Abstract
Background
Effects of antihyperglycemic therapies on cardiovascular and heart failure (HF) risks have varied widely across cardiovascular outcome trials (CVOTs), and underlying factors remain incompletely understood. We aimed to determine the relationships of glycated hemoglobin (HbA1c) or bodyweight changes with these outcomes in all CVOTs of antihyperglycemic therapies.
Methods
We searched PubMed and EMBASE up to 25 January 2023 for all randomized controlled CVOTs of antihyperglycemic therapies reporting both major adverse cardiovascular events (MACE) and HF outcomes in patients with type 2 diabetes or prediabetes. We performed meta-regression analyses following random-effects meta-analyses to evaluate the effects of HbA1c or bodyweight reductions on each outcome.
Results
Thirty-five trials comprising 256,524 patients were included. Overall, antihyperglycemic therapies reduced MACE by 9% [risk ratio (RR): 0.91; 95% confidence interval (CI) 0.88–0.94; P < 0.001; I2 = 36.5%]. In meta-regression, every 1% greater reduction in HbA1c was associated with a 14% reduction in the RR of MACE (95% CI 4–24; P = 0.010), whereas bodyweight change was not associated with the RR of MACE. The magnitude of the reduction in MACE risk associated with HbA1c reduction was greater in trials with a higher baseline prevalence of atherosclerotic cardiovascular disease. On the other hand, antihyperglycemic therapies showed no overall significant effect on HF (RR: 0.95; 95% CI 0.87–1.04; P = 0.28; I2 = 75.9%). In a subgroup analysis based on intervention type, sodium-glucose cotransporter-2 inhibitors (SGLT2i) conferred the greatest HF risk reduction (RR: 0.68; 95% CI 0.62–0.75; P < 0.001; I2 = 0.0%). In meta-regression, every 1 kg bodyweight reduction, but not HbA1c reduction, was found to reduce the RR of HF by 7% (95% CI 4–10; P < 0.001); however, significant residual heterogeneity (P < 0.001) was observed, and SGLT2i reduced HF more than could be explained by HbA1c or bodyweight reductions.
Conclusions
Antihyperglycemic therapies reduce MACE in an HbA1c-dependent manner. These findings indicate that HbA1c can be a useful marker of MACE risk reduction across a wide range of antihyperglycemic therapies, including drugs with pleiotropic effects. In contrast, HF is reduced not in an HbA1c-dependent but in a bodyweight-dependent manner. Notably, SGLT2i have shown class-specific benefits for HF beyond HbA1c or bodyweight reductions.
Similar content being viewed by others
Introduction
People with type 2 diabetes are at high risk of developing cardiovascular events, including cardiovascular death, coronary heart disease, stroke, and heart failure (HF) [1]. To date, various clinical trials have investigated the efficacy of antihyperglycemic therapies on cardiovascular outcomes, and some have provided evidence of a significant reduction in the risk of major adverse cardiovascular events (MACE) and/or hospitalization for HF in patients with type 2 diabetes or prediabetes [2]. Although it is presumed that the cardiovascular protection conferred by antihyperglycemic therapies is attributable to various variables, it is unclear which variables affect the development of cardiovascular diseases in chronic glycemia management.
Antihyperglycemic therapies lower blood glucose levels through various mechanisms to mitigate hyperglycemia symptoms and diabetes-related complication risk. Many cardiovascular outcome trials have shown a significant difference in glycemic control between the intervention (medication or intensive care) and control (placebo or standard care) groups during the observation period, even when they were designed to achieve “glycemic equipoise” between trial arms. Moreover, the effects of antihyperglycemic therapies on total bodyweight have varied substantially across previous clinical trials.
Thus far, various pooled analyses of cardiovascular outcome trials have been reported that have examined the risk modulation of cardiovascular and HF outcomes conferred by alterations in blood glucose levels and bodyweight. For instance, in a previous meta-analysis of 30 large-scale cardiovascular outcome trials that was published in 2020, various glucose-lowering drugs or strategies that significantly reduced the glycated hemoglobin (HbA1c) level (> 0.01%) also reduced MACE risk, but they did not ameliorate HF risk compared with standard care or placebo [3]. In that study, the meta-regression analysis showed that bodyweight reduction was associated with HF risk reduction. In other meta-analyses of cardiovascular outcome trials involving newer antihyperglycemic medications (such as dipeptidyl-peptidase-4 inhibitors [DPP-4i], glucagon-like peptide-1 receptor agonists [GLP-1RA], and sodium-glucose cotransporter-2 inhibitors [SGLT2i]), significant associations were observed between improvements in glycemic control and the reduction of MACE risk [4,5,6,7], whereas no association was identified between glycemic improvement and the risk of HF [5,6,7]. Additionally, despite the acknowledged association between obesity and an increased risk of atherosclerotic cardiovascular disease (ASCVD) [8], the meta-analysis of cardiovascular outcome trials involving GLP-1RA revealed no correlation between bodyweight reduction and the risk of MACE [4]. However, whether and to what extent blood glucose lowering and bodyweight reduction are associated with cardiovascular and HF benefits has not been comprehensively studied and updated to include all trials of antihyperglycemic therapies completed before and after the establishment of the Food and Drug Administration (FDA) guidelines in 2008 [9]. The trials completed before the establishment of the FDA guidelines include the United Kingdom Prospective Diabetes Study (UKPDS), and those completed after the establishment of the FDA guidelines include newer studies of DPP-4i, GLP-1RA, and SGLT2i.
