Abstract
Objective
To evaluate the cardiovascular and renal benefits of finerenone, sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagonlike peptide-1 receptor agonists (GLP-1 RA) in patients with Type 2 Diabetes Mellitus (T2DM) and chronic kidney disease (CKD) with network meta-analysis.
Methods
Systematic literature searches were conducted of PubMed, Cochrane Library, Web of Science, Medline and Embase covering January 1, 2000 to December 30, 2021. Randomized control trials (RCTs) comparing finerenone, SGLT-2i and GLP-1 RA in diabetics with CKD were selected. We performed a network meta-analysis to compare the two drugs and finerenone indirectly. Results were reported as risk ratio (RR) with corresponding 95% confidence interval (CI).
Results
18 RCTs involving 51,496 patients were included. Finerenone reduced the risk of major adverse cardiovascular events (MACE), renal outcome and hospitalization for heart failure (HHF) (RR [95% CI]; 0.88 [0.80–0.97], 0.86 [0.79–0.93], 0.79 [0.67,0.92], respectively). SGLT-2i were associated with reduced risks of MACE (RR [95% CI]; 0.84 [0.78–0.90]), renal outcome (RR [95% CI]; 0.67 [0.60–0.74], HHF (RR [95% CI]; 0.60 [0.53–0.68]), all-cause death (ACD) (RR [95% CI]; 0.89 [0.81–0.91]) and cardiovascular death (CVD) (RR [95% CI]; 0.86 [0.77–0.96]) compared to placebo. GLP-1 RA were associated with a lower risk of MACE (RR [95% CI]; 0.86 [0.78–0.94]). SGLT2i had significant effect in comparison to finerenone (finerenone vs SGLT2i: RR [95% CI]; 1.29 [1.13–1.47], 1.31 [1.07–1.61], respectively) and GLP-1 RA (GLP-1 RA vs SGLT2i: RR [95% CI]; 1.36 [1.16–1.59], 1.49 [1.18–1.89], respectively) in renal outcome and HHF.
Conclusions
In patients with T2DM and CKD, SGLT2i, GLP-1 RA and finerenone were comparable in MACE, ACD and CVD. SGLT2i significantly decreased the risk of renal events and HHF compared with finerenone and GLP-1 RA. Among GLP-1 RA, GLP-1 analogues showed significant effect in reducing cardiovascular events compared with exendin-4 analogues.
Similar content being viewed by others
Background
As the prevalence of diabetes increases over the recent years, approximately 536.6 million are diagnosed with Dabetes Mellitus (DM). It is estimated that by the year of 2045, at least 783.2 million adults will be affected by diabetes [1]. Patients with diabetes are at high risk for adverse outcomes from atherosclerotic cardiovascular disease (ASCVD) [2, 3], heart failure and renal disease [4, 5]. With the increasing prevalence of Type 2 Diabetes Mellitus (T2DM) during recent decades, it has gradually become one of the primary factors accounting for the substantial global increase in end-stage renal disease (ESRD). Even with current therapies available [6,7,8,9,10], patients with T2DM and chronic kidney disease (CKD) still experience a significant cardiovascular and renal morbidity and mortality. Moreover, the risk of patients developing cardiovascular and renal events increase as DM and CKD progresses, potentially reaching renal and cardiac endpoint events such as ESRD, heart failure, myocardial infarction (MI) and stroke [11,12,13,14]. Therefore, the prevention of CKD progression and cardiovascular events is essential for the management of patients with T2DM and CKD.
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1 RA) were at the forefront of research in the field of diabetes. Several large cohort studies and randomized controlled trials (RCTs) have demonstrated cardiovascular and renal benefit for both drugs in patients with diabetes or kidney disease. Thus, the American Diabetes Association (ADA) recommended these two drugs for individuals with T2DM with or at high risk for ASCVD, heart failure, and/or CKD [15].
Finerenone is a nonsteroidal and selective mineralocorticoid receptor antagonist. According to two large randomized placebo-controlled trials targeted at T2DM and CKD patients, finerenone has been demonstrated to significantly reduce the occurrences of composite renal outcome (defined as a composite of a sustained decrease of at least 40% in the estimated glomerular filtration rate (eGFR) from the baseline, kidney failure, or death from renal causes) and composite cardiovascular outcome (defined as a composite of nonfatal MI, nonfatal stroke, death from cardiovascular causes, or hospitalization for heart failure [HHF]), regardless of patients with or without established cardiovascular disease [16, 17]. Consequently, in renin–angiotensin–aldosterone system (RAAS) inhibitions, finerenone represented a new frontier in the treatment of diabetic kidney disease [18]. ADA suggested that in patients with T2DM and CKD who were at increased risk for cardiovascular events or CKD progression or were unable to use the SGLT2i, finerenone was recommended to reduce CKD progression and cardiovascular events,. It was also suggested that the use of GLP-1 RA for individuals with T2DM with or at high risk of ASCVD, and/or CKD was optional [19].
Although finerenone, SGLT2i and GLP-1 RA offered cardiovascular or renal benefits to patients with T2DM and CKD, currently, there was no comparable study focusing on their effects on cardiovascular and renal outcomes. The network meta-analysis based on direct and indirect comparisons is an efficient algorithmically optimized method that can assist in clinical decision making. Even in the absence of head-to-head comparisons, it could still help to produce ranking results. Therefore, we herein investigate the effectiveness of finerenone, SGLT2i and GLP-1 RA in patients with T2DM and CKD by performing network meta-analysis based on RCTs.
