Abstract
Background
Evidence of adverse clinical outcomes for non-vitamin K antagonist oral anticoagulant (NOACs) and warfarin in patients with atrial fibrillation (AF) and diabetes mellitus are limited. We investigated the effectiveness, safety, and major adverse limb events for NOACs versus warfarin among diabetic AF patients.
Methods
In this nationwide retrospective cohort study collected from Taiwan National Health Insurance Research Database, we identified a total of 20,967 and 5812 consecutive AF patients with diabetes taking NOACs and warfarin from June 1, 2012, to December 31, 2017, respectively. We used propensity-score stabilized weighting to balance covariates across study groups.
Results
NOAC was associated with a lower risk of major adverse cardiovascular events (MACE) (adjusted hazard ratio (aHR):0.88; [95% confidential interval (CI) 0.78–0.99]; P = 0.0283), major adverse limb events (MALE) (aHR:0.72;[95% CI 0.57–0.92]; P = 0.0083), and major bleeding (aHR:0.67;[95% CI 0.59–0.76]; P < 0.0001) compared to warfarin. NOACs decreased MACE in patients of ≥ 75 but not in those aged < 75 years (P interaction = 0.01), and in patients with ischemic heart disease (IHD) compared to those without IHD (P interaction < 0.01). For major adverse limb events, the advantage of risk reduction for NOAC over warfarin persisted in high risk subgroups including age ≥ 75 years, chronic kidney disease, IHD, peripheral artery disease, or use of concomitant antiplatelet drugs.
Conclusion
Among diabetic AF patients, NOACs were associated with a lower risk of thromboembolism, major bleeding, and major adverse limb events than warfarin. Thromboprophylaxis with NOACs should be considered in the diabetic AF population with a high atherosclerotic burden.
Similar content being viewed by others
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia globally, and is associated with a five-fold increased risk of stroke compared to patients without AF [1]. Diabetes mellitus (DM), insulin resistance, or obesity is an important risk factor of ischemic stroke and the development of new onset AF [2,3,4]. Around 40% AF patients have comorbid DM, and both are associated with a higher risk of ischemic stroke, acute coronary syndrome, and cardiovascular events [5].
The efficacy and safety of NAOCs have been studied in AF patients in association with several difficult treatment scenarios including the elderly, chronic kidney disease, valvular heart disease, or history of intracranial hemorrhage [6,7,8]. Current international guidelines recommend the use of non-vitamin K antagonist oral anticoagulants (NOACs) as effective, safer and more convenient alternatives to warfarin among patients with non-valvular AF, including those with DM [9, 10]. However, in post hoc analyses of the landmark NOACs trials, the safety profile regarding to the risk of major bleeding for dabigatran 110 mg or apixaban 5/2.5 mg over warfarin was diminished in AF patients comorbid with DM [11, 12]. Specifically, there were no clear benefits of NOACs over warfarin with regard to the risk of major bleeding in diabetic AF patients. In recent non-AF studies [13, 14], the value of NOACs in reducing major adverse limb events in patients at high vascular risk is also apparent.
In this study, we investigated the effectiveness, safety, and major adverse limb events for NOACs versus warfarin among diabetic AF patients, using a large population-based nationwide cohort study.
Methods
We performed a retrospective nationwide cohort study using the Taiwan National Health Insurance Research Database (NHIRD), which contained health care information of more than 23 million Taiwan residents with a > 99% coverage rate of the entire population [15]. The NHIRD database contains each patient’s demographic data, outpatient clinic visits, hospitalizations, interventions and examinations, drug prescriptions, records of outpatient visits, and diagnosis of diseases. By using a consistent encrypting procedure, the original identification number of each patient in the NHIRD is encrypted and de-identified to protect patient privacy; therefore, informed consent was waived in the present study. This study was approved by the Institutional Review Board of the Chang Gung Medical Foundation (104-8079B and 201801427B0).