Recently, the results of more cardiovascular outcome trials with newer antihyperglycemic medications have become available. Therefore, we performed a comprehensive, updated meta-analysis and meta-regression analysis of 35 large-scale cardiovascular outcome trials of antihyperglycemic drugs reporting MACE and HF outcomes published both before and after the establishment of the FDA guidelines in 2008. We aimed to explore the relationships between blood glucose lowering or bodyweight reduction and MACE and HF risks, thereby delineating the potential contribution of blood glucose lowering or bodyweight reduction per se to cardiovascular and HF benefits.
Method
Search strategies and selection criteria
The protocol of this study has been registered at the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42022299075). This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [10]. We followed the eligibility criteria previously established [3]: (i) randomized controlled trials with an enrollment of a minimum of 1000 adults with type 2 diabetes or prediabetes, (ii) compared an antihyperglycemic drug, an intensive glycemic control strategy, or a lifestyle intervention strategy with controls (placebo, standard care, or an active control agent), (iii) reported MACE and HF as outcomes of interest, (iv) a follow-up period of at least one year, and (v) achieved an HbA1c difference greater than 0.01% between trial arms. Trials were excluded if they reported an achieved HbA1c difference of ≤ 0.01%, or if they did not report the difference in achieved HbA1c between the trial arms. We also excluded trials if a multifactorial intervention or non-glycemic medication were examined.
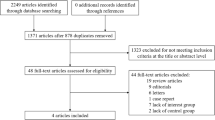
We conducted a literature search on PubMed and EMBASE databases from their inception until 25 January 2023 without language restriction to find relevant studies using the search strategies as follows: (type 2 diabetes OR prediabetes) AND (randomized OR randomly) AND (cardiovascular OR macrovascular OR MACE OR heart failure) AND (antihyperglycemic OR antidiabetic OR intensive glucose control OR intensive blood glucose control OR intensive blood-glucose control OR intensive glucose lowering OR lifestyle intervention OR biguanide* OR sulfonylurea* OR glinide* OR meglitinide* OR peroxisome proliferator-activated receptor* OR thiazolidinedione OR α-glucosidase inhibitor* OR dipeptidyl peptidase 4 OR glucagon-like peptide 1 OR glucagon-like peptide-1 OR sodium-glucose cotransporter 2). We also manually searched the reference lists of previous meta-analyses of cardiovascular outcome trials to identify potentially relevant studies. The PRISMA flow diagram is shown in Fig. 1.
Data extraction and quality assessment
From the eligible trials, we extracted data on study characteristics, baseline characteristics of participants, antihyperglycemic regimens used, control regimens used, mean differences in the achieved HbA1c and bodyweight levels between trial arms, and outcomes of interest that included risk ratios (RRs) with 95% confidence intervals (CIs). We extracted data from primary trial results and their accompanying supplementary materials as the primary data source. To determine the achieved differences in HbA1c or bodyweight levels between the trial arms, we used the time-weighted least squares mean difference over the course of follow-up, at the end of follow-up, or at one year of follow-up. We used the first available follow-up data if none of these values were reported. We used version 2 of the Cochrane Risk of Bias Tool to evaluate the risk of bias in the eligible studies [11]. MH and YK independently conducted the literature search and data extraction. The results were compared, and any discrepancies were resolved by consensus or with input from a third independent reviewer (SY).
Data analysis
The primary outcome was the efficacy of antihyperglycemic therapies on HF and MACE risks (defined as a composite of cardiovascular death, non-fatal myocardial infarction [MI], or non-fatal stroke). We determined the relationships between HbA1c reduction or bodyweight change and HF or MACE risk using meta-regression. If the trials did not report MACE according to our aforementioned definition, we used one of the following alternative definitions: death from cardiovascular or undetermined causes, non-fatal MI, or non-fatal stroke; cardiovascular death, non-fatal MI, or non-fatal ischemic stroke; all-cause death, non-fatal MI, or non-fatal stroke; cardiovascular death, non-fatal MI, non-fatal stroke, or other atherothrombotic events; all-cause death, non-fatal MI, non-fatal stroke, or other atherothrombotic events; fatal and non-fatal MI or stroke; or fatal and non-fatal MI. Detailed definitions of HF and MACE outcomes in each trial are displayed in Additional file 1: Table S1.
For the meta-analysis, pooled RRs with 95% CIs for MACE and HF outcomes were calculated using a random-effects model with the inverse variance method [12]. The between-trial variance was estimated using the DerSimonian–Laird estimator [13]. Heterogeneity among trials was evaluated using Cochran’s Q test and Higgins’s I2 statistics [14]. Thresholds defining the magnitude of heterogeneity based on the I2 index were low (≤ 25%), moderate (26–50%), and high (> 50%) [15]. Subgroup random-effects meta-analysis was performed based on a type of intervention (an intensive glycemic control strategy or each drug class). Additionally, we evaluated publication bias by funnel plots and Egger’s test [16].