Methods
Registration
We prospectively registered this systematic review in the International Prospective Register of Systematic Reviews database (PROSPERO) (registration number: CRD42022301457).
Literature search
Our search strategy was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) extension statement for network meta-analysis [20, 21]. We performed a systematic search of PubMed, Cochrane Library, Web of Science, Medline and Embase from January 1, 2000 to December 30, 2021.
The following keywords were applied:((“Glucagon-Like Peptide 1 receptor[MeSH]” OR “GLP-1” OR “GLP1 receptor agonist” OR “glucagon-like peptide-1 receptor agonist” OR “Exenatide[MeSH]” OR “Liraglutide[MeSH]” OR “Lixisenatide” OR “Albiglutide” OR “Dulaglutide” OR “Semaglutide”) OR (“Sodium-Glucose Transporter 2 Inhibitors[MeSH]” OR “SGLT-2 inhibitor” OR “SGLT-2” OR “Canagliflozin[MeSH]” OR “Dapagliflozin” OR “Sotagliflozin” OR “empagliflozin” OR “Ertugliflozin” OR “Luseoglifozin”) OR “Finerenone”) AND ((“Renal Insufficiency, Chronic[MeSH]” OR “chronic kidney disease” OR “CKD” OR “kidney disease” OR “kidney failure” OR “chronic kidney failure” OR “renal failure” OR “chronic renal disease” OR “chronic renal failure” OR “CRF”) AND (“Diabetes Mellitus[MeSH]” OR “Diabetes Mellitus type 2” OR “type 2 Diabetes Mellitus”)).
The search results were screened separately by two blinded and independent authors (Z and J) to identify studies according to inclusion and exclusion criteria. When the two authors encountered the inconsistencies, a third author (W) was consulted to reach a decision. In addition, we reviewed the list of references included in the meta-analysis studies to minimize missing relevant studies.
Study selection
Studies were selected if they met the following criteria: (1) they were published in peer-reviewed journals; (2) they included adult patients (≥ 18 years old) with T2DM and(or) CKD; (3) they were RCTs that compared finerenone, SGLT2i or GLP-1 RA with a placebo; (4) they compared the risk of cardiovascular and renal outcomes between treatment and placebo groups; and (5) they were published in English. Studies were excluded if data for estimating risk ratio (RR) was insufficient even after contact with the authors.
Outcomes
Five outcomes were assessed in this study, which were MACE, Renal outcome, HHF, all-cause death (ACD) and CVD. The definition of MACE was a composite of CVD, nonfatal MI, or nonfatal stroke. If nonfatal MI and stroke data were unavailable, then the total MI and stroke were used instead. Renal outcome was defined as a composite of a sustained decrease of at least 40% in the eGFR from the baseline or a doubling of the serum creatinine level, kidney failure (a composite of end-stage kidney disease or sustained decrease in eGFR to < 15 ml/min/1.73 m2), or renal death. A similar renal outcome was used instead when this composite outcome was unavailable.
Data extraction and quality assessment
Two researchers (Z and J), independently performed data abstraction and risk of bias assessment from eligible studies. Risk of bias assessment was performed according to the Cochrane risk of bias assessment tool (RoB 2.0) [22]. Any discrepancies in data extraction or quality assessment were resolved by a third reviewer (W). Data regarding cardiovascular and renal outcomes were abstracted from each study group. In this study, we also applied the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method in order to assess the quality of the evidence for each outcome, GRADE method can be found and accessed in GRADEpro GDT software [23]. Evidence quality was graded into four grades, these categories are labelled as High, Moderate, Low, and Very low. To prevent any other factors that may alter the result such as bias and inaccuracies, we have also referred to the five criteria, which are the risk of bias, the inconsistency, the indirectness, the imprecision and the publication bias. The application of these criteria is used as an evaluation to create the summary of evidence table [24, 25]. In addition to the five criteria, this network meta-analysis has also taken intransitivity and incoherence in to consideration, as they are vital when it comes to assess the quality of evidence for each outcome. In parallel, the quality of treatment effect estimation was rated based on the quality ratings of direct and indirect comparisons compliant to the GRADE Working Group approach [26].
Statistical analysis
We performed a network meta-analysis using Stata (version 15.0). Risk ratio (RR) and 95% confidence interval (CI) were used to present the efficacy of treatments. The probability value of the I2 variable was calculated to assess heterogeneity, which was considered to be unimportant (0% < I2 < 40%), moderate heterogeneity (30% < I2 < 60%), substantial heterogeneity (50% < I2 < 90%), considerable heterogeneity (75% < I2 < 100%) [27].
In order to classified each of the intervention's effectiveness, finerenone, SGLT2i and GLP-1 RA were ranked from the most to the least effective or harmful, we used the Minimally Contextualized Framework to perform the results. The placebo was most closely connected to the other interventions and selected as the reference group, with an ineffective value, i.e. a relative effect value of 1, as the decision threshold. Based on the cardiovascular and renal outcomes, we used the 95% CI of the estimate of effect comparing each of the interventions against the placebo. If the interval crosses the decision threshold, then its corresponding intervention can remain in the same group as the placebo. On the other hand, if the interval did not cross the decision threshold, then depending on which side of the threshold the interval lies on, the intervention could be classified as more effective or less effective than the placebo. Based on comparisons made between pairs of interventions, should any intervention proves to be more effective than another category 1 intervention, then that corresponding intervention can be moved to a higher rated group (category 2) [28]. After evaluating the certainty of the evidence from finerenone and other 10 interventions included in SGLT2i and GLP-1 RA, the interventions were classified again into two broad categories: high certainty (moderate to high certainty evidence) and low certainty (low to very low certainty evidence). After checking consistency with pairwise comparisons and rankings, the intervention at the highest classification level could be considered as the most effective choice currently available, while low certainty as might be among the most effective.
