- TAG:
-
triacylglycerol
- POP:
-
1,3-dipalmitoyl-2-oleoyl-glycerol (C16:0-C18:1-C16:0)
- POS:
-
1-palmitoyl-3-stearoyl-2-oleoyl-glycerol (C16:0-C18:1-C18:0)
- SOS:
-
1,3-distearoyl-2-oleoyl-glycerol (C18:0-C18:1-C18:0)
- GPAT:
-
glycerol-3-phosphate acyltransferase
- LPAT:
-
lysophospholipid acyltransferase
- DGAT:
-
diacylglycerol acyltransferase
- Acyl-CoA:
-
acyl-coenzyme A
- DAG:
-
diacylglycerol
- SE:
-
steryl esters
References
Lipp M, Anklam E. Review of cocoa butter and alternative fats for use in chocolate—Part A. Compositional data. Food Chem. 1998;62:73–97.
Beg MS, Ahmad S, Jan K, Bashir K. Status, supply chain and processing of Cocoa—a review. Trends Food Sci Technol. 2017;66:108–16.
Clough Y, Faust H, Tscharntke T. Cacao boom and bust: sustainability of agroforests and opportunities for biodiversity conservation. Conserv Lett. 2009;2:197–205.
Drenth A, Guest DI. Fungal and oomycete diseases of tropical tree fruit crops. Annu Rev Phytopathol. 2016;54:373–95.
Bowers JH, Bailey BA, Hebbar PK, Sanogo S, Lumsden RD. The impact of plant diseases on world chocolate production. Plant Health Prog. 2001. https://doi.org/10.1094/PHP-2001-0709-01-RV.
Jahurul M, Zaidul I, Norulaini N, Sahena F, Jinap S, Azmir J, Sharif K, Omar AM. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J Food Eng. 2013;117:467–76.
Valle-Rodríguez JO, Shi S, Siewers V, Nielsen J. Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid ethyl esters, an advanced biofuel, by eliminating non-essential fatty acid utilization pathways. Appl Energy. 2014;115:226–32.
Ejsing CS, Sampaio JL, Surendranath V, Duchoslav E, Ekroos K, Klemm RW, Simons K, Shevchenko A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc Nat Acad Sci. 2009;106:2136–41.
Wei Y, Gossing M, Bergenholm D, Siewers V, Nielsen J. Increasing cocoa butter-like lipid production of Saccharomyces cerevisiae by expression of selected cocoa genes. AMB Express. 2017;7:34.
Chapman KD, Ohlrogge JB. Compartmentation of triacylglycerol accumulation in plants. J Biol Chem. 2012;287:2288–94.
Zheng Z, Zou J. The initial step of the glycerolipid pathway Identification of glycerol 3-phosphate/dihydroxyacetone phosphate dual substrate acyltransferases in Saccharomyces cerevisiae. J Biol Chem. 2001;276:41710–6.
Ratledge C. Regulation of lipid accumulation in oleaginous micro-organisms. Biochem Soc Trans. 2002;30:1047–9.
Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–76.
de Kroon AI, Rijken PJ, De Smet CH. Checks and balances in membrane phospholipid class and acyl chain homeostasis, the yeast perspective. Prog Lipid Res. 2013;52:374–94.
Benghezal M, Roubaty C, Veepuri V, Knudsen J, Conzelmann A. SLC1 and SLC4 encode partially redundant acyl-coenzyme A 1-acylglycerol-3-phosphate O-acyltransferases of budding yeast. J Biol Chem. 2007;282:30845–55.
Argout X, Salse J, Aury J-M, Guiltinan MJ, Droc G, Gouzy J, Allegre M, Chaparro C, Legavre T, Maximova SN. The genome of Theobroma cacao. Nature Genet. 2011;43:101–8.
Motamayor JC, Mockaitis K, Schmutz J, Haiminen N, Livingstone D III, Cornejo O, Findley SD, Zheng P, Utro F, Royaert S. The genome sequence of the most widely cultivated cacao type and its use to identify candidate genes regulating pod color. Genome Biol. 2013;14:1.
Entian K-D, Kötter P. 25 Yeast genetic strain and plasmid collections. Methods Microbiol. 2007;36:629–66.
Mans R, van Rossum HM, Wijsman M, Backx A, Kuijpers NG, van den Broek M, Daran-Lapujade P, van Maris AJ, Daran JMG. CRISPR/Cas9: a molecular Swiss army knife for simultaneous introduction of multiple genetic modifications in Saccharomyces cerevisiae. FEMS Yeast Res. 2015;15:004.
Verduyn C, Postma E, Scheffers WA, Van Dijken JP. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–17.
Zhou YJ, Buijs NA, Zhu Z, Qin J, Siewers V, Nielsen J. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat Commun. 2016;7:11709. https://doi.org/10.1038/ncomms11709.
Yang X, Jin G, Gong Z, Shen H, Song Y, Bai F, Zhao ZK. Simultaneous utilization of glucose and mannose from spent yeast cell mass for lipid production by Lipomyces starkeyi. Bioresour Technol. 2014;158:383–7.
Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–62.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4.
Riley R, Haridas S, Wolfe KH, Lopes MR, Hittinger CT, Göker M, Salamov AA, Wisecaver JH, Long TM, Calvey CH. Comparative genomics of biotechnologically important yeasts. Proc Nat Acad Sci. 2016;113:9882–7.
Wei Y, Siewers V, Nielsen J. Cocoa butter-like lipid production ability of non-oleaginous and oleaginous yeasts under nitrogen-limited culture conditions. Appl Microbiol Biotechnol. 2017;101:3577–85.