Study design
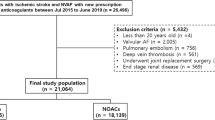
The study identified a total of 296,162 patients diagnosed with AF using (International Classification of Diseases (the ninth revision) Clinical Modification (ICD-9-CM) codes (427.31) between January 1, 2010 and December 31, 2015 or using ICD-10-CM codes (I48)) between January 1, 2016 and December 31, 2018. A total of 92,272 AF patients treated with oral anticoagulants (OACs) after June 1, 2012 were identified. Patients who took more than one NOAC type during their treatment course were excluded from the present study. In order to establish a non-valvular AF cohort treated with OACs for stroke prevention, those patients with a diagnoses indicating deep vein thrombosis or pulmonary embolism, mitral stenosis, post valvular surgery, or joint replacement therapy within 6 months before the index date were excluded. Patients with end-stage renal disease were also excluded because NOACs are absolutely contraindicated in dialyzed patients in Taiwan. Finally, we included 85,641 non-valvular AF patients treated with OACs from June 1, 2012 to December 31, 2017. After excluding 58,864 non-valvular AF patients without a diagnosis of DM, a total of 20,967 and 5812 non-valvular AF patients comorbid with DM treated with NOACs and warfarin, respectively, were enrolled. The index date was defined as the first date of prescription for NOACs or warfarin. For those NOAC users with previous warfarin-exposure before (n = 6399), the index date was defined as the first date of prescription for their NOAC. The follow-up period was defined as the duration from the index date until the first occurrence of any study outcome independently, or until the end date of the study period (December 31, 2017), whichever came first. A flowchart of the study enrollment is shown in Fig. 1.
Enrollment of patients with concomitant non-vlavular atrial fibrillation (AF) and diabetes mellitus (DM). From June 1, 2012, to December 31, 2017, a total of 3249 (16%), 6531 (31%), 1389 (6%), and 9798 (47%) non-valvular AF patients comorbid with DM taking apixaban, dabigatran, edoxaban, and rivaroxaban and 5812 consecutive patients taking warfarin were enrolled in the present study. Abbreviations: AF atrial fibrillation, DM diabetes mellitus, NOAC non-vitamin K antagonist oral anticoagulant, OAC oral anticoagulant
Study outcomes
We reported several outcomes in the present study: (i) effectiveness outcomes: ischemic stroke/systemic embolism (IS/SE), acute myocardial infarction (AMI), and major adverse cardiac events (MACE) (defined as IS/SE or AMI); (ii) major lower limb outcomes: acute or chronic limb ischemia requiring revascularization procedures, lower limb amputation, and major adverse limb events (MALE) (defined as lower limb revascularization or amputation); (iii) safety outcomes: intracranial hemorrhage (ICH), major gastrointestinal bleeding, and all major bleeding events. All major bleeding events are defined as the summation of hospitalized events of ICH, major gastrointestinal bleeding, and other sites of critical bleeding. All study outcomes should be the primary discharge diagnosis to avoid misclassification. The diagnosis codes of NHIRD were shifted from ICD-9-CM to ICD-10-CM after January 1, 2016. The ICD-9-M and ICD-10-CM codes used to identify the baseline covariates and the study outcomes are summarized in Additional file 1: Table S1. The ICD-9-CM and ICD-10-CM codes used to identify the MALE outcomes are summarized in Additional file 1: Table S2 [16]. Patients may have had the same outcomes more than once during the study duration, but we only considered the same study outcome that occurred first.
Covariates
Baseline covariates were obtained from any claim records with the diagnoses, medications, or procedures codes prior to the index date. A history of any prescription medicine was confined to medications taken at least once within 3 months before the index date. The definition of concomitant use of antiplatelet agent (APT) including aspirin, clopidogrel, ticlopidine, or ticagrelor was defined as APT duration > 3 months after drug index date. Bleeding history was confined to events within 6 months before the index date. The CHA2DS2-VASc score (congestive heart failure, hypertension, age 75 years or older for 2 points, DM, previous stroke or transient ischemic attack for 2 points, vascular disease, age 65 to 74 years, and female gender) was computed to predict the risk of thromboembolism in AF patients, [17]. The HAS-BLED score (hypertension, abnormal renal or liver function, stroke, bleeding history, labile INR, age 65 years or older, and APT or alcohol use) was computed to predict the risk of bleeding in AF patients treated with OACs [18].