We conducted a sensitivity analysis excluding “Cherney 2021”, as Cherney 2021 only included diabetics with severe CKD (eGFR: 15–30 ml/min/1.73 m2). In this network meta-analysis, none of the 5 outcomes had a closed loop. Therefore, it means that there was only indirect evidence among finerenone, SGLT2i and GLP-1 RA. Consequently, there was no need to test inconsistency for this network meta-analysis.
Results
Literature search and included studies
The detailed study filtering process is shown in Fig. 1. In brief, we retrieved a total of 5163 articles from PubMed (n = 977), Cochrane Library (n = 74), Web of science (n = 1022), Medline (n = 1470) and Embase (n = 1620) in primary search, during the process another 12 articles were identified through references. A total of 2232 duplicate articles were removed. After review by title and abstract, 2849 articles were removed due to: Non-standard intervention (n = 276), unsuitable population (n = 417), case report (n = 21), non-human (n = 16), design (n = 53), letter or commentary or abstract (n = 476), non-RCT (n = 79), review or meta-analysis (n = 1511). After that, 94 articles remained and entered into full-text assessing section. By assessing full text, 55 additional articles were excluded due to the lack of relevant outcome indicators. Finally, 39 articles (included 18 randomized controlled trials) were included in this network meta-analysis [7, 16, 17, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Out of 18 studies, 3 studies were compared finerenone [16, 17, 29,30,31] with placebo; 8 studies were compared SGLT2i (Empaglifozin [32,33,34,35,36], Canaglifozin [7, 37,38,39,40,41,42,43], Dapaglifozin [44,45,46,47,48], Ertuglifozin [49,50,51], and Sotaglifozin [52, 53]) with placebo; 7 studies compared GLP-1 RA (Dulaglutide [54, 55], Albiglutide [56], Exenatide [57, 58], Semaglutide [59, 60], Liraglutide [61,62,63] and Efpeglenatide [64]) with placebo.
Baseline characteristics of included studies in patients with T2DM and CKD
The characteristics of the included studies are presented in Table 1. The pooled population consisted of 51,496 patients with T2DM and CKD, 14,847 of them were in finerenone studies (7246 in the intervention group and 7601 in control group), 25,098 patients in SGLT-2i studies (13,260 in the intervention group and 11,838 in control group) and 11,551 patients in GLP-1 RA studies (5355 in the group treated with GLP-1 RA and 5796 in the control group). The definition of MACE in the included trials were consistent, except for four of them, EMPA-REG, DECLARE–TIMI 58, EXSCEL trials (data for nonfatal MI and stroke were not available, so we used total MI and stroke instead) and FIGARO-DKD (a composite of CVD, nonfatal MI, nonfatal stroke, or HHF). Whereas renal outcome were defined slightly different across included trials, but they were similar enough that can be used in analysis. The detailed definitions of renal outcome in different trials are shown in Table 2.
Risk of bias
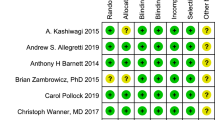
We assessed the risk of bias in those trials using the Revised Cochrane Risk of Bias Tool (RoB 2.0). The quality evaluation of the included studies is shown in Fig. 2. All trials were evaluated as low risk in 5 outcomes. Detailed evaluations are as shown in Additional file 1 (RoB-2 evaluation) for each study.
GRADE assessment
In terms of reducing the MACE, there were 16 direct comparisons in the original articles and they were estimated high quality. In terms of renal outcome, there were 13 direct comparisons in the original articles whose estimated results were high quality. In terms of reducing the HHF and CVD, there were 12 direct comparisons in the original articles and they were rated as high quality. In terms of reducing the ACD, there were 11 direct comparisons in the original articles and they were rated as high quality. The detail was shown in Table 3. Figure 3 shows the network graph. As is shown in Additional file 2 (Publication bias), for the five outcomes, all studies were distributed symmetrically on both sides of the midline.
According to recommendation of GRADE working group, we presented a four-step approach to rate the quality of evidence in each of the direct, indirect, and network meta-analysis estimates based on methods developed by the GRADE working group [26]. In this network meta-analysis, none of the 5 outcomes had a closed loop. Meaning that that no outcomes from both direct and indirect comparisons are included, rendering incoherence assessment unnecessary. The definition of renal outcome varied between studies included in this research, and the baseline eGFR of patients in the “cherney 2021” was different from other studies. For direct comparisons, “cherney 2021” included only 1% of patients in SGLT2i (277/25098). Therefore, risk of bias was not taken in to consideration. As for intransitivity, there was only indirect evidence in the intercomparison of finerenone, SGLT2i and GLP-1 RA. The GRADE working group recommends that situation regarding intransitivity may warrant particular attention, and the threshold for rating down for intransitivity may be lower [26]. Therefore, we downgraded the quality of evidence for the comparison between SGLT2i and finerenone, SGLT2i and GLP-1 RA. The detail was shown in Table 4.
