Abstract
Introduction
Little is known on the pulmonary gradients of oxyhemoglobin, carboxyhemoglobin and methemoglobin in pulmonary arterial hypertension (PAH). We sought to determine these gradients in group 1 PAH and assess their association with disease severity and survival.
Methods
During right heart catheterization (RHC) we obtained blood from pulmonary artery (PA) and pulmonary artery wedge (PAW) positions and used co-oximetry to test their gasometric differences.
Results
We included a total of 130 patients, 65 had group 1 PAH, 40 had pulmonary hypertension (PH) from groups 2–5 and 25 had no PH during RHC. In all groups, PAW blood had higher pH, carboxyhemoglobin and lactate as well as lower pCO2 than PA blood. In group 1 PAH (age 58 ± 15 years, 72% females), methemoglobin in the PAW was lower than in the PA blood (0.83% ± 0.43 vs 0.95% ± 0.50, p = 0.03) and was directly associated with the degree of change in pulmonary vascular resistance (R = 0.35, p = 0.02) during inhaled nitric oxide test. Oxyhemoglobin in PA (HR (95%CI): 0.90 (0.82–0.99), p = 0.04) and PAW (HR (95%CI): 0.91 (0.84–0.98), p = 0.003) blood was associated with adjusted survival in PAH.
Conclusions
Marked differences were observed in the gasometric determinations between PAW and PA blood. The pulmonary gradient of methemoglobin was lower in PAH patients compared to controls and a higher PAW blood methemoglobin was associated with a more pronounced pulmonary vascular response to inhaled nitric oxide. Pulmonary artery and PAW oxyhemoglobin tracked with disease severity and survival in PAH.
Similar content being viewed by others
Introduction
Pulmonary arterial hypertension (PAH) is a condition characterized by progressive narrowing of the small pulmonary arteries that if left untreated leads to right heart failure and death [1]. This pre-capillary involvement results in a distinct hemodynamic profile characterized by a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg, pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg and pulmonary vascular resistance (PVR) > 3 Wood units [2]. During the last three decades, the understanding of the pathobiology of PAH has markedly improved; however, there remains a need to determine whether certain gasometric alterations play a role in the pathogenesis of PAH.
The lungs oxygenate the blood and remove the carbon dioxide (CO2) generated by metabolic processes. It remains unknown how the lungs of patients with PAH process carboxyhemoglobin (COHb) and methemoglobin (metHb); which are compounds potentially involved in the pathogenesis of the disease [3,4,5,6]. Endogenously, carbon monoxide (CO) is produced by the catabolism of heme by heme oxygenase [7]. Carbon monoxide is in part produced in the lungs [8, 9] where it may act as a pulmonary vasodilator [10]. Methemoglobin results from the oxidation of the hemoglobin iron to a ferric (Fe+++) state, a reaction that occurs when oxyhemoglobin reacts with nitric oxide (NO) [11]; therefore levels of metHb may track with levels of NO, a potent vasodilator implicated in the pathogenesis of PAH [12].
Transpulmonary gradients are traditionally measured between pulmonary artery (PA) (mixed venous) and systemic arterial blood (e.g. radial artery) [13,14,15,16,17,18]; however, arterial blood may be affected by right-to-left shunts from the Thebesian veins and potential metabolic processes between the left heart and the arterial sampling site. An approach to prevent these problems is to obtain blood from the pulmonary artery wedge (PAW) position (Fig. 1), indeed PAW blood has been studied in a variety of diseases [19,20,21]. Notably, PAW blood can be obtained at the time of right heart catheterization (RHC) without the need for an arterial puncture. In our practice we routinely obtain PA blood (mixed venous) for indirect/direct Fick cardiac output calculation and PAW blood to support an adequate PAW measurement [22]. Given that there is limited information on the pulmonary gradients of certain gases in PAH, we used co-oximetry to test the differences between blood obtained at the PA and PAW positions. We hypothesize that patients with PAH (group 1) have derangements in COHb and metHb as a result of defects in the NO pathway. We further hypothesize that COHb and metHb gradients may not only provide an insight on the metabolic processes that occur in the lungs of PAH patients but may be associated with disease severity, inhaled NO response and survival.
Materials and methods
Subjects and study design
This cross-sectional study was approved by the Cleveland Clinic institutional review board (study number: 06–245 and 14–1069). Written informed consent was waived given the retrospective nature of the study. We included consecutive patients who underwent RHC either to a) diagnose pulmonary hypertension (PH) or b) manage patients with prior diagnosis of PH. Right heart catheterizations were performed between December 2012 and April 2015.