Khoomrung S, Chumnanpuen P, Jansa-Ard S, Nookaew I, Nielsen J. Fast and accurate preparation fatty acid methyl esters by microwave-assisted derivatization in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2012;94:1637–46.
Khoomrung S, Chumnanpuen P, Jansa-Ard S, Ståhlman M, Nookaew I, Borén J, Nielsen J. Rapid quantification of yeast lipid using microwave-assisted total lipid extraction and HPLC-CAD. Anal Chem. 2013;85:4912–9.
Shukla V, Schiøtz Nielsen W, Batsberg W. A simple and direct procedure for the evaluation of triglyceride composition of cocoa butters by high performance liquid chromatography: a comparison with the existing TLC-GC method. Fett Wiss Technol. 1983;85:274–8.
Barbazuk WB, Fu Y, McGinnis KM. Genome-wide analyses of alternative splicing in plants: opportunities and challenges. Genome Res. 2008;18:1381–92.
Kaneko H, Hosohara M, Tanaka M, Itoh T. Lipid composition of 30 species of yeast. Lipids. 1976;11:837–44.
Czabany T, Athenstaedt K, Daum G. Synthesis, storage and degradation of neutral lipids in yeast. Biochim Biophys Acta. 2007;1771:299–309.
Zheng Z, Xia Q, Dauk M, Shen W, Selvaraj G, Zou J. Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell. 2003;15:1872–87.
Yuzbasheva EY, Mostova EB, Andreeva NI, Yuzbashev TV, Laptev IA, Sobolevskaya TI, Sineoky SP. Co-expression of glucose-6-phosphate dehydrogenase and acyl-CoA binding protein enhances lipid accumulation in the yeast Yarrowia lipolytica. N Biotechnol. 2017. https://doi.org/10.1016/j.nbt.2017.05.008.
Runguphan W, Keasling JD. Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid-derived biofuels and chemicals. Metab Eng. 2014;21:103–13.
Kamisaka Y, Tomita N, Kimura K, Kainou K, Uemura H. DGA1 (diacylglycerol acyltransferase gene) overexpression and leucine biosynthesis significantly increase lipid accumulation in the Δsnf2 disruptant of Saccharomyces cerevisiae. Biochem J. 2007;408:61–8.
Nielsen J, Keasling JD. Engineering Cellular Metabolism. Cell. 2016;164:1185–97.
Authors’ contributions
JN and VS conceived the study. YW designed and performed most of the experiments. DB and MG helped collecting cocoa samples and analyzing the yeast lipid profile. YW wrote the manuscript. JN, VS, MG and DB edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Berit Kristensen, AAK A/S, for performing the TAG analysis, and Morten Emil Møldrup, AAK AB, for scientific discussions during the project. We thank the Gothenburg Botanical Garden for providing cocoa fruits. We thank Julia Karlsson, Jacob Kindbom and Raphael Ferreira for their help in GC and HPLC analyses. We thank Anastasia Krivoruchko for providing the pBS01 plasmid.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional file.
Ethics approval and consent to participate
All authors approved the manuscript.
Funding
This work was funded by AAK AB, the Knut and Alice Wallenberg Foundation, the Novo Nordisk Foundation, the Swedish Foundation for Strategic Research, and the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Genomic Science program (Award number DE-SC0008744).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Figure S1.
Gene cassettes used for gene deletion (A) and gene replacement (B) in CEN.PK 113-11C. Up means upstream sequence of the targeted gene; Down means downstream sequence of the targeted gene; Gene fragment means part of the targeted gene. Figure S2. The cocoa fruit samples in nearly ripe (May 26, 2015) (A) and unripe (October 12, 2015) (B) state at Gothenburg Botanical Garden. Figure S3. Total fatty acid production (A) and relative TAG content (B) in four different S. cerevisiae strains. (A) Others represents the summed content of C12:0, C14:0, C14:1, C20:0, C20:1, C22:0, C24:0 and C26:0 fatty acids. The error bars represent the standard deviation of biological replicates. (B) All TAGs identified in the four yeast strains are shown. The error bars represent the standard deviation of two biological replicates. Shown is the peak area of the respective TAG in comparison to the summed peak areas of all TAGs. Asterisks (*) indicate a significant difference between the yeast strains and the control CEN.PK 113-11C. * indicates p < 0.05; ** indicates p < 0.01. The p-values are calculated based on paired t-tests corrected for multiple comparisons. Figure S4. Relative TAG content (except potential CBL) of CEN.PK 113-11C-derived strains (A) and Y29-derived strains (B). (A) Relative TAG content (except potential CBL) of CEN.PK 113-11C-derived strains harboring cocoa genes. The error bars represent the standard deviation of two biological replicates. Shown is the peak area of the respective TAG in comparison to the summed peak areas of all TAGs. Asterisks (*) indicate significant differences (p-values are based on paired t-tests corrected for multiple comparisons) in comparison to control SYJ0. * indicates p < 0.05; ** indicates p < 0.01. (B) Relative TAG content (except potential CBL) of Y29-derived strains harboring 3 cocoa genes or 1 cocoa gene. Asterisks (*) indicate significant differences (p-values are based on paired t-tests corrected for multiple comparisons) in comparison to reference strain Y29-TcD1. * indicates p < 0.05; ** indicates p < 0.01. Table S1. List of primers used in this study. Table S2. List of plasmids constructed in this study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wei, Y., Bergenholm, D., Gossing, M. et al. Expression of cocoa genes in Saccharomyces cerevisiae improves cocoa butter production. Microb Cell Fact 17, 11 (2018). https://doi.org/10.1186/s12934-018-0866-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-018-0866-2