Statistical analysis
We used the method of propensity score stabilized weights (PSSWs) to balance the differences in baseline characteristics across the study groups [19]. The advantage of PSSWs is to provide an appropriate estimate of the main effect variance and to maintain the designated type I error by preserving the sample size of the original data. The PSSWs among study groups were obtained using the generalized boosted model (GBM), which can automatically determine the best functions of covariates, including interactions or polynomial terms, to obtain the optimal balance among study groups [20]. The advantage of PSSWs obtained by GBM is less affected by large weights [20]. All covariates in Table 1 except for CHA2DS2-VASc and HAS-BLED scores were included in the GBM, because CHA2DS2-VASc and HAS-BLED scores were already a combination of other covariates. The balance of potential confounders at baseline (index date) between each study group was assessed using the absolute standardized mean difference (ASMD) rather than statistical testing, because balance is a property of the sample and not of an underlying population. The value of ASMD ≤ 0.1 indicated an insignificant difference in potential confounders between the two study groups [21]. The incidence rates were computed using the total number of study outcomes during the follow-up period divided by person-years at risk. The risk of study outcomes for NOACs versus warfarin (reference) was obtained using survival analysis (Kaplan–Meier method and log-rank test for univariate analysis and Cox proportional hazards model for multivariate analysis). Subgroup analysis was performed to test whether the NOAC group continued to have a lower risk of clinical outcomes than the warfarin group in specific subgroup. It is noted that the PSSWs were re-estimated for each subgroup analysis so that the NOAC and warfarin subgroup maintained a balance of varied covariates across groups. Statistical significance was defined as a P value < 0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
We identified a total of 20,967 and 5812 diabetic non-valvular AF patients treated with NOACs and warfarin, respectively. Among the NOAC group, there were 16%, 31%, 6%, and 47% patients taking apixaban, dabigatran, edoxaban, and rivaroxaban, respectively (Fig. 1). Among the NOAC group, there were 31% (n = 6399) patients who were warfarin-experienced before starting their NOAC (which was apixaban in 25% (n = 824), dabigatran in 34% (n = 2233), edoxaban in 23% (n = 325), and rivaroxaban in 31% (n = 3017)). Before PSSW, the NOAC group was older, and had a higher prevalence of hypertension, dyslipidemia, and stroke history than the warfarin group (ASMD > 0.1) (Table 1). The NOAC group also had a higher CHA2DS2-VASc and HAS-BLED score than the warfarin group before PSSW. After PSSW, both study groups were well-balanced in most characteristics (all ASMD < 0.1) (Table 2).
For the effectiveness outcome, the DOAC group had a lower cumulative risk of MACE when compared to the warfarin group after PSSW. For the major adverse limb events, DOAC was associated with a lower cumulative risk of lower limb amputation and MALE than warfarin. For the safety outcomes, DOAC use was associated with a lower cumulative risk of ICH, major gastrointestinal bleeding, and all major bleeding than warfarin (Fig. 2). Before PSSW, NOACs were associated with a lower risk of all study outcomes than warfarin (Additional file 2: Figure S1).
Cumulative incidence curves of outcomes for diabetic AF patients taking oral anticoagulants after propensity score stabilized weighting (PSSW). Cumulative incidence curves of effectiveness outcomes including ischemic stroke/systemic embolism (IS/SE), acute myocardial infarction (AMI), and major adverse cardiovascular events (MACE) a, major adverse limb events including lower extremity revascualization procedure, lower limb amputation, and major adverse limb events (MALE) b, and safety outcomes including intracranaial hemorrhage (ICH), major gastrointestinal bleeding, and all major bleeding c for AF patients with concomiant DM taking oral anticoagulants after PSSW are presented. NOAC was associated with a lower risk of MACE, MALE, and all major bleeding events than warfarin among AF patients with concomitant DM. Abbreviations: AF atrial fibrillation, AMI acute myocardial infarction, DM diabetes mellitus, ICH intracranial hemorrhage, IS/SE ischemic stroke/systemic embolism, MACE major adverse cardiovascular events, MALE major adverse limb events, NOAC non-vitamin K antagonist oral anticoagulants, PSSW propensity score stabilized weighting
For the effectiveness outcome, the NOAC group had a lower risk of MACE (hazard ratio (HR): 0.88; 95% confidential interval (CI) [0.78–0.99]; P = 0.0283) when compared to the warfarin group after PSSW. For the major lower limb outcomes, DOAC was associated with a lower risk of lower limb amputation (HR: 0.48; 95% CI [0.33–0.72]; P = 0.0003) and MALE (HR: 0.72; 95% CI [0.57–0.92]; P = 0.0083) than warfarin. For the safety outcomes, NOAC was associated with a lower risk of ICH (HR: 0.44; 95% CI [0.35–0.55]; P < 0.0001), major gastrointestinal bleeding (HR: 0.81; 95% CI [0.69–0.96]; P = 0.0123), and all major bleeding (HR: 0.67; 95% CI [0.59–0.76]; P < 0.0001) than warfarin (Fig. 3).