Network meta‑analysis of treatment groups
MACE
Compared with placebo, finerenone (RR [95% CI]; 0.88 [0.80–0.97]), SGLT-2i (RR [95% CI]; 0.84 [0.78–0.90]) and GLP-1 RA (RR [95% CI]; 0.86 [0.78–0.94]) were associated with a decreased risk of MACE. Finerenone didn`t show a significant difference in reducing the risk of MACE compared with SGLT-2i (RR [95% CI]; 1.05 [0.93–1.19]) and GLP-1 RA (RR [95% CI]; 1.03 [0.90–1.17]). There was also no significant difference in the risk of MACE between SGLT-2i and GLP-1 RA (RR [95% CI]; 1.03 [0.91–1.16]). There was no heterogeneity (I2 = 34.5%, p = 0.087). The detail is shown in Fig. 4.
Renal outcome
Finerenone (RR [95% CI]; 0.86 [0.79–0.93]) and SGLT-2i (RR [95% CI]; 0.67 [0.60–0.74]) significantly decreased the morbidity of renal outcome when compared with placebo, while GLP-1 RA (RR [95% CI]; 0.90 [0.73–1.02]) did not. Compared with finerenone (finerenone vs SGLT2i: RR [95% CI]; 1.31 [1.07–1.61]) and GLP-1 RA (GLP-1 RA vs SGLT2i: RR [95% CI]; 1.49 [1.18–1.89]), SGLT-2i were associated with a decreased morbidity of renal outcome. Finerenone was comparable to GLP-1 RA (RR [95% CI]; 0.95 [0.82–1.10]). There was moderate heterogeneity (I2 = 37.4%, p = 0.085). The detail is shown in Fig. 5.
HHF
Compared with placebo, finerenone (RR [95% CI]; 0.79 [0.67–0.92]) and SGLT2i (RR [95% CI]; 0.60 [0.53–0.68]) were associated with a decreased risk of HHF while GLP-1 RA (RR [95% CI]; 0.90 [0.73–1.09]) did not. Compared with finerenone (finerenone vs SGLT2i: RR [95% CI]; 1.31 [1.07–1.61]) and GLP-1 RA (GLP-1 RA vs SGLT2i: RR [95% CI]; 1.49 [1.18–1.89]), SGLT-2i was shown to be significantly more effective in reducing HHF. But there was no significant difference in the risk of HHF between finerenone and GLP-1 RA (RR [95% CI]; 0.88 [0.68–1.14]). There was moderate heterogeneity (I2 = 44.9%, p = 0.046). The detail is shown in Fig. 6.
ACD
Compared with placebo, finerenone (RR [95% CI]; 0.90 [0.80–1.00) had a tendency to decrease the risk of ACD and SGLT-2i (RR [95% CI]; 0.89 [0.81–0.99]) were associated with a decreased risk of ACD, while GLP-1 RA (RR [95% CI]; 0.89 [0.77–1.02]) did not. There was no significant difference among finerenone, SGLT2i and GLP-1RA (RR 0.99, 95% CI 0.84–1.18; RR 1.00, 95% CI 0.86–1.16; RR 1.01, 95% CI 0.85–1.20, respectively). This analysis showed no heterogeneity (I2 = 0.0%, p = 0.554). The detail is shown in Fig. 7.
CVD
As for CVD, only SGLT-2i were associated with a decreased events (RR [95% CI]; 0.86, [0.77–0.96]) compared with placebo. There was no significant difference between finerenone and placebo, GLP-1 RA and placebo. And finerenone, SGLT2i and GLP-1 RA were comparable in reducing the risk of CVD. (Fig. 8). The analysis of CVD showed no heterogeneity (I2 = 4.4%, p = 0.402). The detail is shown in Fig. 8.
Finerenone vs 10 interventions included in SGLT2i and GLP-1 RA
In order to provide more specific recommendations for clinical drug selection, we further evaluated the efficacy of finerenone and the 10 interventions included in SGLT2i and GLP-1 RA. As for MACE, finerenone was comparable to other interventions, except liraglutide (RR [95% CI]; 1.28 [1.04–1.56]). Canagliflozin, sotagliflozin, efpeglenatide and liraglutide were associated with a decreased risk of MACE compared to ertugliflozin or exenatide. Liraglutide had a tendency to reduce MACE compared to albiglutide (RR [95% CI]; 0.74 [0.55–1.00]), it also showed more positive influence when compared with dapagliflozin (RR [95% CI]; 0.75 [0.58–0.96]). Compared to placebo, finerenone (RR [95% CI]; 0.88 [0.80–0.97]), canagliflozin(RR [95% CI]; 0.78 [0.68–0.89]), sotagliflozin(RR [95% CI]; 0.76 [0.66–0.87]) efpeglenatide(RR [95% CI]; 0.70 [0.53–0.90]) and liraglutide(RR [95% CI]; 0.69 [0.58–0.82]) displayed significant effect when reducing of MACE, while other interventions were not. The detail is shown in Table 5.
In renal outcome, the results of comparison showed that empagliflozin (RR [95% CI]; 0.76 [0.63–0.93]), canagliflozin (RR [95% CI]; 0.81 [0.67–0.99]) and dapagliflozin (RR [95% CI]; 0.70 [0.55–0.87]) significantly reduced the morbidity of renal outcome compared to finerenone. Finerenone, empagliflozin, canagliflozin and dapagliflozin reduced renal events significantly compared to placebo. The detail is shown in Table 6.
