Abstract
Background
Today, diabetes mellitus (DM) has become a worldwide concern. DM is a major risk factor for the development of cardiovascular diseases (CVD). Eligible patients with CVD are treated invasively by percutaneous coronary intervention (PCI) whereby a stent is implanted inside the coronary vessel with the particular lesion to allow sufficient blood flow. Newer scientific research have shown that even though associated with a lower rate of re-stenosis, first-generation drug eluting stents (DES) were associated with a higher rate of late stent thrombosis. Recently, newer stents, namely biodegradable polymer DES (BP-DES) have been developed to overcome the safety issues of earlier generation DES. In this analysis we aimed to systematically compare the long term (≥ 12 months) adverse cardiovascular outcomes observed in DM versus non-DM patients who were implanted with BP-DES.
Methods
Cochrane central, MEDLINE (Subset PubMed), EMBASE, Web of Science, http://www.ClinicalTrials.gov and Google scholar were searched for relevant publications involving BP-DES in patients with DM versus non-DM and their associated adverse cardiovascular outcomes. The mean follow-up time period ranged from 12 to 120 months. Data analysis was carried out with the latest version of the RevMan software (version 5.4). Based on the Mantel-Haenszel test, risk ratios (RR) with 95% confidence intervals (CI) were calculated and used to represent the results following analysis.
Results
Seven (7) studies with a total number of 10,246 participants were included in this analysis. Stents which were implanted during PCI were BP-DES. Participants were enrolled from the year 2006 to 2013. Our current results showed that in patients who were implanted with BP-DES, the risks of major adverse cardiac events (RR: 1.30, 95% CI: 1.18–1.43; P = 0.00001), myocardial infarction (RR: 1.48, 95% CI: 1.14–1.93; P = 0.003), all-cause mortality (RR: 1.70, 95% CI: 1.29–2.23; P = 0.0002), cardiac death (RR: 1.93, 95% CI: 1.28–2.93; P = 0.002), target vessel revascularization (RR: 1.35, 95% CI: 1.03–1.77; P = 0.03), target lesion revascularization (RR: 1.28, 95% CI: 1.07–1.54; P = 0.007) and target lesion failure (RR: 1.79, 95% CI: 1.52–2.12; P = 0.00001) were significantly higher in the DM group. Definite and probable stent thrombosis (RR: 1.80, 95% CI: 1.28–2.55; P = 0.0009) were also significantly higher in the DM group.
Conclusions
Diabetes mellitus was an independent risk factor associated with long term adverse cardiovascular outcomes following PCI with BP-DES.
Similar content being viewed by others
Background
Today, diabetes mellitus (DM) has become a worldwide concern [1]. DM is a major risk factor for the development of cardiovascular diseases (CVD). The global burden of CVD is disproportionately borne by patients with DM [2]. Several mechanisms could explain the effects of DM on the cardiovascular system [3]. High blood sugar levels contribute to oxidative stress through the production of mitochondrial superoxide, NADPH reduction through the accumulation of polyol, and the synthesis of AGE through the non-enzymatic oxidation of glycoproteins, all of which could cause endothelial damage within coronary arteries giving rise to CVD. DM is also associated with high platelet reactivity which might result in acute coronary syndrome (ACS), further complicating CVD [4].
Patients with CVD are often treated invasively by percutaneous coronary intervention (PCI) whereby a stent is implanted inside the coronary vessel with the particular lesion to allow sufficient blood flow to the heart muscles [5]. Several types of coronary stents have been developed [6]. Bare metal stents (BMS) were the first stents which were used during PCI [7]. However, due to the higher risk of re-stenosis and early stent thrombosis with BMS, a drug eluting stent (DES) was developed [8]. Even though patients with DM often have more complicated lesions and more severe disease, including more extensive and diffuse atherosclerosis, with increased risk of triple vessel diseases, and left main coronary occlusions, DES was a good option when the patients were candidates for PCI [9].