Forest plot of hazard ratio (HR) of effectiveness, major lower limb outcomes, and safety outcomes for NOACs vs. warfarin among non-valvular AF patients comorbid with DM after PSSW. NOAC was associated with a lower risk of MACE, major adverse limb events (MALE), and major bleeding than warfarin among non-valvular AF patients with concomitant DM. Abbreviations: AF atrial fibrillation, AMI acute myocardial infarction, CI confidential interval, GI gastrointestinal, HR hazard ratio, ICH intracranial hemorrhage, IS/SE ischemic stroke/systemic embolism, MACE major adverse cardiovascular events, MALE major adverse limb events, NOAC non-vitamin K antagonist oral anticoagulants, PSSW propensity score stabilized weighting
Sensitivity test
Sensitivity analyses were performed by using a multivariate Cox proportional-hazards model, rather than the PSSW, to test if the results were still consistent with the main analysis by using PSSW. The model was adjusted for all baseline characteristics listed in Table 1 except for CHA2DS2-VASc and HAS-BLED scores. Consistent with the main analysis by using PSSW, the use of NOAC was associated with a lower risk of MACE (HR: 0.88; 95% CI [0.78–0.99]; P = 0.0287), MALE (HR: 0.73; 95% CI [0.58–0.92]; P = 0.0099), and major bleeding (HR: 0.70; 95% CI [0.62–0.80]; P < 0.0001) compared to warfarin, after multivariate adjustment (Additional file 3: Figure S2).
Subgroup analysis of different NOACs versus warfarin
Subgroup analysis was performed to determine whether different NOACs were superior to warfarin regarding to the effectiveness, major adverse limb events, and safety among subgroups. There were 66%, 89%, 68%, and 95% patients taking low-dose apixaban (2.5 mg twice daily), dabigatran (110 mg twice daily), edoxaban (30 mg daily), and rivaroxaban (15/10 mg daily) among the NOAC group, respectively. In general, the advantage of effectiveness, major limb outcome, and safety for NOAC over warfarin persisted in four NOAC subgroup (P interaction all > 0.05) (Fig. 4).
Forest plot of HR of effectiveness, major lower limb outcomes, and safety outcomes for each NOAC vs. warfarin among non-valvular AF patients with concomitant DM taking oral anticoagulants after PSSW. In general, the advantage of effectiveness, major adverse limb outcome, and safety for NOAC over warfarin persisted in four NOACs (P interaction all > 0.05). The abbreviations as in Fig. 3
Subgroup analysis
In general, the subgroup analysis showed consistent results for MACE, MALE, and all major bleeding for NOACs versus warfarin among those patients with ≥ 75 years of age, the presence of chronic kidney disease (CKD), ischemic heart disease (IHD), peripheral artery disease (PAD), and use of concomitant APT consistent with the main analysis (Fig. 5). NOACs decreased the risk of MACE in patients aged ≥ 75 years of age but not in patients of < 75 years of age (P interaction = 0.01) and in patients with concomitant IHD than in those without concomitant IHD (P interaction < 0.01). For the subgroup analysis of patients taking concomitant APT, there was a lower risk of major bleeding for NOAC versus warfarin especially in the APT (-) subgroup (P interaction = 0.02).
Subgroup analysis of forest plot of HR for NOAC vs. warfarin among non-valvular AF patients with concomitant DM after PSSW. In general, the subgroup analysis showed consistent results for MACE, MALE, and major bleeding for NOACs vs. warfarin among those patients with ≥ 75 years of age, the presence of chronic kidney disease (CKD), ischemic heart disease (IHD), peripheral artery disease (PAD), and use of concomitant antiplatelet agent (APT) as the main analysis. Furthermore, NOAC reduced MACE more in diabetic AF patients with a high atherosclerotic burden including elderly, the presence of IHD or PAD. Abbreviations: APT antiplatelet agent, CKD chronic kidney disease, IHD ischemic heart disease, PAD peripheral artery disease. Other abbreviations as in Fig. 3
Discussion
To our best knowledge, this is the largest population-based study to investigate the effectiveness, safety, and major limb outcomes for the four NOACs vs. warfarin in Asian population comorbid with AF and DM. Our results indicated that NOACs were associated with a lower risk of MACE, MALE, and all major bleeding when compared to warfarin among AF patients comorbid with DM. Second, the advantage of effectiveness, major limb outcome, and safety for NOAC over warfarin persisted in four NOAC subgroups (P interaction all > 0.05) and in high risk subgroups. Third, NOAC reduced MACE more in diabetic AF patients with a high atherosclerotic burden including the elderly, and the presence of IHD or PAD.