Finerenone (RR [95% CI]; 0.72 [0.52–0.99]), empagliflozin (RR [95% CI]; 0.54 [0.33–0.88]), canagliflozin (RR [95% CI]; 0.55 [0.38–0.78]), dapagliflozin (RR [95% CI]; 0.51 [0.33–0.77]), ertugliflozin (RR [95% CI]; 0.46 [0.28–0.75]), sotagliflozin (RR [95% CI]; 0.61 [0.42–0.88]) and liraglutide (RR [95% CI]; 0.67 [0.45–0.99]) significantly reduced HHF compared to exenatide. At the same time, all 7 interventions mentioned above significantly reduced HHF compared to placebo (Table 5). Another discovery worth noting is that canagliflozin (RR [95% CI]; 0.76 [0.58–1.00]) and dapagliflozin (RR [95% CI]; 0.71 [0.50–1.00]) had a tendency to decrease HHF compared to finerenone, and finerenone was associated with a higher risk of HHF than ertugliflozin (RR [95% CI]; 1.55 [1.01–2.39]). The detail is shown in Table 5.
When it comes to ACD, finerenone was comparable to other interventions. And finerenone (RR [95% CI]; 0.90 [0.80–1.00]) tended to reduce the risk of ACD when compared with placebo, while dapagliflozin (RR [95% CI]; 0.81 [0.66–0.98]) and liraglutide (RR [95% CI]; 0.76 [0.62–0.93]) had significant effect than placebo. As for CVD, liraglutide (RR [95% CI]; 0.69 [0.52–0.90]) was better than placebo, while other interventions were not. And finerenone was also comparable to other interventions. The detail is shown in Table 7.
Conclusions from minimally contextualized framework
As for MACE and CVD, liraglutide could be considered as one of the most effective treatment currently available. Efpeglenatide, sotagliflozin, canagliflozin and finerenone could be considered as inferior to the most effective in reducing the risk of MACE. In renal outcome, dapagliflozin, empagliflozin and canagliflozin could be considered as the most effective, while finerenone could be considered as inferior to the most effective. When it comes to HHF, ertugliflozin could be considered as the most effective. Liraglutide and dapagliflozin could be considered as the most effective in reducing the incidence of ACD. As was presented in Table 8.
Sensitivity analyses
The results of sensitivity analyses are summarized in Table 9. We conducted a sensitivity analysis excluding “Cherney 2021”, as Cherney 2021 only included diabetics with severe CKD (eGFR: 15–30 ml/min/1.73 m2). In MACE, renal outcome and ACD, the results of sensitivity analyses were comparable to non-exclusion of “Cherney 2021”. Compared to sotagliflozin, liraglutide (RR [95% CI]; 0.76 [0.58–0.99]) was associated with a decreased risk of ACD. Whereas the previous results showed liraglutide had a trend towards a reduction in CVD compared to sotagliflozin.
Discussion
In the absence of RCT directly comparing to nonsteroidal and selective mineralocorticoid receptor antagonists, SGLT2i and GLP-1 RA, this network meta-analysis evaluated the relative efficacy of three drugs on cardiovascular and renal outcomes in patients with T2DM and CKD. This network meta-analysis was based on 18 large trials, which included 51,496 patients randomly assigned to finerenone, SGLT2i, GLP-1 RA or placebo. Our results revealed that finerenone can decrease the risk of MACE, renal outcome and HHF, alongside with the tendency to reduce ACD in patients with T2DM and CKD. Our study found that finerenone has the advantage reducing MACE risk just as well as SGLT2i, which was inconsistent with another network meta-analysis [65]. The cause may be that that research only included one trial correlating to finerenone (FIDELIO-DKD) and had the possibility of small-sample bias. SGLT2i was found to be comprehensive in reducing the risk of MACE, renal outcome, HHF, CVD and ACD. It outperformed finerenone in terms of reducing the risk of renal outcome.
This study revealed that GLP-1 RA decreased the risk of MACE compared with placebo, which varied with another network meta-analysis [66]. The inconsistency may be due to the exclusion of ELIXA trial in that article, as its definition of MACE included unstable angina(so why leading to the unsignificant/significant result?). In addition, GLP-1 RA did not show any significant benefit in reducing renal outcome when compared with placebo. Our study also revealed that SGLT-2i were associated with a decreased risk of renal outcome and HHF compared with finerenone and GLP-1 RA. This seemed to imply that GLP1-RA has no significant advantage over SGLT2i, but analysis between finerenone and 10 interventions included in SGLT2i and GLP-1 RA showed different results. Liraglutide, one of GLP-1 RA, was associated with a decreased risk of MACE, ACD, CVD and HHF. Amongst all 11 interventions included in this study, liraglutide was the only intervention to show efficacy in CVD compared with placebo. As shown in minimally contextualized framework, liraglutide also ranked first in MACE, ACD and CVD among all 11 interventions included in this study. This could mean that liraglutide was a more preferable choice for DM patients with CKD who have an elevated risk of cardiovascular events.
Several mechanisms have been proposed for the positive impact of finerenone. As a nonsteroidal, selective mineralocorticoid receptor antagonist, finerenone has been shown to have potent anti-inflammatory and antifibrotic effects while reducing the urinary albumin-to-creatinine ratio, which may be related to its benefits in renal outcome and HHF [67,68,69,70].
As for the morbidity, renal outcome and HHF, it was clear that SGLT-2i had more significant impact than finerenone, which might be explained by the special potency of SGLT-2i such as reducing blood glucose, reducing oxidative stress, losing weight, reducing uric acid, controlling blood pressure and improving renal ultrafiltration and hypoxia [65, 71,72,73,74,75,76,77,78,79].