We carefully assessed each patient to determine the etiology of PH based on the Fifth World Symposium classification [23]. Group 1 PH includes the idiopathic and heritable forms, PH associated with drugs, toxins, connective tissue and congenital heart diseases, portal hypertension, HIV infection and schistosomiasis. Group 2, 3, 4 and 5 includes PH due to left heart disease, lung disease/hypoxia, chronic thromboembolic disease and multifactorial mechanisms, respectively. For the purpose of this study, patients were divided into three groups: a) patients with PAH (PH group 1), 2) patients with PH groups 2–5, and 3) age- and gender-matched subjects without evidence of resting PH on RHC (mPAP < 25 mmHg). Patients without PH underwent RHC due to elevated right ventricular systolic pressure on echocardiography. Patients were excluded (n = 50) if the PAW blood could not be obtained during RHC or if it showed an SO2 < 90%, suggestive of an inadequate balloon wedging with leakage of deoxygenated blood from the proximal (before the balloon) to the distal (after the balloon) aspect of the PA catheter.
Right heart catheterization
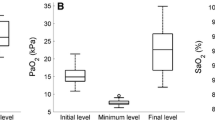
Subjects underwent RHC in the outpatient setting by a single operator (A.R.T.). All RHC were done under local anesthesia using 5 mL of 2% lidocaine. We continued or started oxygen (O2) supplementation in patients in whom the resting pulse oxygen saturation (SpO2) was < 90%. We maintained the same O2 flow during all the study measurements. In the supine position and with the transducer located in the mid-thoracic line (4th intercostal space), we measured the right atrial (RA) pressure, mean PAP and PAWP at end-expiration. We determined cardiac output (CO) by thermodilution. We calculated the cardiac index (CO / body surface area), the transpulmonary pressure gradient (TPG: mean PAP - PAWP), diastolic pulmonary gradient (DPG: diastolic PAP – PAWP) and PVR (TPG / CO). When appropriate, we performed a pulmonary vasodilator challenge using inhaled NO at 40 ppm for 5 min [24].
Laboratory determinations
Pulmonary artery and PAW blood were obtained immediately after fluoroscopic site confirmation and obtaining an adequate waveform. The tip of the pulmonary artery catheter was located in West zone 3 [25]. Ten mL of blood were discarded from each site before a sample was collected in a blood gas syringe. Blood gas samples were immediately analyzed using the ABL 800 Flex analyzer (Radiometer, Copenhagen, Denmark) which uses co-oximetry and provide determinations of COHb, metHb, Hb and lactate.
Other measurements
We collected data regarding demographics, use of PAH-specific medications and smoking status (current smoker was defined as a person who smoked tobacco in the last 30 days). We determined the severity of PH by using the New York Heart Association (NYHA) functional class, plasma N- terminal pro-B type natriuretic peptide (NT-proBNP), distance walked in the six-minute walk test (6MWD), RV size and function on echocardiography and hemodynamic determinations during RHC. Right ventricular function was determined both subjectively by visual inspection and objectively by the tricuspid annular plane systolic excursion (TAPSE) [26]. We also recorded the diffusion lung capacity for carbon monoxide corrected for Hb (DLCOc) [27].
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median (interquartile range (IQR)) as appropriate. Categorical data are summarized as discrete values and percentages (n (%)). Continuous and categorical variables were compared across the groups using analysis of variance (ANOVA) and Chi-square, respectively. Paired data were contrasted with paired t test or Friedman test as appropriate. Associations were tested using the Pearson correlation test. Survival analysis was performed with Cox proportional hazards model adjusted by age, gender and other pre-specified variables. The starting point for the survival analyses was the date of the gasometric determinations. Patients were censored at the time of lung transplantation and followed until death or end of the study in January 2018. Cox proportional hazards model results are expressed as hazard ratios (HR) with the corresponding 95% confidence intervals (CI). All p values are two-tailed and a value of < 0.05 was considered significant. The statistical analyses were performed using the statistical package IBM SPSS, version 20 (IBM; Armonk, New York) and MedCalc, version 14.12.0 (Ostend, Belgium).