Comparisons of four NOACs vs. warfarin in diabetic AF population
The meta-analysis of the four landmark NOAC trials indicated that NOACs significantly reduced the composite efficacy endpoint when compared to warfarin both in non-valvular AF patients with diabetes and in those without diabetes, with no significant interaction by diabetes status and treatment [22,23,24]. In a post hoc analysis of ARISTOTLE trial, a significant interaction was noted between diabetes status and treatment regarding the risk of major bleeding (P interaction = 0.0034), suggesting that apixaban reduces more major bleeding than warfarin only among AF patients without diabetes [12]. A post hoc analysis of the RE-LY trial showed a comparable risk of major bleeding in AF patients with diabetes treated with dabigatran 110 mg twice daily vs. warfarin (HR: 0.91; 95% CI [0.70–1.19]), which was in contrast to a significantly lower risk of major bleeding (HR: 0.76; 95% CI [0.64–0.90]) in non-diabetic patients treated with dabigatran 110 mg twice daily vs. warfarin [11]. In the post hoc analysis of ROCKET-AF study, rivaroxaban showed a comparable risk of major bleeding to warfarin either in the diabetic or non-diabetic subgroup, and there was no significant interaction between diabetic status and the risk of bleeding [25]. Finally, in the post hoc analysis of ENGAGE-AF TIMI 48 trial, edoxaban had a significantly lower risk of major bleeding than warfarin both in the diabetic (HR: 0.78; 95% CI [0.63–0.95]) and non-diabetic subgroups (HR: 0.81; 95% CI [0.69–0.95]) (P interaction > 0.10) [23, 24]. In summary, the advantage of NOACs over warfarin in efficacy generally persisted in diabetic subgroup treated with four NAOCs, whereas the advantage of safety profiles regarding to the risk of major bleeding for NOACs over warfarin had some conflicting results in diabetic AF population treated with NOACs, especially in case of apixaban and dabigatran 110 mg.
The present study indicated that NOACs was associated with a significantly lower MACE than warfarin in those diabetic AF population with a high atherosclerotic burden like the presence of concomitant IHD or PAD (Fig. 4). For AF patient comorbid with IHD or PAD, guidelines recommend the use of oral anticoagulant (OAC) rather than APT [9, 10]. However, there are no data or guideline recommendations specifically focused on the optimal treatment for diabetic AF patients with concomitant IHD or PAD [10, 26, 27]. Previous studies have indicated that warfarin may increase vascular calcification and osteoporotic bone fracture via inhibition of the activation of matrix and bone G1a protein, and may increase coronary or peripheral vascular calcification, thus potentially influencing symptoms and outcomes in patients with IHD or PAD [28,29,30,31,32,33]. Furthermore, patients with IHD or PAD have a higher risk of bleeding events compared to those without IHD or PAD, and the bleeding events may further increase the risk of ischemic events in the IHD or PAD, for example, discontinuation of OAC due to bleeding may cause consequent ischemic event like AMI or critical limb ischemia [34]. Our present study demonstrates the benefit of NOACs over warfarin regarding to the effectiveness and safety outcomes even in a very high risk patient population comorbid with AF, diabetes, and IHD/PAD.
Major limb outcomes for NOACs vs. warfarin in diabetic AF population
Data regarding to the major adverse limb events for AF patients treated with NOAC vs. warfarin are limited. We are only aware of one retrospective study investigating the major limb outcomes for diabetic AF patients treated with NOAC vs. warfarin [35]. Baker et al. studied 10,700 and 13,946 diabetic AF patients treated with rivaroxaban and warfarin, respectively, by using a claims database in USA, whereby rivaroxaban was associated with a 25% reduced risk of MACE and a 63% reduced risk of MALE compared to warfarin, with no difference in major bleeding [35]. Our present study shows that NOACs, with nearly 50% of whom treated with rivaroxaban, were also associated with a significantly lower risk of MALE than warfarin in diabetic AF patients. Although there is no difference for the advantage on MALE for different NOACs over warfarin (P interaction = 0.81 within four NOACs), the other three NOACs except for rivaroxaban showed non-significantly lower risk of MALE than warfarin among the diabetic patients, possibly due to a smaller sample size of other three NOACs when compared to rivaroxaban (Fig. 3).
Recently, the COMPASS trial showed a strategy of combined therapy with aspirin and rivaroxaban (2.5 mg twice per day) or rivaroxaban alone (5 mg twice per day) was associated with a significantly lower risk of major adverse limb events than aspirin alone in ~ 27,000 patients with stable atherosclerotic vascular disease, nearly 45% of whom had comorbid diabetes [14]. Although the COMPASS trial differ from our present study in that it did not enroll AF patients, used a lower dose of rivaroxaban, and used aspirin but not warfarin as a comparator, the COMPASS trial firstly demonstrated that anticoagulant regimen with rivaroxaban indeed provide an extra benefit in improving MALE outcome in patients with a high atherosclerotic burden when compared to the current standard treatment. Until now, there are no large randomized controlled studies evaluating the major limb outcome for AF patients with a high atherosclerotic burden or AF treated with other three NOACs.