Interestingly, in the three observed outcomes of ACD, HHF and CVD, GLP-1 RA did not show a significant advantage over placebo, but liraglutide, a GLP-1 RA did. In addition, liraglutide had a more outstandig effect than exenatide in the morbidity of MACE, ACD, HHF, and CVD. Based on chemical structure, GLP-1 RA could be divided into two groups: incretin-mimetics (exendin-4 analogs) and human GLP-1 analogues. Exenatide is a synthetic exendin‑4 analogue and liraglutide is an acylated analogue of GLP‑1.
The mechanism of renoprotective action of GLP-1 analogues is not entirely clear. It was believed that GLP-1 analogues are metabolized in target tissues via the common proteolytic pathway of large proteins. Their large molecular size or noncovalent attachment to albumin can prevent them from being eliminated by the kidneys. However, exendin-4 analogues are metabolized and eliminated by the kidneys. Moreover, exendin-4 analogues are resistant to inactivation of dipeptidyl peptidase-4, while GLP-1 analogues are partially metabolized to metabolites, which may be related to the better benefits in cardioprotective effects of liraglutide than exenatide [80, 81].
Major strengths of this network meta-analysis are of the follwowing: first and foremost, it was the first to investigate the effect of finerenone, SGLT-2 inhibitors and GLP-1 RA on cardiovascular and renal outcomes in patients with T2DM and CKD. Secondly, the number of included studies and sample size was large and the statistical efficiency was reliable, which provided evidence for individualized drug administration in clinical practice of patients with T2DM and CKD. Last but not least, in the Chronic Kidney Disease and Risk Management: Standards of Medical Care in Diabetes-2022 [19], ADA preferably recommended SGLT-2 inhibitors and finerenone over GLP-1 RA in vulnerable population who were at increased risk for cardiovascular events or CKD progression. They also emphasized that finerenone should only be recommended when the patient has CKD, that are at an increased risk for cardiovascular events, chronic kidney disease progression or are unable to use SGLT2i. They also suggest the use of GLP-1 RA or SGLT-2i for individuals with T2DM with or at high risk for ASCVD, and/or CKD in the Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2022 [15]. Our study supported their recommendations, with additional evidence that finerenone is comparable with SGLT2i in reducing the risk of MACE, meaning that if cardiovascular risks become prominent, then SGLT2i, finerenone and GLP-1 analogues are all suitable options. When the risk of renal events rises, the SGLT2i becomes the appropriate recommendation. The GLP-1 analogues could reduce the risk of MACE, HHF, CVD, especially ACD, suggesting that GLP-1 analogues can be an alternative option in patients with T2DM and CKD. GLP-1 RA may be suggested for cardiovascular risk reduction if such risk is a predominant problem, as they reduce risks of cardiovascular events appear to possibly slow CKD progression. While there is clear cardiovascular risk reduction associated with GLP-1 RA use in patients with T2DM and CKD, the proof of benefit on renal outcome will come with the results of the ongoing FLOW (A Research Study to See How Semaglutide Works Compared with Placebo in People With Type 2 Diabetes and Chronic Kidney Disease) trial with injectable semaglutide [82].
This study had several limitations. Firstly, we conducted this network meta-analysis on the basis of indirect comparisons. Therefore, our results require validation by head-to-head trials comparing finerenone with SGLT2i and GLP-1 RA. Secondly, partial studies included in this paper are subgroup analysis of RCTs, there is still a concern that patients with T2DM and CKD may not be completely randomized. Thirdly, there were more patients involved in SGLT2i than GLP-1 RA and finerenone. In addition, the baseline eGFR of patients in “Cherney 2021” was different from other studies. Although we did not observe high heterogeneity, these imbalances may limit the statistical capabilities of network meta-analysis. Finally, we did not pay attention to albuminuria, so we could not investigate the effects of finerenone, SGLT2i and GLP-1 RA for albuminuria in diabetics with CKD.
Conclusion
In patients with T2DM and CKD, finerenone led to a risk reduction in MACE, renal outcome and HHF, SGLT2i were associated with a decreased risk of cardiovascular and renal events. Finerenone had a tendency to decrease the risk of ACD. GLP-1 RA were associated with a decreased risk of MACE. Finerenone was comparable to SGLT2i in reducing the risk of MACE, CVD and ACD. As for renal outcome and HHF, SGLT2i had significant effect over finerenone and GLP-1 RA. Among GLP-1 RA, GLP-1 analogues showed significantly reduced cardiovascular events compared with exendin-4 analogues. Cardiovascular risks are common within diabetic patients with CKD, when such risk jeopardize the wellbeing of the patient, SGLT2i, finerenone and GLP-1 analogues are all apposite recommendations, but when the risk of renal events heightens, then SGLT2i will be the sole recommendation available.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- SGLT2i:
-
Sodium-glucose cotransporter-2 inhibitors
- GLP-1 RA:
-
Glucagonlike peptide-1 receptor agonists
- T2DM:
-
Type 2 diabetes mellitus
- CKD:
-
Chronic kidney disease
- RCTs:
-
Randomized control trials
- RR:
-
Risk ratio
- CI:
-
Confidence interval
- HHF:
-
Hospitalization for heart failure
- MACE:
-
Major adverse cardiovascular events
- ACD:
-
All-cause death
- CVD:
-
Cardiovascular death
- DM:
-
Diabetes mellitus
- ASCVD:
-
Atherosclerotic cardiovascular disease
- ESRD:
-
End-stage renal disease
- MI:
-
Myocardial infarction
- ADA:
-
American Diabetes Association
- eGFR:
-
Estimated glomerular filtration rate
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- GRADE:
-
Grading of recommendations assessment, development, and evaluation
- CrCl:
-
Creatinine clearance
- UACR:
-
Urinary albumin-to-creatinine ratio
References
International Diabetes Federation. IDF diabetes atlas. 10th ed. Brussels: International Diabetes Federation; 2021.
Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–209.
Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298(7):765–75.
Ahmad FS, Ning H, Rich JD, et al. Hypertension, obesity, diabetes, and heart failure-free survival: the Cardiovascular Disease Lifetime Risk Pooling Project. JACC Heart Fail. 2016;4(12):911–9.
Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341(15):1127–33.
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45.
Perkovic V, Jardine MJ, Neal B, CREDENCE Trial Investigators, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Agarwal R, Anker SD, Bakris G, FIDELIO-DKD and FIGARO-DKD Investigators, et al. Investigating new treatment opportunities for patients with chronic kidney disease in type 2 diabetes: the role of finerenone. Nephrol Dial Transplant. 2020. https://doi.org/10.1093/ndt/gfaa294.
Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–54.
Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022–31.
Fox CS, Matsushita K, Woodward M, Chronic Kidney Disease Prognosis Consortium, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–73.
Jiang G, Luk AOY, Tam CHT, et al. Progression of diabetic kidney disease and trajec_tory of kidney function decline in Chinese patients with type 2 diabetes. Kidney Int. 2019;95(1):178–87.
Yandrapalli S, Jolly G, Horblitt A, et al. Cardiovascular benefits and safety of non-insulin medications used in the treatment of type 2 diabetes mellitus. Postgrad Med. 2017;129(8):811–21.
Briasoulis A, Bakris GL. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep. 2013;15(3):340.
American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S125–43.
Bakris GL, Agarwal R, Anker SD, FIDELIO-DKD Investigators, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29.
Pitt B, Filippatos G, Agarwal R, FIGARO-DKD Investigators, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–63.
Sridhar VS, Liu H, Cherney DZI. Finerenone—a new frontier in renin-angiotensin-aldosterone system inhibition in diabetic kidney disease. Am J Kidney Dis. 2021;78(2):309–11.
American Diabetes Association Professional Practice Committee. Chronic kidney disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Suppl. 1):S175–84.
Moher D, Liberati A, Tetzlaf J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535.
Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Pang B, Lian FM, Zhao XY, et al. Prevention of type 2 diabetes with the traditional Chinese patent medicine: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2017;131:242–59.
Liu JP. GRADE methods in traditional medicine. Integr Med Res. 2022;11(2): 100836.
Brignardello-Petersen R, Guyatt GH, Mustafa RA, et al. GRADE guidelines 33: addressing imprecision in a network meta-analysis. J Clin Epidemiol. 2021;139:49–56.
Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349: g5630.
Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook.
Brignardello-Petersen R, Florez ID, Izcovich A, et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ. 2020;371: m3900.
Filippatos G, Anker SD, Agarwal R, FIDELIO-DKD Investigators, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143(6):540–52.
Qiu M, Zhao LM. Long-term cardiorenal efficacy of finerenone in patients with chronic kidney disease and type 2 diabetes. Ann Palliat Med. 2021;10(10):11239–41.
Bakris GL, Agarwal R, Chan JC, Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884–94.
Zinman B, Wanner C, Lachin JM, EMPA-REG OUTCOME Investigators, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME™). Cardiovasc Diabetol. 2014;13:102.
Wanner C, Inzucchi SE, Lachin JM, EMPA-REG OUTCOME Investigators, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34.
Wanner C, Lachin JM, Inzucchi SE, EMPA-REG OUTCOME Investigators, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137(2):119–29.
Wanner C, Inzucchi SE, Zinman B, EMPA-REG OUTCOME Investigators, et al. Consistent effects of empagliflozin on cardiovascular and kidney outcomes irrespective of diabetic kidney disease categories: insights from the EMPA-REG OUTCOME trial. Diabetes Obes Metab. 2020;22(12):2335–47.
Neal B, Perkovic V, Mahaffey KW, CANVAS Program Collaborative Group, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138(15):1537–50.
Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704.
Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140(9):739–50.
Ye N, Jardine MJ, Oshima M, et al. Blood pressure effects of canagliflozin and clinical outcomes in type 2 diabetes and chronic kidney disease: insights from the CREDENCE trial. Circulation. 2021;143(18):1735–49.
Bakris G, Oshima M, Mahaffey KW, et al. Effects of canagliflozin in patients with baseline eGFR < 30 ml/min per 1.73 m2: subgroup analysis of the randomized CREDENCE trial. Clin J Am Soc Nephrol. 2020;15(12):1705–14.
Jardine MJ, Zhou Z, Mahaffey KW, CREDENCE Study Investigators, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020;31(5):1128–39.
Wiviott SD, Raz I, Bonaca MP, DECLARE–TIMI 58 Investigators, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, DAPA-CKD Trial Committees and Investigators, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
Persson F, Rossing P, Vart P, DAPA-CKD Trial Committees and Investigators, et al. Efficacy and safety of dapagliflozin by baseline glycemic status: a prespecified analysis from the DAPA-CKD trial. Diabetes Care. 2021;44(8):1894–7.
Heerspink HJL, Sjöström CD, Jongs N, DAPA-CKD Trial Committees and Investigators, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021;42(13):1216–27.
Wheeler DC, Stefánsson BV, Jongs N, DAPA-CKD Trial Committees and Investigators, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22–31.
Cannon CP, Pratley R, Dagogo-Jack S, VERTIS CV Investigators, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35.
