Abstract
Introduction
New oral anticoagulants (NOACs) are approved for use in nonvalvular atrial fibrillation (AF).
Objectives
This study aimed to evaluate the efficacy and safety of NOACs compared with warfarin in AF and valvular heart disease (VHD).
Methods
We identified randomized controlled trials (RCTs) and post-hoc analyses comparing NOACs and warfarin in AF and VHD, including biological and mechanical heart valves (MHV). Through systematic review and meta-analysis, with the aid of the “Rev Man” program 5.3, the primary effectiveness endpoints were stroke and systemic embolism (SE). The primary safety outcome was major bleeding, and the secondary outcome included intracranial hemorrhage. Data were analyzed using risk ratios (RRs) and 95% confidence intervals (CIs), and heterogeneity was assessed using the I2 statistic.
Results
Six RCTs were included, involving 13,850 patients with AF and VHD. NOACs significantly reduced the risk of stroke/SE (RR 0.78; 95% CI 0.66–0.91; P = 0.002) and intracranial hemorrhage (RR 0.51; 95% CI 0.33–0.79; P = 0.003) and lowered the risk of major bleeding (RR 0.77; 95% CI 0.58–1.02; P = 0.07) compared with warfarin.
Conclusions
The efficacy and safety of NOACs as thromboprophylaxis for AF and VHD are similar to those of warfarin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
NOACS significantly reduced the risk of stroke/systemic embolism in AF and VHD. |
NOACS have similar safety when compared to Warfarin in AF and VHD. |
There are innumerable advantages of the use of the NOACs to Vitamin K Antagonists. |
However, there are very limited data on the use of NOACs for AF patients with VHD. |
1 Introduction
Valvular heart disease (VHD) affects more than 100 million people worldwide [1]. At least 0.5–1% of the general population experience atrial fibrillation (AF), a sustained arrhythmia frequently seen in clinical practice, that significantly increases the incidence of thromboembolism when associated with VHD [2]. This association often requires the use of oral anticoagulation (OAC) to reduce the risk of thromboembolism.
Among the most commonly prescribed OACs in the prophylaxis of primary and secondary thromboembolism events are vitamin K antagonists (VKAs), particularly warfarin [3,4,5]. Over the last few years, alternatives to VKAs have been explored in thromboprophylaxis for AF, with and without VHD. These new oral anticoagulants (NOACs) do not require regular monitoring of hemostatic parameters. To date, the US FDA has approved dabigatran (the direct inhibitor of Factor IIa—thrombin) and rivaroxaban, apixaban and edoxaban (inhibitors of Factor Xa) [6]. Studies of NOACs in nonvalvular AF have demonstrated efficacy and safety similar to that of warfarin [7].
Recent guidelines regarding anticoagulation have supported the use of NOACs in specific native valve conditions when associated with AF, including aortic stenosis (AS), aortic regurgitation (AR), tricuspid regurgitation (TR) and mitral regurgitation (MR). NOACs are not currently recommended in moderate to severe mitral stenosis (MS) and mechanical heart valves (MHVs), where the use of VKAs for the prevention of thromboembolic events is the only established option. NOACs are considered reasonable alternatives to VKAs in patients with bioprosthetic valves (after the third month of implantation) and AF, particularly in the 2017 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines [8], given their previously demonstrated efficacy in AF (class IIA; level of evidence: C). The lack of robust data on this issue justifies the absence of specific recommendations in the American Heart Association (AHA)/American College of Cardiology (ACC) guidelines [9].
Published meta-analyses have concluded that the safety and efficacy of NOACs are similar to those of VKA in patients with VHD and AF [10,11,12], but patients with MHVs were excluded from these analyses. Given this gap in knowledge, we performed a quantitative and qualitative analysis of publications related to AF and VHD using systematic review and meta-analysis to evaluate the use of NOACs, focusing on efficacy (reduction of stroke and systemic embolism [SE]) and safety (rates of major bleeding and intracranial hemorrhage), compared with warfarin in adult patients with AF and VHD.
2 Methods
This is a systematic review and meta-analysis carried out according to the standards established by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [13]. More details are available in Table E1 in the Electronic Supplementary (ESM).
2.1 Eligibility Criteria
We included randomized controlled trials (RCTs) that compared NOACs (dabigatran, rivaroxaban, apixaban and/or edoxaban) and warfarin in adult humans with AF and VHD (including patients with MHV ≥ 3 months postoperatively).