For the meta-regression analysis with a mixed-effects model, we analyzed the association between the differences in the achieved HbA1c or bodyweight difference and the corresponding estimated log RR [Ln(RR)] of MACE and HF outcomes. To obtain the relative RR reduction of each outcome for every 1% HbA1c reduction or every 1 kg bodyweight reduction, we used the following formula with the regression coefficient (slope) in the meta-regression (Additional file 1: Fig. S1):
We used the restricted maximum likelihood as an estimator [17]. If a significant association was identified in the primary meta-regression analyses, we conducted trial-level subgroup meta-regression, stratified by (1) type of intervention (an intensive glycemic control strategy or each drug class) and (2) baseline prevalence of ASCVD (≥ 70% vs. < 70%). Trials without reported baseline proportions of patients with ASCVD were excluded from the latter subgroup analysis. Sensitivity meta-regression was performed to adjust for trial-level mean age, proportion of female participants, and person-years (calculated as the number of participants multiplied by the median follow-up duration in years, divided by 1000) to assess the consistency of the significant association found in the primary analysis.
Results with two-sided P-values less than 0.05 were considered significant for the pooled RR meta-analysis and meta-regression analysis. P-values less than 0.05 in Cochran’s Q test were also considered significant. All analyses were performed using R statistical software (version 4.1.2; the R Foundation for Statistical Computing, Vienna, Austria) with the ‘meta’ (version 4.18.2) and ‘metafor’ (version 3.02) packages.
Results
Search results and baseline study characteristics
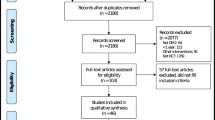
The initial search identified 1661 trials after removing duplicates. Screening for eligibility using titles and abstracts yielded 44 trials for detailed assessment, of which nine trials did not meet the inclusion criteria and were excluded (Fig. 1, Additional file 1: Table S2). Trials excluded through the full-text assessment included the CAROLINA trial, which compared linagliptin with glimepiride and showed no significant difference in both the achieved HbA1c and the MACE/ HF outcomes [18]. Therefore, 35 trials comprising 256,524 patients were included in the meta-analysis [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Among the 35 included trials, four assessed an intensive glycemic control strategy, one assessed intensive lifestyle intervention focusing on weight loss, one assessed insulin glargine (long-acting insulin analog), one assessed acarbose (α-glucosidase inhibitor), eight assessed peroxisome proliferation-activated receptor (PPAR) agonists, five assessed DPP-4i, nine assessed GLP-1RA, and six assessed SGLT2i. The main characteristics of the included trials are shown in Table 1. The mean follow-up duration was 1.3–10.0 years, and the average age of the patients was 53.3–69.0 years. A total of 164,276 of 248,306 (66.2%) assessable patients had established atherosclerotic cardiovascular disease, and 30,708 of 229,343 (13.4%) assessable patients had a history of HF at baseline. Although the trials designed as open-label had a high risk of bias in the domain of deviations from intended interventions because of the inability to blind the intervention, the evaluation of eligible trials showed no obvious risk of bias in most other domains. The risk of bias of each eligible trial is summarized in Additional file 1: Table S3. A visual inspection of funnel plots and the results of Egger’s test for outcomes of interest both indicated no evidence of publication bias (P Egger’s test = 0.42 and 0.79 for MACE and HF outcome, respectively); however, the ADOPT and DREAM trials, both of which investigated the efficacy of rosiglitazone, were observed as outliers in the funnel plot for MACE (Additional file 1: Fig. S2) [21, 22]. DREAM was also identified as an outlier in the funnel plot for HF (Additional file 1: Fig. S2) [22].
Major adverse cardiovascular events
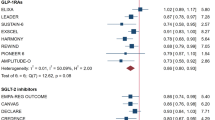
Overall, 25,475 patients (9.9%) experienced MACE outcomes during the follow-up period. In the pooled analysis of 35 trials, antihyperglycemic therapies decreased MACE risk by 9%, with moderate heterogeneity between trials (RR: 0.91; 95% CI 0.88–0.94; P < 0.001; I2 = 36.5%) (Fig. 2). In a subgroup random-effects meta-analysis based on the type of intervention, intensive glycemic control strategies (RR: 0.90; 95% CI 0.83–0.97; P = 0.008; I2 = 0.0%), PPAR agonists (RR: 0.91; 95% CI 0.84–0.97; P = 0.006; I2 = 25.2%), GLP-1RA (RR: 0.87; 95% CI 0.81–0.94; P = 0.001; I2 = 53.3%), and SGLT2i (RR: 0.88; 95% CI 0.82–0.94; P < 0.001; I2 = 28.1%) conferred a significantly lower risk of MACE to a similar extent, whereas the others showed null effects on MACE risk (Additional file 1: Fig. S3).
Efficacy of antihyperglycemic drugs on the risk of major adverse cardiovascular events (MACE). UKPDS 33, ACCORD, ADVANCE, VADT: trials comparing an intensive glycemic control strategy with standard care; Look AHEAD: a trial comparing intensive lifestyle intervention for weight loss with standard care; ORIGIN: a trial comparing insulin glargine with standard care; ACE: a trial comparing acarbose (α-glucosidase inhibitor [α-GI]) with placebo; PROactive, ADOPT, DREAM, BARI 2D, RECORD, AleCardio, IRIS, TOSCA.IT: trials comparing peroxisome proliferation-activated receptor (PPAR) agonists with placebo or active control drug; EXAMINE, SAVOR-TIMI 53, TECOS, OMNEON, CARMELINA: trials comparing dipeptidyl-peptidase-4 inhibitors (DPP-4i) with placebo; ELIXA, LEADER, SUSTAIN-6, EXSCEL, Harmony Outcomes, REWIND, PIONEER 6, AMPLITUDE-O, FREEDOM-CVO: trials comparing glucagon-like peptide-1 receptor agonists (GLP-1RA) with placebo; EMPAREG-OUTCOME, CANVAS-Program, DECLARE-TIMI 58, CREDENCE, VERTIS CV, SCORED: trials comparing sodium-glucose cotransporter-2 inhibitors (SGLT2i) with placebo
The univariate meta-regression analysis revealed a significant association between the HbA1c reduction from baseline and the Ln(RR) of MACE (slope: − 0.15; 95% CI − 0.27 to − 0.04; P = 0.010; variance explained: 52%) (Fig. 3A). Accordingly, every 1% greater reduction in HbA1c was associated with a 14% (95% CI 4–24) relative reduction in the RR of MACE. In contrast, bodyweight change from baseline was not significantly associated with the Ln(RR) of MACE (slope: − 0.006; 95% CI − 0.020 to 0.008; P = 0.41) (Fig. 3B).