Cherney DZI, McGuire DK, Charbonnel B, VERTIS CV Investigators, et al. Gradient of risk and associations with cardiovascular efficacy of ertugliflozin by measures of kidney function: observations from VERTIS CV. Circulation. 2021;143(6):602–5.
Dagogo-Jack S, Pratley RE, Cherney DZI, et al. Glycemic efficacy and safety of the SGLT2 inhibitor ertugliflozin in patients with type 2 diabetes and stage 3 chronic kidney disease: an analysis from the VERTIS CV randomized trial. BMJ Open Diabetes Res Care. 2021;9(1): e002484.
Bhatt DL, Szarek M, Pitt B, SCORED Investigators, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39.
Cherney DZI, Ferrannini E, Umpierrez GE, et al. Efficacy and safety of sotagliflozin in patients with type 2 diabetes and severe renal impairment. Diabetes Obes Metab. 2021;23(12):2632–42.
Gerstein HC, Colhoun HM, Dagenais GR, REWIND Investigators, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–8.
Gerstein HC, Colhoun HM, Dagenais GR, REWIND Investigators, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30.
Hernandez AF, Green JB, Janmohamed S, Harmony Outcomes Committees and Investigators, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29.
Holman RR, Bethel MA, Mentz RJ, EXSCEL Study Group, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39.
Bethel MA, Mentz RJ, Merrill P, et al. Microvascular and cardiovascular outcomes according to renal function in patients treated with once-weekly exenatide: insights from the EXSCEL trial. Diabetes Care. 2020;43(2):446–52.
Husain M, Birkenfeld AL, Donsmark M, PIONEER 6 Investigators, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51.
Marso SP, Bain SC, Consoli A, SUSTAIN-6 Investigators, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Mann JFE, Ørsted DD, Brown-Frandsen K, LEADER Steering Committee and Investigators, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–48.
Marso SP, Daniels GH, Brown-Frandsen K, LEADER Steering Committee, LEADER Trial Investigators, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Mann JFE, Fonseca V, Mosenzon O, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. 2018;138(25):2908–18.
Gerstein HC, Sattar N, Rosenstock J, AMPLITUDE-O Trial Investigators, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907.
Zhao LM, Zhan ZL, Ning J, et al. Network meta-analysis on the effects of SGLT2 inhibitors versus finerenone on cardiorenal outcomes in patients with type 2 diabetes and chronic kidney disease. Front Pharmacol. 2022;12: 751496.
Yamada T, Wakabayashi M, Bhalla A, et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2021;20(1):14.
Grune J, Beyhoff N, Smeir E, et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone’s antifibrotic activity. Hypertension. 2018;71(4):599–608.
Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78.
Kolkhof P, Jaisser F, Kim SY, et al. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol. 2017;243:271–305.
Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152–61. https://doi.org/10.1093/eurheartj/ehaa736.
Ren DY, Zhang Y. Cardiovascular benefit of SGLT2 inhibitors in the therapeutics of diabetes mellitus: a close look beyond the horizon. Curr Drug Targets. 2018;19(9):1051–7.
Chaudhuri A, Ghanim H, Arora P. Improving the residual risk of renal and cardiovascular outcomes in diabetic kidney disease: a review of pathophysiology, mechanisms, and evidence from recent trials. Diabetes Obes Metab. 2022;24(3):365–76.
Wang J, Xiang H, Lu Y, et al. New progress in drugs treatment of diabetic kidney disease. Biomed Pharmacother. 2021;141: 111918.
Imprialos KP, Sarafdis PA, Karagiannis AI. Sodium-glucose cotransporter-2 inhibitors and blood pressure decrease: a valuable efect of a novel antidiabetic class? J Hypertens. 2015;33(11):2185–97.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Ojima A, Matsui T, Nishino Y, et al. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47(9):686–92.
Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–97.
Kitada M, Hirai T, Koya D. Significance of SGLT2 inhibitors: lessons from renal clinical outcomes in patients with type 2 diabetes and basic researches. Diabetol Int. 2020;11(3):245–51.
Gupta V. Glucagon-like peptide-1 analogues: an overview. Indian J Endo-crinol Metab. 2013;17(3):413–21.
Gorriz JL, Soler MJ, Navarro-Gonzalez JF, et al. GLP-1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020;9(4):947.
Novo Nordisk A/S. A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). In: ClinicalTrials.gov. Bethesda, MD, National Library of Medicine. 2019. https://clinicaltrials.gov/ct2/show/NCT03819153. Accessed 13 Oct 2021.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No.: 82074354) and the Natural Science Foundation of Beijing Municipality (No.: 7222278).
Funding
The author Jinxi Zhao received a fund from the National Natural Science Foundation of China and the Natural Science Foundation of Beijing Municipality. The funding agency had no role in designing the study, conducting the analysis, interpreting the data or writing the manuscript.
Author information
Authors and Affiliations
Contributions
YZ, LJ and TW designed and monitored the whole analysis. JW and WH contributed to study selection. YZ, LJ and TW contributed to data extraction. CC provided the methodological support. YX, YZ, QF and XF contributed to the data analysis and paper writing. SW and JZ provided the project fund. JZ and SW were responsible for the data review. All authors provided critical review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
RoB-2 evaluation.
Additional file 2.
Publication bias.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y., Jiang, L., Wang, J. et al. Network meta-analysis on the effects of finerenone versus SGLT2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Cardiovasc Diabetol 21, 232 (2022). https://doi.org/10.1186/s12933-022-01676-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01676-5