2.2 Exclusion Criteria
Exclusion criteria were as follows: articles not focused on the use of NOACs in VHD and AF, observational studies, nonrandomized controlled trials, studies performed in animals, reviews and duplicate publications reporting the same trials.
2.3 Research Strategy for Identification of Studies
We searched the PubMed, LILACS, MEDLINE, SciELO and Cochrane Library (October 2017–June 2018) databases without year restrictions. We also reviewed pharmaceutical industry sites for additional data and the references of the selected publications to identify other potentially eligible articles. The search strategy is detailed in the ESM.
2.4 Data Collection
Two reviewers (YdSLB and ARD) independently evaluated the list of titles and abstracts from each data source. We obtained the full text of articles considered eligible to verify that they met inclusion criteria prior to data extraction. A data extraction form was prepared for the retrieval of information, including year of publication, authors, type of RCT, main population characteristics, types of VHD included and excluded, type and dose of NOAC, outcomes (total efficacy rate and safety) and follow-up time. The data were extracted and summarized independently by the same reviewers.
2.5 Evaluated Outcomes
We considered the primary endpoint of efficacy, stroke composition and SE, and the primary safety outcome was the presence of major bleeding (according to the International Society of Thrombosis and Haemostasis definition) [14]. Intracranial hemorrhage was a secondary outcome.
2.6 Risk of Bias in Individual Studies
We used the Cochrane Collaboration’s tool to assess the risk of bias and the methodological quality of the included trials. The following domains were evaluated: selection bias (random sequence generation method and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data) and reporting bias (selective reporting) [15]. The quality of each item was classified as either “yes” (low risk of bias), “no” (high risk of bias) or “unclear” (unclear risk of bias).
2.7 Statistical Analysis
Statistical analysis was performed using the Review Manager tool (version 5.3; The Cochrane Collaboration, 2011). We used the random-effects model as the standard in our meta-analysis, with data analyzed using risk ratios (RRs) and 95% confidence intervals (CIs). The results were considered statistically significant when the P value was < 0.05. Studies that presented the use of different dosages were independently grouped in different estimates using the random-effects model in the meta-analysis. As a quantitative measure of inconsistency, the I-squared (I2) statistic was used to assess heterogeneity.
2.8 Certainty in the Evidence and Strength of Recommendations
In our meta-analysis, we assessed the certainty of evidence and strength of recommendations for the outcomes stroke and SE composition, the presence of major bleeding and intracranial hemorrhage after the use of NOACs and warfarin using the GRADEpro (Grading of Recommendations, Assessment, Development and Evaluation profiler) software [16]. The GRADE approach classifies the quality of evidence as high, moderate, low, or very low based on the following considerations: risk of bias, consistency, directness, precision and publication bias [17].
The evidence for each item was classified as “none” (no reduction in points), “serious” (reduction of 1 point) or “very serious” (reduction of 2 points) according to the interference biases detected in these items. We resolved disagreements between reviewers during the data extraction and assessments of risk of bias or quality of evidence by discussion and, if needed, by third-party adjudication.
3 Results
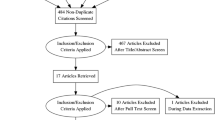
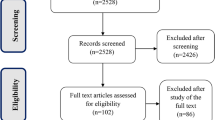
We identified six studies that met the eligibility criteria [18,19,20,21,22,23]. Two studies tested two different doses, so we performed specific analyses for each, giving rise to four substudies and a total of eight studies (Fig. E1 in the ESM).
3.1 Study Characteristics
Four (66.6%) of the included studies were phase III RCTs and two (33.3%) were experimental studies characterized as phase II RCTs and a prospective pilot study. Table 1 describes the main characteristics of the included studies.
Three of the included publications evaluated the use of dabigatran (the RE-ALIGN study [18], post-hoc analysis of the RE-LY study [22] and the DAWA study [21]), with the first exclusively involving patients with MHV and the third involving a group of patients with bioprostheses (aortic or mitral). The remaining studies are as follows: one evaluated the use of apixaban through a post-hoc study of ARISTOTLE [20], one evaluated the use of rivaroxaban (post-hoc analysis of the ROCKET-AF study [19]) and one analyzed the use of edoxaban (post-hoc analysis of the ENGAGE AF-TIME-48 trial) [23].