Association between the risk of major adverse cardiovascular events (MACE) and A HbA1c reduction or B bodyweight change. The thicker line shows meta-regression with 95% CI as shading. The circle size of each trial reflects the study weight. HbA1c glycated hemoglobin, CI confidence interval, Ln(RR) estimated log risk ratio
We further evaluated the robustness of the relationship between HbA1c reduction and the risk of MACE through subgroup and sensitivity meta-regression analyses. In the stratified meta-regression by type of intervention, trials involving intensive glycemic control strategies, DPP-4i, and GLP-1RA showed a trend of reducing the Ln(RR) of MACE in association with a decrease in HbA1c; however, the relationship between HbA1c reduction and MACE risk was not statistically significant for all intervention types (Additional file 1: Table S4). In the stratified meta-regression based on the baseline prevalence of ASCVD (≥ 70% vs. < 70%), HbA1c reduction was associated with a decrease in the Ln(RR) of MACE for both subgroups. Notably, the decrease in MACE risk associated with HbA1c reduction was significant and greater in trials with ≥ 70% of patients with ASCVD at baseline (Additional file 1 Fig. S4). On the sensitivity meta-regression analysis with adjustment for multiple confounders (trial-level age, sex, and person-years), HbA1c reduction was significantly associated with the risk reduction of MACE, in agreement with the results of the main univariate analysis (slope: − 0.15; 95% CI − 0.26 to − 0.04; P = 0.008; variance explained: 89%).
Heart failure
Overall, 9163 patients (3.6%) experienced HF outcomes during the follow-up period. In the pooled analysis of 35 trials, antihyperglycemic therapies conferred no overall significant effect on HF risk with high heterogeneity across studies (RR: 0.95; 95% CI 0.87–1.04; P = 0.28; I2 = 75.9%) (Fig. 4). In a subgroup analysis based on the type of intervention, GLP-1RA (RR: 0.90; 95% CI 0.83–0.98; P = 0.019; I2 = 0.0%) and SGLT2i (RR: 0.68; 95% CI 0.62–0.75; P < 0.001; I2 = 0.0%) significantly reduced HF risk with a greater reduction of risk with SGLT2i. PPAR agonists significantly increased HF risk by 38% (RR: 1.38; 95% CI 1.19–1.60; P < 0.001; I2 = 53.0%). The others showed neutral effects on HF risk (Additional file 1: Fig. S5). Notably, SGLT2i lowered HF risk more than any other type of intervention, as indicated by the non-overlapping CI.
Efficacy of antihyperglycemic drugs on the risk of heart failure (HF). UKPDS 33, ACCORD, ADVANCE, VADT: trials comparing an intensive glycemic control strategy with standard care; Look AHEAD: a trial comparing intensive lifestyle intervention for weight loss with standard care; ORIGIN: a trial comparing insulin glargine with standard care; ACE: a trial comparing acarbose (α-glucosidase inhibitor [α-GI]) with placebo; PROactive, ADOPT, DREAM, BARI 2D, RECORD, AleCardio, IRIS, TOSCA.IT: trials comparing peroxisome proliferation-activated receptor (PPAR) agonists with placebo or active control drug; EXAMINE, SAVOR-TIMI 53, TECOS, OMNEON, CARMELINA: trials comparing dipeptidyl-peptidase-4 inhibitors (DPP-4i) with placebo; ELIXA, LEADER, SUSTAIN-6, EXSCEL, Harmony Outcomes, REWIND, PIONEER 6, AMPLITUDE-O, FREEDOM-CVO: trials comparing glucagon-like peptide-1 receptor agonists (GLP-1RA) with placebo; EMPAREG-OUTCOME, CANVAS-Program, DECLARE-TIMI 58, CREDENCE, VERTIS CV, SCORED: trials comparing sodium-glucose cotransporter-2 inhibitors (SGLT2i) with placebo
In meta-regression analyses, the Ln(RR) of HF was not significantly associated with HbA1c reduction (slope: − 0.12; 95% CI − 0.42 to 0.19; P = 0.46) (Fig. 5A), in contrast to the results from the meta-regression analysis of MACE outcomes. Instead, bodyweight reduction was significantly associated with the Ln(RR) of HF (slope: − 0.07; 95% CI − 0.10 to − 0.04; P < 0.001) (Fig. 5B). Accordingly, every 1 kg greater reduction in bodyweight was associated with a 7% (95% CI: 4–10) relative RR reduction of HF. However, significant residual heterogeneity (P < 0.001) was observed despite the variance explained (52%) was comparable with the variance explained by HbA1c for MACE (52%). Notably, all trials involving SGLT2i did not align well with the regression slope.