Results
Baseline characteristics
We included a total of 130 patients, of whom 65 had group 1 PAH, 40 had PH from groups 2–5 and 25 had no PH during RHC. Of the patients with PAH, 38 (58%) had idiopathic or heritable PAH, 17 (26%) had PAH associated with connective tissue diseases, 5 (8%) had porto-pulmonary hypertension and 5 (8%) had PAH due to other etiologies. Patients with non-group 1 PH belonged to PH groups 2 (n = 20, 50%), 3 (n = 10, 25%), 4 (n = 5, 12.5%) and 5 (n = 5, 12.5%). All patients without PH (n = 25, 19%) had an elevated RVSP (≥ 40 mmHg) and associated diseases such as scleroderma, cirrhosis, interstitial lung disease, obstructive sleep apnea or suggestion of left ventricular diastolic dysfunction by echocardiogram.
Of the patients with PAH, 18 (28%), 15 (23%), 20 (31%) and 12 (19%) were on none, 1, 2, and 3 PAH-specific therapies, respectively. These PAH-specific therapies were phosphodiesterase-5 inhibitors (n = 39, 60%), endothelin receptor antagonists (n = 25, 39%), soluble guanylate cyclase stimulator (n = 1, 2%), and prostacyclin analogues (n = 26, 40%). Baseline characteristics of the three groups of patients are shown in Table 1.
Comparison of PAW and PA blood in patients with group 1 PAH
We observed significant differences between the PAW and PA blood in PAH patients. PAW blood had higher pH, COHb and lactate as well as lower pCO2, bicarbonate and metHb when compared to the PA blood (Table 2).
Comparison of PAW and PA blood gradients among study groups
A comparison among the three study groups (group 1 PAH, non-group 1 PH and no PH) showed that the pH increase in the PAW compared to the PA blood was more pronounced in group 1 PAH patients (Table 3). We also noted that the metHb was lower in the PAW relative to PA blood in patients with PH (group 1 PAH and non-group 1 PH) compared to individuals without PH (Table 3). In fact, in individuals without PH, metHb was higher in the PAW than in the PA blood (mean (95% CI) difference: 0.12 (+ 0.01, + 0.23), p = 0.04).
Association between blood gases, gradients and disease severity in group 1 PAH patients
The gradient of O2Hb between PAW and PA was directly associated with RA pressure (R: 0.34, p = 0.006), PVR (R: 0.35, p = 0.006) and NT-proBNP (R: 0.36, p = 0.03) and inversely associated with CI (R: − 0.43, p < 0.001) and TAPSE (R: -0.32, p = 0.013). Other gasometric gradients were not significantly associated with markers of disease severity.
Response to inhaled NO challenge and gasometric determinations in group 1 PAH patients
In group 1 PAH patients: the PVR decreased a mean of 16.3% ± 16.9% (n = 45). The percentage drop in PVR was inversely associated with Hb (R = − 0.34, p = 0.02) and directly associated with metHb (R = 0.35, p = 0.02) and O2Hb (R = 0.321, p = 0.03) in the PAW blood.
Relationship between blood determinations, gradients and survival in PH patients
The median (IQR) follow-up for patients with type 1 PAH was 41 (22–51) months, with a 2-year survival of 78%. When adjusted for age and gender, variables associated with mortality in group 1 PAH included: PA O2Hb (HR (95%CI): 0.87 (0.81–0.94), p = 0.001) and PAW O2Hb (HR (95%CI): 0.93 (0.87–0.98), p = 0.01). Pulmonary artery O2Hb was associated with mortality even when adjusted by supplemental oxygen therapy (HR (95%CI): 0.89 (0.82–0.96), p = 0.003) and adding PVR, 6MWD and number of PAH-specific therapies (HR (95%CI):0.90 (0.82–0.99), p = 0.04). Pulmonary artery wedge O2Hb continued to be a significant predictor when adjusted by supplemental oxygen therapy (HR (95%CI): 0.89 (0.83–0.96), p = 0.001), PA O2Hb (HR (95%CI): 0.88 (0.82–0.95), p = 0.001), and adding PVR, 6MWD and number of PAH-specific therapies (HR (95%CI):0.91 (0.84–0.98), p = 0.003). Methemoglobin or COHb levels measured in the PA or PAW, or their pulmonary gradients, did not predict survival.
Discussion
In the present study we reported results on gasometric gradients between PAW and PA blood and compared findings in patients with group 1 PAH, PH groups 2–5 and age- and gender-matched disease controls. In all groups, we noted that the PAW blood showed higher pH, COHb and lactate compared to the PA blood. Interestingly, the level of metHb was lower in PAW blood than in PA blood in patients with PH, in contrast to higher levels in the PAW blood in patients without PH. In patients with group 1 PAH, we noted that a higher PAW metHb was associated with a more pronounced PVR drop during inhaled nitric oxide challenge. In patients with group 1 PAH, a lower PA and PAW O2Hb levels were independently associated with worse survival.