3.2 Patient Characteristics
Table 2 describes the main clinical characteristics and risk factors for bleeding and thromboembolism events in patients with AF and VHD who used some type of NOAC. Overall, approximately 13,850 subjects with different VHD status were involved in these studies. Of these, 13,826 were from post-hoc analyses of phase III clinical trials that compared NOACs and warfarin in nonvalvular AF.
The lowest and highest mean (± standard deviation) ages were 45.7 ± 6 and 71.8 ± 9.4 years, respectively. The most frequent comorbidities reported and risk factors for thromboembolism events were systemic arterial hypertension (SAH), heart failure (HF), prior stroke, SE or transient ischemic attack (TIA), coronary artery disease (CAD) and diabetes mellitus (DM). The most commonly cited classes of medications concomitant with chronic and/or continuous use of NOAC therapy included antihypertensives, diuretics, β-blockers and antiplatelet agents.
The subtype of VHD most frequently identified in the populations involved in these studies were as follows: 7842 individuals with MR and 2559 with AR, 3303 with TR, 1235 with AS, 708 with MS, 393 with some type of valve repair or repair, 252 with MHV and 218 with bioprostheses.
3.3 Outcomes
The primary and secondary outcomes included the safety and efficacy of dabigatran 150 and 110 mg twice daily compared with warfarin in the prevention of thromboembolism in those with AF and VHD (Table E3 in the ESM). Table E4 in the ESM describes the outcomes of the other available NOACs in AF and VHD.
3.4 Stroke and Systemic Embolism
NOACs were more effective than warfarin, with a lower relative risk of stroke and SE, in patients with VHD (RR 0.78; 95% CI 0.66–0.91; P = 0.002; high-quality evidence; see Table E2 in the ESM) (Fig. 1a). Heterogeneity among the studies was low.
Forest plot with individual and pooled estimates of the risk of stroke/systemic embolism, major bleeding and intracranial hemorrhage in patients with atrial fibrillation and valvular heart disease using new oral anticoagulants at different dosages compared with warfarin. CI confidence interval, M–H Mantel–Haenszel, NOAC new oral anticoagulant, SE systemic embolism. Asterisk indicates in the RE-ALIGN study performed by Eikelboom et al. [18], events in major bleeding and intracranial hemorrhage, for both Warfarin and Dabigatran groups, were not reported for population B (late postoperative period), therefore, they were not included in this analysis
3.5 Major Bleeding
The use of NOACs had a statistically significant favorable effect on the risk of major bleeding in patients with VHD compared with warfarin (RR 0.77; 95% CI 0.58–1.02; P = 0.07; low-quality evidence; Table E2 in the ESM), and the I2 was 79% (P < 0.0001), demonstrating a high level of heterogeneity (Fig. 1b).
3.6 Intracranial Hemorrhage
NOAC use was associated with a significant reduction in the risk of intracranial hemorrhage in patients with VHD compared with the use of warfarin (RR 0.51; 95% CI 0.33–0.79; P = 0.003, moderate-quality evidence; Table E2 in the ESM), with an estimated I2 of 36% (P = 0.16) (Fig. 1c).
3.7 Risk of Bias Across Studies and Quality of Evidence
The overall risk of reporting bias was low according to our analysis using the Cochrane Collaboration Tool (details in Table E2 in the ESM). Table E5 in the ESM presents the quality of evidence according to the GRADE system, and Table E6 in the ESM summarizes the main pharmacological characteristics of NOACs approved by the FDA for use in the USA.
4 Discussion
Our meta-analysis suggests that NOACs significantly reduced the risk of stroke/SE and intracranial hemorrhage in patients with AF and VHD compared with warfarin, even after inclusion of patients with MHV. In addition, the overall risk of major bleeding was lower. To our knowledge, this is the first systematic review and meta-analysis to separately evaluate subgroups of patients with VHD stratified by different doses of anticoagulants and to include patients with MHV ≥ 3 months postoperatively.
Prior RCTs included more than 72,000 individuals and compared the use of NOACs with warfarin in nonvalvular AF [24]. Post-hoc analyses of these studies revealed that a significant number of study individuals had at least some degree of VHD associated with AF, with our evaluation verifying the presence of at least 13,826 patients with AF and VHD.
Recent meta-analyses of these studies, as well as the results presented here, indicate that NOACs are as effective as warfarin in OAC in reducing the risk of thromboembolism events in AF and VHD. Furthermore, NOACs appear to have a lower association with major bleeding, when considering the analyses of the combined results [10, 11, 25]. In particular, apixaban appears considerably safer in this regard [7].