Association between heart failure (HF) risk and A HbA1c reduction or B bodyweight change. The thicker line shows meta-regression with 95% CI as shading. The circle size of each trial inversely reflects the study weight. HbA1c glycated hemoglobin, CI confidence interval, Ln(RR) estimated log risk ratio
We assessed the consistency of the relationship between bodyweight loss and the risk of HF through subgroup and sensitivity meta-regression analyses. In the stratified meta-regression by type of intervention, trials involving intensive glycemic control strategies, PPAR agonists, DPP-4i, and GLP-1RA showed a trend towards reducing the Ln(RR) of HF in association with a decrease in bodyweight; however, the association between bodyweight reduction and HF risk was not statistically significant for all intervention types (Additional file 1: Table S5). In the stratified meta-regression based on the baseline prevalence of ASCVD (≥ 70% vs. < 70%), bodyweight reduction was significantly associated with a decrease in the Ln(RR) of HF for both subgroups, in line with the overall results (Additional file 1: Fig. S6). In the sensitivity meta-regression analysis with adjustment for multiple confounders (trial-level age, sex, and person-years), bodyweight reduction was significantly associated with the risk reduction of HF consistent with the results of the main univariate analysis (slope: − 0.07; 95% CI − 0.10 to − 0.03; P < 0.001; variance explained: 48%).
Discussion
The current meta-analysis with meta-regression enrolled 256,524 patients with type 2 diabetes or prediabetes from 35 large-scale cardiovascular outcome trials and explored the associations between glycemic improvement or bodyweight change and MACE and HF risks. To our knowledge, this is the largest meta-analysis and meta-regression analysis of randomized controlled trials of antihyperglycemic therapies reporting MACE and HF outcomes. With respect to MACE outcomes, antihyperglycemic therapies significantly reduced MACE risk in the random-effects model meta-analysis, and the HbA1c reduction, not bodyweight reduction, was significantly correlated with a decline in the Ln(RR) of MACE in the univariate and multivariate meta-regression analyses; every 1% additional reduction in HbA1c was associated with a 14% relative reduction in MACE risk. The subgroup analysis further demonstrated that the relationship between HbA1c reduction and MACE risk reduction was more pronounced in patients with advanced atherosclerosis. These findings indicated potential effect modifications of MACE outcomes through glycemic control and reiterated the utility of HbA1c reduction as a marker of MACE risk reduction.
Regarding HF, antihyperglycemic therapies demonstrated a trend towards reducing HF risk compared with controls, with a RR of 0.95 (95% CI 0.87–1.04) in the meta-analysis, whereas the results showed high heterogeneity across trials. Contrary to MACE, bodyweight change, not HbA1c reduction, was associated with the Ln(RR) of HF in the univariate and multivariate meta-regression analyses. However, residual heterogeneity was high, and all trials involving SGLT2i did not align well with the regression slope; these findings indicated that while bodyweight reduction can partially contribute to HF risk reduction, other factors still influence HF risk modulation. In particular, the data on SGLT2i suggest a greater influence of residual contributors beyond the reduction in bodyweight and HbA1c on HF risk amelioration.
Glycemic control, bodyweight reduction, and major adverse cardiovascular events
In our study, antihyperglycemic therapies reduced MACE risk by 9%, which is almost identical to the MACE risk reduction reported by the previous meta-analyses of cardiovascular outcome trials with intensive glycemic control [54] and those with newer antihyperglycemic medications conducted after the establishment of the FDA guidelines in 2008 [5]. The significant association between HbA1c decline and attenuation of the MACE risk indicates that blood glucose lowering would proportionally decrease the risk of MACE, in agreement with the observations of the post hoc studies of the LEADER and REWIND trials, which suggested that HbA1c was a major and significant mediator of the cardiovascular benefits [55, 56]. Moreover, the findings in those mediation analyses that bodyweight was not a significant mediator of cardiovascular benefits for GLP-1RA are consistent with the non-significant association between bodyweight change and MACE risk in our meta-regression analysis [54, 55]. Additionally, most of the cardiovascular benefits associated with SGLT2i were presumed to be attributed to HbA1c reduction in three large cardiovascular outcome trials (EMPA-REG OUTCOME, CANVAS Program, and DECLARE-TIMI 58 trials) [57]. The previous meta-analysis results also corroborate the significant association between glycemic improvement and reduced risk of MACE [4,5,6,7]. Our finding supports the hypothesis that HbA1c reduction can be a useful clinical marker of MACE risk reduction across a wide range of antihyperglycemic therapies. Although high hypoglycemia risk associated with antihyperglycemic therapies may dilute the cardiovascular benefit conferred by glycemic reduction [58], blood glucose lowering remains a crucial aspect of cardiovascular risk management and is likely to contribute significantly to reducing MACE risk, as supported by a recent causal directed acyclic graphs study [7]. However, we note that the observed HbA1c reduction can be a marker representing multiple factors, including pleiotropic effects, rather than a single marker of improvement in glycemic control. Pleiotropic effects may include mitigation of endothelial dysfunction and oxidative stress, as observed with GLP-1RA and SGLT2i administration [59, 60]. Further studies are required to disentangle the contribution of glycemic and non-glycemic effects.