Unfortunately, with recent progress in medicine and technology, newer scientific research have shown that even though associated with a lower rate of re-stenosis, first-generation DES were associated with a higher rate of late stent thrombosis [10]. Explanations which were given were based on the fact that the permanent polymers of the DES which contained the anti-proliferative drug partially contributed to the delayed vascular healing, which might be a potential cause for the late stent thrombosis in patients who were revascularized with first generation DES [11]. In addition, DM was associated with platelet dysfunction [12], and antiplatelet hyporesponsiveness [13] which could further increase the risk of stent thrombosis in such patients. Therefore, in CVD patients with DM, the development of a more effective and safer DES could potentially decrease adverse outcomes following PCI.
Recently, newer stents, namely biodegradable polymer DES (BP-DES) were developed to overcome the safety issues of earlier generation DES [14]. The BP-DES system incorporates a biodegradable polymer carrier containing the anti-proliferative drug which is only applied on the luminal surface of the stent platform, thus, limiting its exposure to blood and therefore, the abluminal coating might enhance the attachment of endothelial progenitor cells from the peripheral circulation, or in-growth of the endothelial tissue from the proximal and the distal edges of the stents [15]. Thus, a major advantage of this BP-DES could be the fact that it could be associated with similar long term outcomes as the first generation DES, but with a more favorable safety profile based on a reduction of late stent thrombosis in patients with DM. However, this benefit has still not been confirmed [16].
All around the globe, DM is rising at an alarming rate, resulting in an increase in cardiovascular complications and deaths. Therefore, scientists and medical professionals should focus on further clinical research to provide the best treatment and management of patients with DM. The most recent BP-DES have seldom systematically been compared in patients with DM versus patients without DM.
In this analysis we aimed to systematically compare the long term (≥ 12 months) adverse cardiovascular outcomes observed in DM versus non-DM patients with CVD who were implanted with BP-DES.
Methods
Search databases
Cochrane central, MEDLINE (Subset PubMed), EMBASE, Web of Science, http://www.ClinicalTrials.gov and Google scholar were searched for relevant publications involving BP-DES in patients with DM versus non-DM.
Search strategies
The above mentioned databases were searched for relevant publications using the following search terms: ‘biodegradable polymer drug eluting stents and diabetes mellitus’, ‘biodegradable polymer DES and diabetes mellitus’, ‘biodegradable polymer drug eluting stents and DM’.
After going through the relevant publications, their reference lists were also searched for suitable publications.
This meta-analysis was performed in accordance with the Preferred Reporting Items in Systematic reviews and Meta-Analyses (PRISMA) guideline.
Inclusion and exclusion criteria
The following inclusion and exclusion criteria were considered:
-
(a)
Inclusion criteria:
-
Studies comparing biodegradable polymer DES in patients with DM and non-DM;
-
Reported adverse cardiovascular outcomes as the endpoints;
-
English publications.
-
(b)
Exclusion criteria:
-
Systematic reviews, meta-analyses and literature reviews;
-
Studies which were not based on patients with DM;
-
Studies that did not include a comparison of biodegradable polymer DES between DM and non-DM;
-
Studies that did not report adverse cardiovascular outcomes as the endpoints;
-
Duplicated studies or studies which were based on the same trial.
Outcomes and the duration of follow-up time period
The outcomes which were reported in the original studies have been listed in Table 1. The follow up time period of each studies has been listed in the same table.
The endpoints which have been assessed in this analysis were:
-
(a)
Major adverse cardiac events (MACEs) consisting of a combination of all-cause mortality, myocardial infarction and revascularization;
-
(b)
All-cause mortality;
-
(c)
Cardiac death;
-
(d)
Myocardial infarction (MI);
-
(e)
Definite and probable stent thrombosis;
-
(f)
Target vessel revascularization (TVR);
-
(g)
Target lesion revascularization (TLR);
-
(h)
Target lesion failure (TLF).
The duration of the follow-up time period ranged from 12 to 120 months (1–10 years).
Data extraction and quality assessment
The authors independently extracted data including the time period of participants’ enrollment, the type of study, the outcomes which were reported, the total number of events associated with each outcome, the type of biodegradable polymer, the total number of patients with DM and non-DM, the baseline features including the mean age, percentage of male patients, percentage of participants with hypertension, dyslipidemia and current smoker, the cardiovascular and antiplatelet drugs which were used as well as the angiographic features of the lesions including the percentage of patients with lesions in the left main coronary artery, left anterior descending artery, left circumflex and right coronary artery.
