Abstract
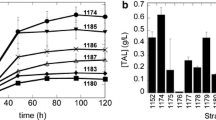
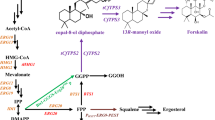
To develop microbial production method for prenyl alcohols (e.g., (E,E)-farnesol (FOH), (E)-nerolidol (NOH), and (E,E,E)-geranylgeraniol (GGOH)), the genes encoding enzymes in the mevalonate and prenyl diphosphate pathways were overexpressed in Saccharomyces cerevisiae, and the resultant transformants were evaluated as to the production of these alcohols. Overexpression of the gene encoding hydroxymethylglutaryl (HMG)-CoA reductase was most effective among the genes tested. A derivative of S. cerevisiae ATCC 200589, which was selected through screening, was found to be the most suitable host for the production. On cultivation of the resultant transformant, in which the HMG-CoA reductase gene was overexpressed, in a 5-liter bench-scale jar fermenter for 7 d, the production of FOH, NOH, and GGOH reached 145.7, 98.8, and 2.46 mg/l, respectively.




Similar content being viewed by others
References
Bard M, Downing JF (1981) Genetic and biochemical aspects of yeast sterol regulation involving 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Gen Microbiol 125:415–420
Benford HL, Frith JC, Auriola S, Mönkkönen J, Rogers MJ (1999) Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol Pharmacol 56:131–140
Bergstrom JD, Kurtz MM, Rew DJ, Amend AM, Karkas JD, Bostedor RG, Bansal VS, Dufresne C, Vanmiddlesworth FL, Hensens OD, Liesch JM, Zink DL, Wilson KE, Onishi J, Milligan JA, Bill G, Kaplan L, Nallin Omstead M, Jenkins RG, Huang L, Meinz MS, Quinn L, Burg RW, Kong YL, Mochales S, Mojena M, Martin I, Pelaez F, Diez MT, Alberts AW (1993) Zaragozic acids: a family of fungal metabolites that are picomolar competitive inhibitors of squalene synthase. Proc Natl Acad Sci USA 90:80–84
Chambon C, Ladeveze V, Oulmouden A, Servouse M, Karst F (1990) Isolation and properties of yeast mutants affected in farnesyl diphosphate synthetase. Curr Genet 18:41–46
Chambon C, Ladeveze V, Servouse M, Blanchard L, Javelot C, Vladescu B, Karst F (1991) Sterol pathway in yeast. Identification and properties of mutant strains defective in mevalonate diphosphate decarboxylase and farnesyl diphosphate synthetase. Lipids 26:633–636
Dimsterdenk D, Thorsness MK, Rine J (1994) Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in Saccharomyces cerevisiae. Mol Biol Cell 5:655–665
Donald KAG, Hampton RY, Fritz IB (1997) Effects of overproduction of the catalytic domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase on squalene synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol 63:3341–3344
Hashida-Okado T, Ogawa A, Kato I, Takesako K (1998) Transformation system for prototrophic industrial yeasts using the AUR1 gene as a dominant selection marker. FEBS LETT 425:117–122
Hayashi C (1952) On the prediction of phenomena from qualitative data on the quantification of qualitative data from the mathelective cervical lymph node dissection in patients ematico-statistical point of view. Annals of the Institute of Statistical Mathematics 3:69–98
Hemmi H, Ohnuma S, Nagaoka K, Nishino T (1998) Identification of genes affecting lycopene formation in Escherichia coli transformed with carotenoid biosynthetic genes: Candidates for early genes in isoprenoid biosynthesis. J Biochem 123:1088–1096
Hiser L, Basson ME, Rine J (1994) ERG10 from Saccharomyces cerevisiae encodes acetoacetyl-CoA thiolase. J Biol Chem 269:31383–31389
Hyatt JA, Kottas GS, Effler J (2002) Development of synthetic routes to d,l-α-tocopherol (vitamin E) from biologically produced geranylgeraniol. Org Process Res Dev 6:782–787
Jackson BE, Hart-Wells EA, Matsuda SPT (2003) Metabolic engineering to produce sesquiterpenes in yeast. Org Lett 5:1629–1632
Jiang Y, Proteau P, Poulter D, Ferro-Novick S (1995) BTS1 encodes a geranylgeranyl diphosphate synthase in Saccharomyces cerevisiae. J Biol Chem 270:21793–21799
Kuroda M, Hashida-Okado T, Yasumoto R, Gomi K, Kato I, Takesako K (1999) An aureobasidin A resistance gene isolated from Aspergillus is a homolog of yeast AUR1, a gene responsible for inositol phosphorylceramide (IPC) synthase activity. Mol Gen Genet 261:290–296
Mumberg D, Muller R, Funk M (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119–122
Negishi E, Liou SY, Xu CD, Huo SQ (2002) A novel, highly selective, and general methodology for the synthesis of 1,5-diene-containing oligoisoprenoids of all possible geometrical combinations exemplified by an iterative and convergent synthesis of coenzyme Q(10). Org Lett 4:261–264
Ogura K, Koyama T (1998) Enzymatic aspects of isoprenoid chain elongation. Chem Rev 98:1263–1276
Polakowski T, Stahl U, Lang C (1998) Overexpression of a cytosolic hydroxymethylglutaryl-CoA reductase leads to squalene accumulation in yeast. Appl Microbiol Biotechnol 49:66–71
Servouse M, Karst F (1986) Regulation of early enzymes of ergosterol biosynthesis in Saccharomyces cerevisiae. Biochem J 240:541–547
Shimada H, Kondo K, Fraser PD, Miura Y, Saito T, Misawa N (1998) Increased carotenoid production by the food yeast Candida utilis through metabolic engineering of the isoprenoid pathway. Appl Environ Microbiol 64:2676–2680
Szkopinska A, Grabinska K, Delourme D, Karst F, Rytka J, Palamarczyk G (1997) Polyprenol formation in the yeast Saccharomyces cerevisiae: Effect of farnesyl diphosphate synthase overexpression. J Lipid Res 38:962–968
Veen M, Stahl U, Lang C (2003) Combined overexpression of genes of the ergosterol biosynthetic pathway leads to accumulation of sterols in Saccharomyces cerevisiae. FEMS Yeast Res 4:87–95
Wang GY, Keasling JD (2002) Amplification of HMG-CoA reductase production enhances carotenoid accumulation in Neurospora crassa. Metab Eng 4:193–201
Yu JS, Kleckley TS, Wiemer DF (2005) Synthesis of farnesol isomers via a modified Wittig procedure. Org Lett 7:4803–4806
Acknowledgments
We thank Ms. Chiharu Kato and Ms. Yoshie Tsukahara for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohto, C., Muramatsu, M., Obata, S. et al. Overexpression of the gene encoding HMG-CoA reductase in Saccharomyces cerevisiae for production of prenyl alcohols. Appl Microbiol Biotechnol 82, 837–845 (2009). https://doi.org/10.1007/s00253-008-1807-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1807-5