Glycemic control, bodyweight reduction, and heart failure
Regarding HF, antihyperglycemic therapies numerically but not significantly reduced HF risk by 5%, and both primary analysis and subgroup analysis showed high heterogeneity across studies. Contrary to MACE risk, HbA1c reduction was not associated with HF risk. Subgroup analyses show differing effects on HF based on the type of intervention, with SGLT2i and GLP-1RA reducing risk and PPAR agonists increasing it. This suggests glycemic control may not be critical for short-term (< 10 years) prevention or treatment of HF in dysglycemia.
In agreement with our meta-regression analysis results, the previous meta-regression analysis results showed that bodyweight reduction was significantly associated with a reduced HF risk [3]. This is theoretically reasonable because the two drugs that reduced HF risk (SGLT2i and GLP-1RA) decrease bodyweight through specific mechanisms [61], and PPAR agonists, which increased HF risk, increase bodyweight via fluid retention [62]. Given the favorable hemodynamic effects, such as ameliorated high blood pressure and fluid congestion, associated with bodyweight loss [63], the significant association we discovered between bodyweight loss and decreased risk of HF is biologically plausible. However, high residual heterogeneity (P < 0.001) and disproportionate reduction of HF risk by SGLT2i (Fig. 5B) suggest the involvement of other important factors in reducing HF risk. Considering that multiple post hoc analyses of the trials involving SGLT2i revealed that changes in markers of volume status and hemoconcentration (e.g., hematocrit), but not in bodyweight, are the most important mediators of cardiovascular death and HF, the clinical markers of the plasma volume status might be the more reliable markers of HF benefits [64,65,66,67]. This hypothesis is supported by an observational study reporting that lower hematocrit levels are associated with an increased risk of hospitalization for patients with HF [68].
Strengths and weaknesses
The main strength of our study is the inclusion of the largest number of cardiovascular outcome trials investigating various antihyperglycemic therapies conducted both before and after the establishment of the FDA guidelines in 2008, thereby allowing the most comprehensive evaluation of the contribution of blood glucose and bodyweight control to MACE and HF risks. Our study has important clinical implications–we highlight the utility of HbA1c and bodyweight changes as useful surrogates for cardiovascular and HF benefits, respectively, and also show class-specific benefits of SGLT2i beyond HbA1c or bodyweight reduction.
However, this study has several limitations. First, we did not use individual participant data, thus precluding our ability to adjust for some potential confounders. A meta-analysis performed with individual participant data could illustrate the independent effect of potential mediators of HF risk reduction, such as plasma volume, vascular resistance, and ketone bodies [59, 60, 64, 69]. Second, we did not evaluate the relationship of MACE or HF with conventional cardiovascular risks such as hypertension [70] and dyslipidemia [71] due to the limited availability of these data. Third, the included trials varied in their design, population, controls, and definitions of MACE and HF outcomes (Table 1, Additional file 1: Table S1); therefore, the pooled effects have to be interpreted with caution. However, the inclusion of a wider variety of trials, many of which represent the basis for the international clinical practice guidelines, allowed for more robust insights into the relationship between HbA1c or bodyweight change and cardiovascular outcomes. Fourth, it is essential to exercise caution in interpreting the results of the stratified meta-regression by type of intervention (Additional file 1: Tables S4 and S5), as a limited number of trials in each subgroup analysis increases the risk of overfitting and magnifies the variability of individual trial results, including any random error.
Conclusions
The updated meta-analysis and meta-regression analysis of 35 cardiovascular outcome trials show that glycemic control conferred by a wide range of antihyperglycemic drugs decreases MACE risk in an HbA1c-dependent manner, and the degree of HbA1c reduction is a useful surrogate of cardiovascular benefits. Contrary to MACE risk reduction, HF risk modulation was not associated with HbA1c reduction but was associated with bodyweight reduction. However, high residual heterogeneity suggests the contributions of other factors. Importantly, SGLT2 inhibitors reduced the risk of HF more than could be explained by HbA1c or bodyweight reduction, highlighting the drug class-specific benefits for HF.
Availability of data and materials
All data were extracted from publicly available sources and are included in this published article and its Additional file.
Change history
24 April 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12933-023-01825-4
Abbreviations
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CI:
-
Confidence interval
- CVOTs:
-
Cardiovascular outcome trials
- DPP-4i:
-
Dipeptidyl-peptidase-4 inhibitors
- Ln(RR):
-
Estimated log risk ratio
- FDA:
-
Food and Drug Administration
- GLP-1RA:
-
Glucagon-like receptor agonists
- HbA1c:
-
Glycated hemoglobin
- HF:
-
Heart failure
- MACE:
-
Major adverse cardiovascular events
- MI:
-
Myocardial infarction
- PPAR:
-
Peroxisome proliferation-activated receptor
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- RR:
-
risk ratio
- SGLT2i:
-
Sodium-glucose cotransporter-2 inhibitors
- UKPDS:
-
United Kingdom Prospective Diabetes Study
References
Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–44. https://doi.org/10.1056/NEJMoa1800256.
Davies MJ, Drexel H, Jornayvaz FR, Pataky Z, Seferović PM, Wanner C. Cardiovascular outcomes trials: a paradigm shift in the current management of type 2 diabetes. Cardiovasc Diabetol. 2022;21(1):144. https://doi.org/10.1186/s12933-022-01575-9.
Ghosh-Swaby OR, Goodman SG, Leiter LA, Cheng A, Connelly KA, Fitchett D, et al. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: an updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2020;8(5):418–35. https://doi.org/10.1016/S2213-8587(20)30038-3.