Any disagreement which occurred during this data extraction process was carefully discussed among the authors and if a decision could not be reached, the corresponding author was contacted and he was the one to make a final decision.
The methodological quality of the trials and observational studies was assessed by the recommendations of the Cochrane collaboration [24] and the Newcastle Ottawa Scale (NOS) [25] respectively.
Grades were allotted to denote a low, moderate or high risk of bias for the randomized trials, whereas a ‘star system’ was used to represent bias risk assessment for the observational studies (Table 2). This assessment of observational studies was based on the design of the respective study, content and ease of use directed to the task of incorporating the quality assessments on three broad perspectives: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies respectively. The higher the number of stars, the better the methodological quality of the study.
Statistical analysis
This is a meta-analysis, and the data analysis was carried out with the latest version of the RevMan software (version 5.4).
Studies which have been included in this analysis will differ from each other. Any kind of variability among studies in a meta-analysis is termed heterogeneity.
Two simple statistic test were used to assess heterogeneity among the subgroups: (1) the Q statistic test whereby a P value less or equal to 0.05 associated with a particular subgroup was considered statistically significant, (2) the I2 statistic test whereby heterogeneity decreased with a decreased I2 value. For a subset having an I2 value less than 50%, a fixed effect statistical model was used, whereas for a subset having an I2 value above 50%, a random effect statistical model was used.
Based on the Mantel-Haenszel test, risk ratios (RR) with 95% confidence intervals (CI) were calculated and used to represent the results following analysis.
Sensitivity analysis was also carried out. Sensitivity analysis was conducted to countercheck whether the final result was influenced by one particular study. This sensitivity analysis was carried out by an exclusion method whereby among all the studies which were involved with one particular outcome, each study was excluded one by one, and a new analysis was carried out each time and compared with the main analytical result.
Publication bias was also assessed through funnel plots.
Ethical approval
An ethical approval or board review approval was not required for this study since data were obtained from previously published original studies, and no experiment on animals or humans was carried out by any of the authors.
Results
Search outcomes
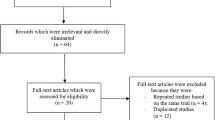
The PRISMA guideline was followed in this systematic review and meta-analysis [26]. A total number of 235 publications were obtained through search databases. The authors carefully assessed the titles and abstracts, and therefore an initial elimination of studies was carried out prior to assessing the full text articles.
Therefore, following this initial elimination whereby 179 publications were eliminated, we finally short-listed 56 full text articles which we assessed for eligibility. Further elimination were carried out based on the following:
-
Studies that did not involve non-DM group as the control group (n = 13);
-
Studies which reported platelet reactivity instead of adverse cardiovascular outcomes (n = 3);
-
Case studies, editorials and erratum (n = 5);
-
Duplicated studies or studies which were based on the same trial (n = 28).
Finally, only 7 studies [17,18,19,20,21,22,23] were included in this meta-analysis. The flow diagram of the study selection has been illustrated in Fig. 1.
General and baseline features of the studies
Seven (7) studies with a total number of 10,246 participants were included in this analysis whereby 2747 participants were DM patients and 7499 participants were non-DM as shown in Table 3. The stents which were implanted during PCI were BP-DES. Participants were enrolled from the year 2006 to 2013.
The baseline features of the participants were listed in Table 4. The mean age of the participants with DM varied from 59.7 to 68.6 years, whereas the mean age of the non-DM participants ranged from 58.5 to 66.5 years. The mean percentage of male participants ranged from 69.6 to 80.3% as shown in Table 4. The percentage of DM and non-DM participants with hypertension, dyslipidemia and smokers were also listed in Table 4.