Regarding safety outcomes, the high heterogeneity identified in the present study is due to the results obtained through the ROCKET-AF study, in which rivaroxaban was associated with a higher risk of major bleeding than was warfarin, especially in patients with VHD. Intracranial hemorrhage did not reach statistical significance [10]. The VHD population involved in this study, in addition to having a higher thromboembolic risk, with a mean CHA2DS2-Vasc score of 3.5, was older (mean 75 years) and had a mean HAS-BLED score of 2.8, indicating considerable risk of bleeding.
On the other hand, Caldeira et al. [10] reported that NOACs such as apixaban, dabigatran and edoxaban, unlike rivaroxaban, offer an advantage in reducing the risk of intracranial bleeding compared with warfarin, independent of the presence or absence of VHD. These same authors, after analysis of cumulative evidence assessed through trial sequential analysis, identified a robust relation in stroke prevention and reduction of intracranial hemorrhage events. Similarly, our study showed a protective effect of approximately 50% with NOACs compared with warfarin in the case of intracranial hemorrhage, despite the moderate nonsignificant heterogeneity.
Notably, the current evidence argues against the use of dabigatran in MHV, because of the study by Eikelboom et al. [18] (RE-ALIGN), which ended prematurely after finding an absence of benefit and an increased risk of thromboembolism events with dabigatran in this population. However, the negative results of that study may result from subtherapeutic dosing, with dabigatran 50 ng/mL as a target level. In addition, this study included patients early postoperatively (a population in which the negative effects were fundamentally observed), a period with a high incidence of thromboembolism events.
Finally, it is possibile that dabigatran induced downstream effects in the coagulation cascade that impaired its ability to bypass the hypercoagulable state of the postoperative period in relation to warfarin. According to Ahmad and Wilt [26], the pathogenesis of thrombus formation in MHV does not resemble the mechanism involved in AF.
In vitro and animal models have shown promising results in the efficacy of rivaroxaban as thromboprophylaxis in MHV [27, 28]. Recently, we reported promising results from the first experience of a Factor Xa inhibitor (rivaroxaban) in humans, where we followed seven patients with MHV over 3 months [29]. An RCT is currently comparing rivaroxaban and warfarin in patients with MHV [30].
According to Ha et al. [31], the use of NOACs in the prevention of thromboembolism events in bioprostheses and AF remain a gray zone in contemporary practice. To date, only three RCTs (involving 280 patients) describe this group of patients in relation to the use of NOACs and VKA (ARISTOTLE [interim report], ENGAGE AF [high-dose edoxaban vs. warfarin; conference paper], DAWA study [dabigatran 110 mg twice daily vs. warfarin]), finding that NOACs were similar to VKA in terms of thromboembolic events and risks of major bleeding [10].
Recent guidelines have not yet made formal recommendations about the use of NOACs in AF and VHD, although they are not contraindicated. In this sense, in 2015, the European Heart Rhythm Association [32] stated that patients with AF and bioprostheses could be eligible for NOACs as long as they are > 3 months postoperative. However, studies regarding the efficacy in this population are lacking [31].
Given that patients with moderate to severe MS were not included in the RCTs in our meta-analysis, it was not possible to obtain specific results for this population. The current AHA/ACC and ESC/EACTS recommendations maintain the use of VKAs, in line with existing evidence. Further studies are necessary to elucidate the safety and efficacy of NOACs compared with VKAs in this population [8, 9].
Kim et al. [33] recently conducted an observational, retrospective study of patients with AF associated with MS, with the objective of validating the efficacy of NOACs (off-label) compared with warfarin. The authors found that the incidence of ischemic stroke/embolisms was lower with NOACs than with warfarin (2.2 vs. 4.19% per year, respectively; P < 0.0001). Furthermore, the incidence of intracranial hemorrhage was estimated at 0.49 and 0.93% per year with NOACs and warfarin, respectively [33]. This retrospective study supports our findings that NOACs appear to be more effective than and have a similar safety profile to warfarin. However, such results require reproduction in future RCTs to evaluate the efficacy of NOACs in patients with MS and AF. Until then, VKA remains the only proven alternative for the prevention of thromboembolic events in this population and for patients with MHV.