Yoshiji S, Minamino H, Tanaka D, Yamane S, Harada N, Inagaki N. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular and renal outcomes: a meta-analysis and meta-regression analysis. Diabetes Obes Metab. 2022;24(6):1029–37. https://doi.org/10.1111/dom.14666.
Maiorino MI, Longo M, Scappaticcio L, Bellastella G, Chiodini P, Esposito K, et al. Improvement of glycemic control and reduction of major cardiovascular events in 18 cardiovascular outcome trials: an updated meta-regression. Cardiovasc Diabetol. 2021;20(1):210. https://doi.org/10.1186/s12933-021-01401-8.
Giugliano D, Bellastella G, Longo M, Scappaticcio L, Maiorino MI, Chiodini P, et al. Relationship between improvement of glycaemic control and reduction of major cardiovascular events in 15 cardiovascular outcome trials: a meta-analysis with meta-regression. Diabetes Obes Metab. 2020;22(8):1397–405. https://doi.org/10.1111/dom.14047.
Huang CJ, Wang WT, Sung SH, Chen CH, Lip GYH, Cheng HM, et al. Revisiting ‘intensive’ blood glucose control: a causal directed acyclic graph-guided systematic review of randomized controlled trials. Diabetes Obes Metab. 2022;24(12):2341–52. https://doi.org/10.1111/dom.14819.
Cercato C, Fonseca FA. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11:74. https://doi.org/10.1186/s13098-019-0468-0.
Food and Drug Administration. Guidance for industry: diabetes mellitus-evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Silver Spring, Maryland: Food and Drug Administration, December 2008. https://www.fda.gov/media/71297/download. Accessed 4 Feb 2023.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. https://doi.org/10.1002/jrsm.12.
DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. https://doi.org/10.1016/j.cct.2015.09.002.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Wolfgang V. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. https://doi.org/10.18637/jss.v036.i03.
Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: The CAROLINA Randomized Clinical Trial. JAMA. 2019;322(12):1155–66. https://doi.org/10.1001/jama.2019.13772.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–89. https://doi.org/10.1016/S0140-6736(05)67528-9.
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–43. https://doi.org/10.1056/NEJMoa066224.
Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096–105. https://doi.org/10.1016/S0140-6736(06)69420-8.
Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. https://doi.org/10.1056/NEJMoa0802743.
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. https://doi.org/10.1056/NEJMoa0802987.
Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–15. https://doi.org/10.1056/NEJMoa0805796.
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–35. https://doi.org/10.1016/S0140-6736(09)60953-3.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. https://doi.org/10.1056/NEJMoa0808431.
Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28. https://doi.org/10.1056/NEJMoa1203858.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35. https://doi.org/10.1056/NEJMoa1305889.
Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54. https://doi.org/10.1056/NEJMoa1212914.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26. https://doi.org/10.1056/NEJMoa1307684.
Lincoff AM, Tardif JC, Schwartz GG, Nicholls SJ, Rydén L, Neal B, et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311(15):1515–25. https://doi.org/10.1001/jama.2014.3321.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. https://doi.org/10.1056/NEJMoa1509225.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42. https://doi.org/10.1056/NEJMoa1501352.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31. https://doi.org/10.1056/NEJMoa1506930.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141.
Gantz I, Chen M, Suryawanshi S, Ntabadde C, Shah S, O’Neill EA, et al. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2017;16(1):112. https://doi.org/10.1186/s12933-017-0593-8.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. https://doi.org/10.1056/NEJMoa1611925.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. https://doi.org/10.1056/NEJMoa1612917.
Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):877–86. https://doi.org/10.1016/S2213-8587(17)30309-1.
Vaccaro O, Masulli M, Nicolucci A, Bonora E, Del Prato S, Maggioni AP, et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCAI.T): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017;5(11):887–97. https://doi.org/10.1016/S2213-8587(17)30317-0.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. https://doi.org/10.1016/S0140-6736(18)32261-X.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. https://doi.org/10.1056/NEJMoa1812389.
Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019;321(1):69–79. https://doi.org/10.1001/jama.2018.18269.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. https://doi.org/10.1056/NEJMoa1811744.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. https://doi.org/10.1016/S0140-6736(19)31149-3.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. https://doi.org/10.1056/NEJMoa1901118.
Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35. https://doi.org/10.1056/NEJMoa2004967.
Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39. https://doi.org/10.1056/NEJMoa2030186.
Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. https://doi.org/10.1056/NEJMoa2108269.
Ruff CT, Baron M, Im K, O’Donoghue ML, Fiedorek FT, Sabatine MS. Subcutaneous infusion of exenatide and cardiovascular outcomes in type 2 diabetes: a non-inferiority randomized controlled trial. Nat Med. 2022;28(1):89–95. https://doi.org/10.1038/s41591-021-01584-3.
Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288–98. https://doi.org/10.1007/s00125-009-1470-0.
Buse JB, Bain SC, Mann JFE, Nauck MA, Nissen SE, Pocock S, et al. Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care. 2020;43(7):1546–52. https://doi.org/10.2337/dc19-2251.
Konig M, Riddle MC, Colhoun HM, Branch KR, Atisso CM, Lakshmanan MC, et al. Exploring potential mediators of the cardiovascular benefit of dulaglutide in type 2 diabetes patients in REWIND. Cardiovasc Diabetol. 2021;20(1):194. https://doi.org/10.1186/s12933-021-01386-4.
Shao H, Shi L, Fonseca VA. Using the BRAVO risk engine to predict cardiovascular outcomes in clinical trials with sodium-glucose transporter 2 inhibitors. Diabetes Care. 2020;43(7):1530–6. https://doi.org/10.2337/dc20-0227.