Table 5 lists the antiplatelet medications as well as the other cardiac drugs which are used by the participants. All the participants were on dual antiplatelet therapy including aspirin and a P2Y12 inhibitor (clopidogrel or prasugrel or ticagrelor) as shown in Table 5. Other medications included angiotensin receptor blockers, angiotensin converting enzyme inhibitors, statins, beta-blockers, calcium channel blockers as the main drugs.
Table 6 lists the angiographic features including the location of the coronary lesions, the number of treated lesions per patients, and the number of stents implanted per lesion.
Main results of this analysis
During a mean follow-up time period ranging from one to ten years, our current results showed that in patients who were implanted with BP-DES, the risks of MACEs and MI were significantly higher in patients with DM with RR: 1.30, 95% CI: 1.18–1.43; P = 0.00001; I2 = 0% and RR: 1.48, 95% CI: 1.14–1.93; P = 0.003; I2 = 42% when compared to non-DM participants as shown in Fig. 2. All-cause mortality (RR: 1.70, 95% CI: 1.29–2.23; P = 0.0002); I2 = 58%, and cardiac death (RR: 1.93, 95% CI: 1.28–2.93; P = 0.002); I2 = 61% were also significantly higher in the DM group as shown in Fig. 3.
The risk of TVR (RR: 1.35, 95% CI: 1.03–1.77; P = 0.03); I2 = 3%, TLR (RR: 1.28, 95% CI: 1.07–1.54; P = 0.007); I2 = 35% and TLF (RR: 1.79, 95% CI: 1.52–2.12; P = 0.00001); I2 = 0% were also significantly higher in the DM group as shown in Fig. 4.
Definite and probable stent thrombosis (RR: 1.80, 95% CI: 1.28–2.55; P = 0.0009); I2 = 0%, specifically definite stent thrombosis (RR: 2.11, 95% CI: 1.01–4.42; P = 0.05); I2 = 0% and probable stent thrombosis (RR: 1.92, 95% CI: 1.13–3.26; P = 0.02); I2 = 25% were also significantly higher in the DM group as shown in Fig. 5.
No significant difference in result was observed during sensitivity analysis, implying that the results of this analysis were not influenced by any particular study. Publication bias was visually observed through the funnel plots. Based on this assessment, there was little evidence of publication bias among the studies that were included in this analysis as shown in Figs. 6, 7 and 8.
Discussion
DM patients with CVD are at greater risk of long term stent thrombosis following PCI with first generation DES [28]. Considering the fact that the polymer in DES could be a source of stent thrombosis in these patients with DM [29], the new BP-DES were developed. In this analysis, we aimed to systematically assess the cardiovascular outcomes in DM versus non-DM patients who were implanted with BP-DES.
The results of this analysis showed that MACEs, MI, all-cause mortality, repeated revascularization, TLF, and stent thrombosis were all significantly higher in the DM group compared to the DM group. The studies which were included in this analysis had a duration of follow up time period ranging from 12 months to 120 months.
Our current analysis showed that clinical outcomes including stent thrombosis continued to be worse in patients with DM despite of undergoing PCI with newer BP-DES whereby the polymer which might have been one of the causes of stent thrombosis, was eliminated with time. In addition, all patients were on dual antiplatelet drugs following stent implantation. Therefore, it could be possible that other potential factors in these patients with DM, including antiplatelet hyporesponsiveness and platelet hyperactivity and endothelial dysfunction could be more influential and have a greater impact compared to the polymer in DES to cause late stent thrombosis.
Scientific reports have shown diabetic status to affect the short and long term outcomes following PCI with any type of stents, through several pathophysiological mechanisms including the acceleration of atherosclerosis and promotion of endothelial dysfunction by hyperglycemia and insulin resistance. In addition, impaired vasodilation, exaggerated neointimal hyperplasia and platelet hyperactivity were observed in DM patients in comparison to non-DM patients, and these could have contributed to significantly higher adverse cardiovascular outcomes in DM patients compared to non-DM patients who were implanted with BP-DES. Furthermore, the proinflammatory environment of DM enhances the vasculoproliferative cascade in response to stent-mediated arterial injury and therefore, as a consequence, patients with DM experience both a higher recurrence of ischemic events related to new atherosclerotic lesions and worse cardiovascular outcomes for stent related complications including re-stenosis and stent thrombosis. Even though BP-DES have significantly reduced cardiovascular outcomes including late stent thrombosis in patients following PCI, our analysis showed that when DM and non-DM were compared following implantation with BP-DES, a significantly higher risk of adverse cardiovascular outcomes was still associated with DM patients. Could newer potent antiplatelet agents improve stent thrombosis in these patients with DM?