Limitations
The present study has several limitations. First, our study is a retrospective cohort study. Although the use of inverse propensity score weighting with adjustment of several variables allowed the balance of baseline comorbidities among the study groups, selection bias and residual confounding by unobserved or unmeasured variables could not be excluded in the present study. Second, misclassification and miscoding of the baseline comorbidities and study outcomes is a potential limitation. Third, laboratory data such as international normalized ratio (INR) for patients treated with warfarin were not obtained in the NHIRD database; indeed, Asian populations treated with warfarin generally have a much lower time in therapeutic range (TTR) for an INR target 2.0 to 3.0 compared to other regions of the world [36, 37]. Hence, the superiority of NOACs over warfarin may be partly due to low TTR for those patients treated with warfarin in the present study [38]. Fourth, four NOACs and warfarin prescribed had varying rates of renal excretion and, thus, decisions regarding the use of a specific NOAC or warfarin would have been guided by the renal function of each patient (e.g., a perceived risk may result in conscious avoidance in use of NOACs in specific patient populations). Renal function lab data for each patient are lacking in the NHIRD, and ICD coding indicating an impaired renal function was dependent on each physician’s choice. Therefore, residual confounding factors including the renal function across the exposure groups could not be excluded. In addition, there was a high prevalence of low-dose NOAC prescriptions in the present AF cohort. The lack of both body weight and renal function data makes us difficult to determine if those AF patients treated with low-dose NOACs were correctly prescribed with an “adjusted” or “off-label” low-dose NOACs. Fifth, glycated hemoglobin is directly associated with risk of stroke in diabetic patients with AF [39]; however, we are unable to determine the quality of glycemic control for each diabetic AF patient due to lack of glycated hemoglobin data. Finally, for the issue of MALE outcome, we were unable to differentiate whether the outcome of lower limb amputation or revascularization was due to a pure cardio-embolic event caused by AF itself or an atherothrombotic event caused by DM related atherosclerosis and PAD. Diabetic patients have a higher prevalence of PAD than other populations, and the underlying pathology for PAD related critical limb ischemia is mainly mediated by atherothrombotic events [40]. Nevertheless, the COMPASS trial has demonstrated that use of a NOAC was beneficial in reducing atherothrombotic events in PAD patients, whereby nearly 45% had comorbid diabetes [14].
Conclusions
Among diabetic AF patients, NOACs were associated with a lower risk of thromboembolism, major bleeding, and major adverse limb events than warfarin. Thromboprophylaxis with NOACs should be considered in the diabetic AF population with a high atherosclerotic burden.
Availability of data and materials
The datasets used in this study were only available in the Applied Health Research Data Integration Service from National Health Insurance Administration, Taiwan. The SAS programs (codes) involved for this study are available from the corresponding author on reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- AMI:
-
Acute myocardial infarction
- APT:
-
Antiplatelet agent
- CHA2DS2-VASc:
-
Congestive heart failure, hypertension, age 75 years or older, diabetes mellitus, previous stroke/transient ischemic attack, vascular disease, age 65 to 74 years, female
- CKD:
-
Chronic kidney disease
- DM:
-
Diabetes mellitus
- HAS-BLED:
-
Hypertension, abnormal renal or liver function, stroke, bleeding history, labile INR, age 65 years or older, and antiplatelet drug or alcohol use
- ICH:
-
Intracranial hemorrhage
- IHD:
-
Ischemic heart disease
- IS/SE:
-
Ischemic stroke/systemic embolism
- MACE:
-
Major adverse cardiovascular event
- MALE:
-
Major adverse limb event
- NOAC:
-
Non-vitamin K antagonist oral anticoagulants
- PAD:
-
Peripheral artery disease
- PSSW:
-
Propensity score stabilized weighting
References
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962.
Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383(9933):1973–80.
Chan YH, Chang GJ, Lai YJ, Chen WJ, Chang SH, Hung LM, Kuo CT, Yeh YH. Atrial fibrillation and its arrhythmogenesis associated with insulin resistance. Cardiovasc Diabetol. 2019;18(1):125.
Kim YG, Han KD, Choi JI, Boo KY, Kim DY, Oh SK, Lee KN, Shim J, Kim JS, Kim YH. The impact of body weight and diabetes on new-onset atrial fibrillation: a nationwide population based study. Cardiovasc Diabetol. 2019;18(1):128.
Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500–10.
Chang TY, Liao JN, Chao TF, Vicera JJ, Lin CY, Tuan TC, Lin YJ, Chang SL, Lo LW, Hu YF, et al. Oral anticoagulant use for stroke prevention in atrial fibrillation patients with difficult scenarios. Int J Cardiol Heart Vasc. 2018;20:56–62.
de Souza Lima Bitar Y, Neto MG, Filho JAL, Pereira LV, Travassos KSO, Akrami KM, Roever L, Duraes AR. Comparison of the new oral anticoagulants and warfarin in patients with atrial fibrillation and valvular heart disease: systematic review and meta-analysis. Drugs R D. 2019;19(2):117–26.
Nielsen PB, Skjoth F, Sogaard M, Kjaeldgaard JN, Lip GYH, Larsen TB. Non-vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients with intracerebral hemorrhage. Stroke. 2019;50(4):939–46.
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93.
Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121–201.
Brambatti M, Darius H, Oldgren J, Clemens A, Noack HH, Brueckmann M, Yusuf S, Wallentin L, Ezekowitz MD, Connolly SJ, et al. Comparison of dabigatran versus warfarin in diabetic patients with atrial fibrillation: results from the RE-LY trial. Int J Cardiol. 2015;196:127–31.
Ezekowitz JA, Lewis BS, Lopes RD, Wojdyla DM, McMurray JJ, Hanna M, Atar D, Cecilia Bahit M, Keltai M, Lopez-Sendon JL, et al. Clinical outcomes of patients with diabetes and atrial fibrillation treated with apixaban: results from the ARISTOTLE trial. Eur Heart J Cardiovasc Pharm. 2015;1(2):86–94.
Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without Aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319–30.
Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219–29.
National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. National Health Insurance Annual Statistical Report 2018. https://www.nhi.gov.tw/English/Content_List.aspx?n=AB41B66610EAC01A&topn=616B97F8DF2C3614. Accessed 2 Dec 2019
Lee HF, See LC, Li PR, Liu JR, Chao TF, Chang SH, Wu LS, Yeh YH, Kuo CT, Chan YH, et al. Non-vitamin K antagonist oral anticoagulants and warfarin in atrial fibrillation patients with concomitant peripheral artery disease. Eur Heart J Cardiovasc Pharm. 2019. https://doi.org/10.1093/ehjcvp/pvz072.
Pamukcu B, Lip GY, Lane DA. Simplifying stroke risk stratification in atrial fibrillation patients: implications of the CHA2DS2-VASc risk stratification scores. Age Ageing. 2010;39(5):533–5.
Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–100.
Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273–7.
McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–414.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
Plitt A, McGuire DK, Giugliano RP. Atrial fibrillation, type 2 diabetes, and non-vitamin K antagonist oral anticoagulants: a review. JAMA Cardiol. 2017;2(4):442–8.
Itzhaki Ben Zadok O, Eisen A. Use of non-vitamin K oral anticoagulants in people with atrial fibrillation and diabetes mellitus. Diabet Med. 2018;35(5):548–56.
Patti G, Di Gioia G, Cavallari I, Nenna A. Safety and efficacy of nonvitamin K antagonist oral anticoagulants versus warfarin in diabetic patients with atrial fibrillation: a study-level meta-analysis of phase III randomized trials. Diabetes Metab Res Rev. 2017;33(3):e2876.
Bansilal S, Bloomgarden Z, Halperin JL, Hellkamp AS, Lokhnygina Y, Patel MR, Becker RC, Breithardt G, Hacke W, Hankey GJ, et al. Efficacy and safety of rivaroxaban in patients with diabetes and nonvalvular atrial fibrillation: the Rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF Trial). Am Heart J. 2015;170(4):675–82.
Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763–816.
Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C, Healey JS, Bell A, Cairns J, Connolly S, et al. 2018 focused update of the canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34(11):1371–92.
Han KH, O’Neill WC. Increased peripheral arterial calcification in patients receiving Warfarin. J Am Heart Assoc. 2016;5(1):e002665.
Lomashvili KA, Wang X, Wallin R, O’Neill WC. Matrix Gla protein metabolism in vascular smooth muscle and role in uremic vascular calcification. J Biol Chem. 2011;286(33):28715–22.
Andrews J, Psaltis PJ, Bayturan O, Shao M, Stegman B, Elshazly M, Kapadia SR, Tuzcu EM, Nissen SE, Nicholls SJ, et al. Warfarin use is associated with progressive coronary arterial calcification: insights from serial intravascular ultrasound. JACC Cardiovasc Imag. 2018;11(9):1315–23.
Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51(20):1967–74.
Zettervall SL, Marshall AP, Fleser P, Guzman RJ. Association of arterial calcification with chronic limb ischemia in patients with peripheral artery disease. J Vasc Surg. 2018;67(2):507–13.
Yamagishi SI. Concerns about clinical efficacy and safety of warfarin in diabetic patients with atrial fibrillation. Cardiovasc Diabetol. 2019;18(1):12.
van Hattum ES, Algra A, Lawson JA, Eikelboom BC, Moll FL, Tangelder MJ. Bleeding increases the risk of ischemic events in patients with peripheral arterial disease. Circulation. 2009;120(16):1569–76.
Baker WL, Beyer-Westendorf J, Bunz TJ, Eriksson D, Meinecke AK, Sood NA, Coleman CI. Effectiveness and safety of rivaroxaban and warfarin for prevention of major adverse cardiovascular or limb events in patients with non-valvular atrial fibrillation and type 2 diabetes. Diabetes Obes Metab. 2019;21(9):2107–14.
Oh S, Goto S, Accetta G, Angchaisuksiri P, Camm AJ, Cools F, Haas S, Kayani G, Koretsune Y, Lim TW, et al. Vitamin K antagonist control in patients with atrial fibrillation in Asia compared with other regions of the world: real-world data from the GARFIELD-AF registry. Int J Cardiol. 2016;223:543–7.
Chan YH, Lee KT, Kao YW, Huang CY, Chen YL, Hang SC, Chu PH. The comparison of non-vitamin K antagonist oral anticoagulants versus well-managed warfarin with a lower INR target of 1.5 to 2.5 in Asians patients with non-valvular atrial fibrillation. PLoS One. 2019;14(3):e0213517.
Wang KL, Lip GY, Lin SJ, Chiang CE. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46(9):2555–61.
Saliba W, Barnett-Griness O, Elias M, Rennert G. Glycated hemoglobin and risk of first episode stroke in diabetic patients with atrial fibrillation: a cohort study. Heart Rhythm. 2015;12(5):886–92.
Narula N, Dannenberg AJ, Olin JW, Bhatt DL, Johnson KW, Nadkarni G, Min J, Torii S, Poojary P, Anand SS, et al. Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol. 2018;72(18):2152–63.
Acknowledgements
This study is based in part on National Health Insurance Research Database provided by the Applied Health Research Data Integration Service from National Health Insurance Administration, Taiwan. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes.
Disclosures
None directly related to this paper. GYHL: Consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally. The remaining authors have nothing to disclose.
Funding
This study was supported by grants 108-2314-B-182-053-MY2 from the Ministry of Science and Technology and grants CMRPG3J1371, CMRPD1K0031, CMRPG3K0021 and CORPG3G0351 from Chang Gung Memorial Hospital, Linkou, Taiwan.
Author information
Authors and Affiliations
Contributions
YHC, HFL, and TFC contributed to conception and design of the study, analysis and interpretation of the data, wrote the manuscript and approved submission. PRL, JRL, and LCS contributed data acquisition and analysis. YHC and LCS contributed to analysis of data and provided critical revision of the paper. LSW, SHC, YHY, CTK, LCS, and GYHL contributed to conception/design, and provided critical revision of the paper for the important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol complies with the Declaration of Helsinki and was approved by the Institutional Review Board of the Chang Gung Medical Foundation.
Consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
International Classification of Disease (9thand 10thedition) Clinical Modification (ICD 9-CMand ICD 10-CM) codes used to define the co-morbidities and clinical outcome in the study cohort. Table S2. International Classification of Disease (9thand 10thedition) Clinical Modification (ICD 9-CM and ICD 10-CM) codes used to define the major adverse limb outcome in the study cohort.
Additional file 2: Figure S1.
Cumulative incidence curves of outcomes for atrial fibrillation (AF) patients with concomitant diabetes mellitus (DM) taking oral anticoagulants before propensity score stabilized weighting (PSSW).
Additional file 3: Figure S2.
Forest plot of hazard ratio (HR) of effectiveness, major lower limb outcomes, and safety outcomes for NOACs vs. warfarin among non-valvular AF patients comorbid with DM, after multi-variate adjustment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chan, YH., Lee, HF., Li, PR. et al. Effectiveness, safety, and major adverse limb events in atrial fibrillation patients with concomitant diabetes mellitus treated with non-vitamin K antagonist oral anticoagulants. Cardiovasc Diabetol 19, 63 (2020). https://doi.org/10.1186/s12933-020-01043-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-020-01043-2