Indications for NOAC use in those without AF are still not completely established, primarily in the context of VHD. However, emerging evidence highlights practical considerations in the presence of certain patient characteristics (elderly, polypharmacy, presence of gastrointestinal bleeding, presence of CAD, etc.) that may guide the selection of a certain NOAC in AF, with or without VHD, for the purpose of reducing thromboembolism events.
As with previously published analyses, the lack of specific details in the literature regarding valve disease or surgery made it difficult to analyze the efficacy and safety of NOACs compared with warfarin by subtype of native valve disease or valve surgery.
4.1 Study Limitations
Our study has several important limitations. First, in the absence of absolute consensus regarding the terms “valvular AF” and “nonvalvular AF”, the lack of inclusion and exclusion criteria homogeneity in the included studies may underlie the presence of heterogeneity in some evaluated outcomes.
Most of our results were produced through information obtained in post-hoc analyses of large RCTs. The only studies that focused on patients with VHD were the DAWA study, which evaluated the use of dabigatran versus warfarin in patients with bioprosthesis, and the RE-ALIGN study, evaluating dabigatran versus warfarin in patients with MHV. We recognize that the populations involved in the studies included in our analysis are relatively heterogeneous and analyze different drugs, albeit of the same class. Combined outcome analyses may overestimate or underestimate the benefit of the results found.
Our results highlight the possible efficacy of NOACs, though less convincingly the safety profile because of the moderate and high heterogeneities identified in our investigation. Further studies are required to establish prospectively the efficacy and safety of NOACs in patients with AF and VHD with careful consideration of the implications of different subtypes of disease.
4.2 Future Directions
NOACs emerged as an excellent alternative to VKAs, mainly because of their practicality of use, limited drug interactions, and similar efficacy and safety profile in the prevention of stroke and SE. Patients with heart valve prostheses may be the last frontier to overcome, especially with the use of Factor Xa inhibitors. An ongoing open-label, noninferiority phase II RCT at our institution is evaluating the efficacy and safety of rivaroxaban compared with warfarin in patients with AF and MHV. In the future, the use of NOACs in AF and VHD may significantly influence the quality of life of millions of individuals through thromboembolism prevention.
5 Conclusion
NOACs have a number of advantages over VKAs and appear to significantly reduce the risk of stroke/SE and intracranial hemorrhage compared with warfarin in patients with AF and VHD, with a lower overall risk of major bleeding. New RCTs are needed to establish the efficacy and safety of NOACs compared with VKAs, particularly in patients with MS and those with mechanical and bioprostethic valves.
References
Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11.
Magalhães L, Figueiredo M, Cintra F, Saad E, Kuniyoshi R, Teixeira R, et al. II Diretrizes Brasileiras de Fibrilação Atrial. Arq Bras Cardiol. 2016;106.
Wittkowsky AK, Boccuzzi SJ, Wogen J, Wygant G, Patel P, Hauch O. Frequency of concurrent use of warfarin with potentially Interacting drugs. Pharmacotherapy. 2004;24:1668–74.
Lorga Filho AM, Azmus A, Soeiro A, Quadros A, Avezum Junior A, Marques A, et al. Diretrizes Brasileiras de Antiagregantes Plaquetários e Anticoagulantes em Cardiologia. Arq Bras Cardiol. 2013;101:01–93.
Erwin JP, Iung B. Current recommendations for anticoagulant therapy in patients with valvular heart disease and atrial fibrillation: the ACC/AHA and ESC/EACTS Guidelines in Harmony…but not Lockstep! Heart 2018;heartjnl-2017-312758.
Unger EF. News & Events—Atrial fibrillation, oral anticoagulant drugs, and their reversal agents [Internet]. U.S. Food Drug Adm. Center for Drug Evaluation and Research; 2015. https://www.fda.gov/drugs/newsevents/ucm467203.htm. Accessed 05 Jul 2018.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62.
ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2017;1–53.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–95.
Caldeira D, David C, Costa J, Ferreira JJ, Pinto FJ. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease: systematic review and meta-analysis. Eur Hear J Cardiovasc Pharmacother. 2017;4(2):111–18.
Pan K, Singer DE, Ovbiagele B, Wu Y, Ahmed MA, Lee M. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005835.
Renda G, Ricci F, Giugliano RP, De Caterina R. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease. J Am Coll Cardiol. 2017;69:1363–71.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Schulman S, Anger SU, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2005;8:202–4.
Higgins J, Altman D, Gotzsche P, Juni P, Moher D, Oxman A, et al. Chapter 8: Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions version 5.1.0. Cochrane Collab. BMJ Publishing Group; 2011;
Schünemann HJ, Schünemann AHJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–10.
Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–51.
Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–14.
Breithardt G, Baumgartner H, Berkowitz SD, Hellkamp AS, Piccini JP, Stevens SR, et al. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. 2014;35:3377–85.
Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna M, et al. Apixaban compared with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the ARISTOTLE trial. Circulation. 2015;132(8):624–32.
Durães AR, de Souza Roriz P, de Almeida Nunes B, Albuquerque FP, de Bulhões FV, de Souza Fernandes AM, et al. Dabigatran versus warfarin after bioprosthesis valve replacement for the management of atrial fibrillation postoperatively: DAWA Pilot Study. Drugs R&D. 2016;16(2):149–54.
Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M, et al. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease clinical perspective. Circulation. 2016;134:589–98.
De Caterina R, Renda G, Carnicelli AP, Nordio F, Trevisan M, Mercuri MF, et al. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol. 2017;69:1372–82.
Verheugt FWA, Granger CB. Oral anticoagulants for stroke prevention in atrial fibrillation: current status, special situations, and unmet needs. Lancet. 2015;386:303–10.
Hicks T, Stewart F, Eisinga A. NOACs versus warfarin for stroke prevention in patients with AF: a systematic review and meta-analysis. Open Hear. 2016;3:e000279.
Ahmad S, Wilt H. Stroke prevention in atrial fibrillation and valvular heart disease. Open Cardiovasc Med J. 2016;10:110–6.
Kaeberich A, Reindl I, Raaz U, Maegdefessel L, Vogt A, Linde T, et al. Comparison of unfractionated heparin, low-molecular-weight heparin, low-dose and high-dose rivaroxaban in preventing thrombus formation on mechanical heart valves: results of an in vitro study. J Thromb Thrombolysis. 2011;32:417–25.
Greiten LE, McKellar SH, Rysavy J, Schaff HV. Effectiveness of rivaroxaban for thromboprophylaxis of prosthetic heart valves in a porcine heterotopic valve model. Eur J Cardio-Thoracic Surg. 2014;45:914–9.
Durães AR, Bitar YD, Lima MLG, Santos CC, Schonhofen IS, Filho JAL, et al. Usefulness and safety of rivaroxaban in patients following isolated mitral valve replacement with a mechanical prosthesis. Am J Cardiol. 2018;122(6):1047–50.
Durães AR. Rivaroxaban vs. warfarin in patients with metallic prosthesis (RIWAMP) [Internet]. ClinicalTrial.gov. 2018. https://clinicaltrials.gov/ct2/show/NCT03566303. Accessed 2018 Aug 12.
Ha ACT, Verma A, Verma S. Oral anticoagulation for stroke prevention amongst atrial fibrillation patients with valvular heart disease. Curr Opin Cardiol. 2017;32:1.
Heidbuchel H, Verhamme P, Alings M, Antz M, Diener H-C, Hacke W, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–507.
Kim JY, Kim S-H, Myong J-P, Kim YR, Kim T-S, Kim J-H, et al. Outcomes of direct oral anticoagulants in patients with mitral stenosis. J Am Coll Cardiol. 2019;73:1123–31.
Breithardt G, Baumgartner H, Berkowitz SD, Hellkamp AS, Piccini JP, Lokhnygina Y, et al. Native valvedisease in patients with non-valvular atrial fibrillation on warfarin or rivaroxaban. Heart. 2016;102(13):1036–43. https://doi.org/10.1136/heartjnl-2015-308120.
Acknowledgements
The authors thank Professors Paulo Novis Rocha and Pedro Antônio Pereira de Jesus for their great collaboration in the revision of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
Yasmin de Souza Lima Bitar, Mansueto Gomes Neto, Jose Admirço Lima Filho, Larissa Vitória Pereirab, Kethyren Santos Oliveira Travassosd, Kevan M. Akrami, Leonardo Roever and Andre Rodrigues Duraes have no conflicts of interest that are directly relevant to the content of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
de Souza Lima Bitar, Y., Neto, M.G., Filho, J.A.L. et al. Comparison of the New Oral Anticoagulants and Warfarin in Patients with Atrial Fibrillation and Valvular Heart Disease: Systematic Review and Meta-Analysis. Drugs R D 19, 117–126 (2019). https://doi.org/10.1007/s40268-019-0274-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-019-0274-z