In a pre-specified subgroup analysis including participants from Germany, the authors demonstrated that during a follow-up time period of 10 years, BP-DES were associated with less stent thrombosis compared to durable polymer everolimus eluting stents in patients with DM and non-DM [19]. In patients with DM who were implanted with BP-DES, the percentage of stent thrombosis was 2.2% whereas in those DM patients who were implanted with durable polymer everolimus eluting stents, the percentage of stent thrombosis was 2.7%. But when stent thrombosis was compared in DM and non-DM participants who were implanted with BP-DES, stent thrombosis was 2.2% in DM population versus 1.6% in non-DM showing that DM participants were still at higher risk of stent thrombosis compared to non-DM participants whatever be the type of stents. A meta-analysis of randomized trial comparing BP-DES versus contemporary durable polymer DES in patients with DM, the authors showed that overall, BP-DES had similar efficacy and safety profiles in comparison to contemporary durable polymer DES in patients with DM [29]. The difference was observed when patients with DM were compared with non-DM patients.
In the ISAR-Test 5 trial whereby the authors aimed to show 10 year clinical outcomes of polymer free versus durable polymer new generation DES in patients with coronary artery disease with and without DM [30], 3002 participants were randomly assigned to polymer free sirolimus eluting stents or durable polymer zotarolimus eluting stents. Results of their study showed that at 10 years, both new generation DES showed comparable outcomes irrespective of diabetic status or polymer strategy. However, events rate after PCI in patients with DM were considerably higher when compared to patients without DM.
It is still not clear whether more potent antiplatelet agents could partially resolve this issue by decreasing stent thrombosis in these patients with DM [31]. When DES were upgraded to BP-DES and this new stent was compared in patients with DM and non-DM, the former were still at higher risk of adverse cardiovascular outcomes including stent thrombosis. It would now be the turn of antiplatelet agents to play the card. More potent antiplatelet agents could help to better manage such patients and reduce the rate of stent thrombosis [32]. A recent publication was based on the treatment in this new era showing the long term ticagrelor monotherapy use for the treatment of patients with DM following PCI [33]. The authors concluded that long term ticagrelor monotherapy after a short course of dual antiplatelet therapy was better, without significantly increasing bleeding risk in those patients with DM.
At last, several meta-analysis have compared BP-DES with durable polymer DES [34, 35], however, this is the first meta-analysis till date to compare the cardiovascular outcomes in DM versus non-DM participants who were treated with BP-DES.
This study has several limitations. First of all, even though all BP-DES were used, there were minor variations with those BP-DES which could have had an influence on the results. Another limitation could be the fact that other medications including antiplatelet drugs, which were ignored in this analysis, could have had an impact on the results. Moreover, the intensity of coronary artery disease was not similar in all the patients, and this could have had an impact on the outcomes too. Furthermore, even though the follow up time period varied from 12 months to 120 months, each study reported different follow up time periods. The angiographic features were also different for each patient and this could also have had an impact on the final result.
Conclusion
Diabetes mellitus was an independent risk factor associated with long term adverse cardiovascular outcomes following PCI with BP-DES.
Data Availability
All data and materials used in this research are freely available in electronic databases (Cochrane central, MEDLINE (Subset PubMed), EMBASE, Web of Science, http://www.ClinicalTrials.gov and Google scholar). References have been provided.
Abbreviations
- BP DES:
-
Biodegradable polymer drug eluting stents
- DES:
-
Drug eluting stents
- DM:
-
Diabetes mellitus
- NDM:
-
Non-diabetes mellitus
- RR:
-
Risk ratios
- PCI:
-
Percutaneous coronary intervention
- CVD:
-
Cardiovascular diseases
References
Khan MAbdulB, Hashim MJ, King JK, Govender RD, Mustafa H. Juma Al Kaabi. Epidemiology of type 2 diabetes - global burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10(1):107–11.