Huang CJ, Wang WT, Sung SH, Chen CH, Lip GYH, Cheng HM, et al. Blood glucose reduction by diabetic drugs with minimal hypoglycaemia risk for cardiovascular outcomes: evidence from meta-regression analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(9):2131–9. https://doi.org/10.1111/dom.13342.
Koska J, Sands M, Burciu C, D’Souza KM, Raravikar K, Liu J, et al. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: Evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes. 2015;64(7):2624–35. https://doi.org/10.2337/db14-0976.
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180–93. https://doi.org/10.1111/dom.12572.
Brown E, Wilding JPH, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obes Rev. 2019;20(6):816–28. https://doi.org/10.1111/obr.12841.
Home P. Safety of PPAR agonists. Diabetes Care. 2011;34(Suppl 2):S215–9. https://doi.org/10.2337/dc11-s233.
Reddy YNV, Anantha-Narayanan M, Obokata M, Koepp KE, Erwin P, Carter RE, et al. Hemodynamic effects of weight loss in obesity: a systematic review and meta-analysis. JACC Heart Fail. 2019;7(8):678–87. https://doi.org/10.1016/j.jchf.2019.04.019.
Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41(2):356–63. https://doi.org/10.2337/dc17-1096.
Fitchett D, Inzucchi SE, Zinman B, Wanner C, Schumacher M, Schmoor C, et al. Mediators of the improvement in heart failure outcomes with empagliflozin in the EMPA-REG OUTCOME trial. ESC Heart Fail. 2021;8(6):4517–27. https://doi.org/10.1002/ehf2.13615.
Li J, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail. 2020;8(1):57–66. https://doi.org/10.1016/j.jchf.2019.08.004.
Segar MW, Kolkailah AA, Frederich R, Pong A, Cannon CP, Cosentino F, et al. Mediators of ertugliflozin effects on heart failure and kidney outcomes among patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2022;24(9):1829–39. https://doi.org/10.1111/dom.14769.
Kosiborod M, Curtis JP, Wang Y, Smith GL, Masoudi FA, Foody JM, et al. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med. 2005;165(19):2237–44. https://doi.org/10.1001/archinte.165.19.2237.
Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–22. https://doi.org/10.2337/dc16-0542.
Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2(7):775–81. https://doi.org/10.1001/jamacardio.2017.1421.
Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. https://doi.org/10.1001/jama.2009.1619.
Acknowledgements
SY and HM are research fellows of the Japan Society for the Promotion of Science.
Funding
There was no direct funding for performing this study.
Author information
Authors and Affiliations
Contributions
SY conceived the study concept and design and conducted the statistical analysis. MH and YK performed the literature search and data collection from relevant studies. MH, SY, and YK contributed to the interpretation of the findings and writing the first draft of the manuscript, which was critically revised by HM, TM, DT, YF, NH, AH, and NI. NI acted as the guarantor of this work. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Does not involve ethical approval and consent.
Consent for publications
Not applicable.
Competing interests
NI received research funds from Terumo Corp., Drawbridge, Inc., and Asken Inc. NI received speaker honoraria from Kowa Co., Ltd., MSD K. K, Astellas Pharma Inc., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Sumitomo Dainippon Pharma Co., Ltd., Sanofi K.K., and Eli Lilly Japan K.K.; received a scholarship grant from Kissei Pharmaceutical Co., Ltd., Sanofi K.K., Daiichi-Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corp., Takeda Pharmaceutical Co., Ltd., Japan Tobacco Inc., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., MSD K.K., Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Novartis Pharma K.K., and Life Scan Japan K.K. NI is an advisory board member of Novo Nordisk. NI and NH were investigators for the SUSTAIN and PIONEER programs. Other authors declare no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The errors in Figure 2 had been corrected.
Supplementary Information
Additional file 1
: Table S1. Definition of heart failure and major adverse cardiovascular events of the included trials. Table S2. Excluded trials through detailed full-text assessment. Table S3. Risk of bias of the included trials. Table S4. Univariate meta-regression analyses of HbA1c reduction and the estimated log risk ratio of major adverse cardiovascular events based on intervention type. Table S5. Univariate meta-regression analyses of bodyweight change and the estimated log risk ratio of heart failure based on intervention type. Figure S1. Derivation of formula to obtain the relative risk ratio reduction of outcomes with meta-regression results. Figure S2. Funnel plots for assessing publication bias of major adverse cardiovascular events (MACE) and heart failure (HF) outcomes. Figure S3. Efficacy of antihyperglycemic therapies on the risk of major adverse cardiovascular events (MACE) in each subgroup. Figure S4. Association between the risk of major adverse cardiovascular events (MACE) and HbA1c reduction stratified by the baseline prevalence of ASCVD. Figure S5. Efficacy of antihyperglycemic therpies on the risk of heart failure (HF) in each subgroup. Figure S6. Association between heart failure (HF) risk and bodyweight change stratified by the baseline prevalence of ASCVD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hasebe, M., Yoshiji, S., Keidai, Y. et al. Efficacy of antihyperglycemic therapies on cardiovascular and heart failure outcomes: an updated meta-analysis and meta-regression analysis of 35 randomized cardiovascular outcome trials. Cardiovasc Diabetol 22, 62 (2023). https://doi.org/10.1186/s12933-023-01773-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01773-z