Ma C-X, Ma X-N, Guan C-H, Li Y-D. Dídac Mauricio, Song-Bo Fu. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21(1):74.
Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19(32):5695–703.
Raminderjit Kaur M, Kaur J, Singh. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121.
Tamim, Akbari. Rasha Al-Lamee. Percutaneous coronary intervention in Multi-Vessel Disease. Cardiovasc Revasc Med. 2022;44:80–91.
Stevens JR, Zamani A, Osborne JIanA, Zamani R, Mohammad Akrami. Critical evaluation of stents in coronary angioplasty: a systematic review. Biomed Eng Online. 2021;20(1):46.
William B, Hillegass. End of the bare metal stent era? Catheter Cardiovasc Interv. 2016;88(1):49–50.
Joshua Feinberg EE, Nielsen J, Greenhalgh J, Hounsome NJ, Sethi S, Safi C, Gluud, Janus C, Jakobsen. Drug-eluting stents versus bare-metal stents for acute coronary syndrome. Cochrane Database Syst Rev. 2017;8(8):CD012481.
Hao P-P, Chen Y-G, Wang X-L, Zhang Y. Efficacy and safety of drug-eluting stents in patients with acute ST-segment-elevation myocardial infarction: a meta-analysis of randomized controlled trials. Tex Heart Inst J. 2010;37(5):516–24.
Huang KN, Sonia M, Grandi KB, Filion, Mark J, Eisenberg. Late and very late stent thrombosis in patients with second-generation drug-eluting stents. Can J Cardiol. 2013;29(11):1488–94.
Pedro R, Moreno V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004;44(12):2293–300.
Zhang Y, Zhou H. Hyper-reactive platelets and type 2 diabetes. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47(3):374–83.
Yang T-H, Kim D-I, Kim D-K, et al. Detection of clopidogrel hyporesponsiveness using a point-of-care assay and the impact of additional cilostazol administration after coronary stent implantation in diabetic patients. Korean J Intern Med. 2011;26(2):145–52.
Sripal Bangalore. The Elusive Late Benefit of Biodegradable Polymer Drug-Eluting Stents. Circulation. 2019;139(3):334–6.
Danzi GB, Chevalier B, Ostojic M, Hamilos M, Wijns W. Nobori™ drug eluting stent system: clinical evidence update. Minerva Cardioangiol. 2010;58(5):599–610.
Yanyu Wang P, Dong L, Li X, Li H, Wang X, Yang S, Wang Z, Li X. Shang. Biodegradable polymer drug-eluting stents versus second-generation drug-eluting stents for patients with coronary artery disease: an update meta-analysis. Cardiovasc Drugs Ther. 2014;28(4):379 – 85.
Juan F, Iglesias D, Heg M, Roffi, et al. Five-year outcomes in patients with diabetes Mellitus treated with Biodegradable Polymer Sirolimus-Eluting Stents Versus durable polymer everolimus-eluting stents. J Am Heart Assoc. 2019;8(22):e013607.
Lee DH, Park TK, Song YB Clinical outcomes of biodegradable polymer biolimus-eluting BioMatrix stents versus durable polymer everolimus-eluting Xience stents. PLoS One. 2017 Aug 10;12(8):e0183079.
Lenz T, Koch T, Joner M et al. Ten-Year Clinical Outcomes of Biodegradable Versus Durable Polymer New-Generation Drug-Eluting Stent in Patients With Coronary Artery Disease With and Without Diabetes Mellitus. J Am Heart Assoc. 2021;10(12):e020165.
Li Y, Han Y, Zhang L, et al. Clinical efficacy and safety of biodegradable polymer-based sirolimus-eluting stents in patients with diabetes mellitus insight from the 4-year results of the create study. Catheter Cardiovasc Interv. 2013;81(7):1127–33.
Piotr Rola A, Włodarczak M, Barycki, et al. Biodegradable polymer DES (Ultimaster) vs. Magnesium Bioresorbable Scaffold (BRS Magmaris) in Diabetic Population with NSTE-ACS: a one-year clinical outcome of two Sirolimus-Eluting Stents. J Diabetes Res. 2021;2021:8636050.
Tang X-F, Ma Y-L, Song Y, et al. Biodegradable polymer drug-eluting stents versus second-generation drug-eluting stents in patients with and without diabetes mellitus: a single-center study. Cardiovasc Diabetol. 2018;17(1):114.
Marcus Wiemer S, Stoikovic A, Samol, et al. Third generation drug eluting stent (DES) with biodegradable polymer in diabetic patients: 5 years follow-up. Cardiovasc Diabetol. 2017;16(1):23.
Julian PT, Higgins DG, Altman, Peter C, Gøtzsche, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Andreas Stang. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Matthew J, Page JE, McKenzie, Patrick M, Bossuyt, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Xiang Wang X, Chen W, Sun et al. Very late stent thrombosis in drug-eluting stents New Observations and clinical implications. Cardiol Rev 2019 Nov/Dec;27(6):279–85.
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R. Kristi Skorija, Herman K Gold, Renu Virmani. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202.
Chirag Bavishi Y, Chugh T, Kimura, et al. Biodegradable polymer drug-eluting stent vs. contemporary durable polymer drug-eluting stents in patients with diabetes: a meta-analysis of randomized controlled trials. Eur Heart J Qual Care Clin Outcomes. 2020;6(1):81–8.
Tobias Koch T, Lenz M, Joner, et al. Ten-year clinical outcomes of polymer-free versus durable polymer new-generation drug-eluting stent in patients with coronary artery disease with and without diabetes mellitus: results of the Intracoronary Stenting and Angiographic results: test efficacy of Sirolimus- and probucol- and zotarolimus-eluting stents (ISAR-TEST 5) trial. Clin Res Cardiol. 2021;110(10):1586–98.
Tufano A, Cimino E, Minno MNDD, Ieranò P, Marrone E, Strazzullo A, Di Minno G, Cerbone AM. Diabetes mellitus and cardiovascular prevention: the role and the limitations of currently available antiplatelet drugs. Int J Vasc Med. 2011;2011:250518.
Erik L, Grove S. Antiplatelet therapy in patients with diabetes mellitus. Curr Vasc Pharmacol. 2012;10(4):494–505.
Wang H, Xie X, Zu Q, et al. Treatment of the New era: long-term Ticagrelor Monotherapy for the treatment of patients with type 2 diabetes Mellitus following percutaneous coronary intervention: a Meta-analysis. Diabetes Ther. 2023;14(1):47–61.
Lin Zhu YN, Lv L. Stent thrombosis with biodegradable polymer drug-eluting stents versus durable polymer sirolimus-eluting stents: an update meta-analysis. Cardiology. 2015;130(2):96–105.
Lupi A, Secco GG, Rognoni A, et al. Meta-analysis of bioabsorbable versus durable polymer drug-eluting stents in 20,005 patients with coronary artery disease: an update. Catheter Cardiovasc Interv. 2014;83(6):E193–206.
Acknowledgements
Not applicable.
Funding
This research was supported by the Guangxi Key Research and Development Program (Grant No. AB22035078); Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project (Grant No. S2017077) and the Guangxi Nanning Qingxiu District Science and Technology Development Project (Grant No. 2014S06).
Author information
Authors and Affiliations
Contributions
Hong Wang, Quannan Zu, Hairong Tang, Ming Lu, Rongfa Chen, and Zhiren Yang were responsible for the conception and design, drafting the initial manuscript and revising it critically for important intellectual content. Hong Wang wrote the final draft. All the authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethical approval was not applicable for this systematic review and meta-analysis.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Zu, Q., Tang, H. et al. Long-term cardiovascular outcomes of biodegradable polymer drug eluting stents in patients with diabetes versus non-diabetes mellitus: a meta-analysis. Cardiovasc Diabetol 22, 228 (2023). https://doi.org/10.1186/s12933-023-01962-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01962-w