As shown by others [19, 20], the pH was higher in the PAW blood compared to the PA blood, in association with a lower pCO2. This pH gradient was more pronounced in patients with PAH than the other 2 groups of patients. Patients with PAH tend to have lower arterial pCO2 [28, 29] given an increase in the respiratory drive, an effect that becomes more evident in the PAW blood. Interestingly, the levels of lactate were higher in the PAW than PA blood suggesting lung production, as seen in other diseases such as acute respiratory distress syndrome [14, 30, 31]. We speculate that the differences in PAW blood are due to a) double lung passage of blood, initially from the PA through the alveolar capillaries and pulmonary veins to the left atrium, and then backwards from the left atrium through the pulmonary veins and alveolar capillaries to the PA catheter, leading to longer exposure to alveolar gas and intensification of the lung processes (Fig. 1) and/or b) prolonged exposure of the PAW blood to alveolar gases, given the temporal immobility of blood in a functional portion of the lung.
Nitric oxide is a potent vasodilator [32] that plays an important role in the pathogenesis of PH [12]. The reaction with O2Hb is one of the catabolic pathways for NO, a process that generates nitrate and metHb [33]. Naples et al. showed that venous metHb correlated with the levels of NO in the plasma of asthmatic patients [34]. In our study, we showed that blood metHb levels are lower in the PAW compared to PA position in PH patients; in contrast, we found higher levels of metHb in PAW position in patients without PH. These findings insinuate a reduced production or an enhanced metabolism of metHb in the lung of PH patients. Interestingly, in PAH patients, a higher metHb in PAW blood was associated with a more pronounced decline in PVR during inhaled NO challenge, suggesting a greater diffusion of NO, a healthier pulmonary vasculature and/or an earlier stage of the disease in these patients. Further investigations may determine whether the level of metHb in the PAW position is associated with the response to PH-medications that target the nitric oxide pathway.
Carboxyhemoglobin is produced endogenously during the oxidation of heme compounds by heme oxygenase [35]. The carbon monoxide released from this catabolic reaction has vasodilatory [36], anti-inflammatory [37] and anti-proliferative properties [4]. Hypoxia transiently increases expression of heme oxygenase [38, 39]. The increased expression of heme oxygenase prevented the development of PH induced by hypoxia [38, 39] a mechanism that may involve the antiproliferative action of carbon monoxide [39]. In the present study, we found that COHb is higher in the PAW compared to PA blood in all groups of patients, suggesting pulmonary production and systemic metabolism of carbon monoxide. A finding consistent with prior studies which showed positive arteriovenous COHb difference, with higher COHb in arterial compared to venous blood [8, 34, 40].
Analysis of survival in group 1 PAH patients showed that PA and PAW O2Hb adjusted by age, gender, use of supplemental oxygen, PVR and 6MWD are independent predictors of long-term survival. The association between PA O2Hb and survival was expected given previous research [41, 42]. The association between PAW blood O2Hb and survival is novel and consistent with our prior study showing that hypoxemia (determined by pulse oximetry) in patients with idiopathic and heritable PAH is associated with worse survival [43].
Our study has limitations: a) retrospective analysis, b) even though the gasometric differences of metHb are small and might not be clinically significant, they provide an insight on the complex physiological processes involving nitric oxide and c) although patients had a detailed smoking history, surreptitious smoking or other environmental exposure that increased carbon monoxide could not be ruled out. Smoking may affect the pulmonary endothelium, leading to pulmonary vascular remodeling and PH, even in the absence of hypoxemia and destructive emphysematous lung disease [44,45,46,47,48]. Despite these limitations, our study is the first to assess the gasometric gradients between PAW and PA blood in patients with PAH.
Conclusions
Pulmonary artery wedge blood samples had higher pH and lower pCO2 than PA, suggesting that PAW blood was exposed to alveolar gas for a longer period of time. Methemoglobin levels were lower in the PAW than PA blood in patients with PAH and a higher PAW metHb was associated with a more pronounced pulmonary vascular response to inhaled NO. Pulmonary artery and PAW O2Hb tracked with disease severity and survival in PAH patients.
References
Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, Dweik RA. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:365–9.
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50.
Pabalan MJ, Nayak SP, Ryan RM, Kumar VH, Lakshminrusimha S. Methemoglobin to cumulative nitric oxide ratio and response to inhaled nitric oxide in PPHN. J Perinatol. 2009;29:698–701.
Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475–9.
Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem. 1997;272:32804–9.
Kourembanas S. Hypoxia and carbon monoxide in the vasculature. Antioxid Redox Signal. 2002;4:291–9.
Montellano PR. The mechanism of heme oxygenase. Curr Opin Chem Biol. 2000;4:221–7.
Meyer J, Prien T, Van Aken H, Bone HG, Waurick R, Theilmeier G, Booke M. Arterio-venous carboxyhemoglobin difference suggests carbon monoxide production by human lungs. Biochem Biophys Res Commun. 1998;244:230–2.
Scharte M, Bone HG, Van Aken H, Meyer J. Increased carbon monoxide in exhaled air of critically ill patients. Biochem Biophys Res Commun. 2000;267:423–6.
Dubuis E, Gautier M, Melin A, Rebocho M, Girardin C, Bonnet P, Vandier C. Chronic carbon monoxide enhanced IbTx-sensitive currents in rat resistance pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L120–9.
Privat C, Lantoine F, Bedioui F, Millanvoye van Brussel E, Devynck J, Devynck MA. Nitric oxide production by endothelial cells: comparison of three methods of quantification. Life Sci. 1997;61:1193–202.
Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–21.
Johnson ML, Emhoff CA, Horning MA, Brooks GA. Transpulmonary lactate shuttle. Am J Physiol Regul Integr Comp Physiol. 2012;302:R143–9.
De Backer D, Creteur J, Zhang H, Norrenberg M, Vincent JL. Lactate production by the lungs in acute lung injury. Am J Respir Crit Care Med. 1997;156:1099–104.
Bendjelid K, Treggiari MM, Romand JA. Transpulmonary lactate gradient after hypothermic cardiopulmonary bypass. Intensive Care Med. 2004;30:817–21.
Wang TL, Hsu KL, Chiang FT, Tseng CD, Tseng YZ. Anaerobic metabolism in patients undergoing intra-aortic balloon counterpulsation for cardiogenic shock. J Formos Med Assoc. 1995;94:379–85.
Inoue T, Sakai Y, Morooka S, Hayashi T, Takayanagi K, Yamaguchi H, Takabatake Y. Venoarterial carbon dioxide tension gradient in acute heart failure. Cardiology. 1993;82:383–7.
Harris P, Bailey T, Bateman M, Fitzgerald MG, Gloster J, Harris EA, Donald KW. Lactate, pyruvate, glucose, and free fatty acid in mixed venous and arterial blood. J Appl Physiol. 1963;18:933–6.
Williams WH Jr, Olsen GN, Allen WG, Yergin BM. Use of blood gas values to estimate the source of blood withdrawn from a wedged flow-directed catheter in critically ill patients. Crit Care Med. 1982;10:636–40.
Brewster H, McIlroy MB. Blood gas tensions and pH of pulmonary “wedge” samples in patients with heart disease. J Appl Physiol. 1973;34:413–6.
Safian RD, Come SE, Kadin M, Lorell BH. Use of pulmonary capillary wedge aspirates for the antemortem diagnosis of pulmonary microvascular tumor. Catheter Cardiovasc Diagn. 1989;17:112–5.
Tonelli AR, Mubarak KK, Li N, Carrie R, Alnuaimat H. Effect of balloon inflation volume on pulmonary artery occlusion pressure in patients with and without pulmonary hypertension. Chest. 2011;139:115–21.
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41.
Tonelli AR, Alnuaimat H, Mubarak K. Pulmonary vasodilator testing and use of calcium channel blockers in pulmonary arterial hypertension. Respir Med. 2010;104:481–96.
West JB, Dollery CT. Distribution of blood flow and the pressure-flow relations of the whole lung. J Appl Physiol. 1965;20:175–83.
Ahmed M, Dweik RA, Tonelli AR. What is the best approach to a high systolic pulmonary artery pressure on echocardiography? Cleve Clin J Med. 2016;83:256–60.
Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35.
Hoeper M, Pletz M, Golpon H, Welte T. Prognostic value of blood gas analyses in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2007;29:944–50.
Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–23.
Douzinas EE, Tsidemiadou PD, Pitaridis MT, Andrianakis I, Bobota-Chloraki A, Katsouyanni K, Sfyras D, Malagari K, Roussos C. The regional production of cytokines and lactate in sepsis-related multiple organ failure. Am J Respir Crit Care Med. 1997;155:53–9.
Brown SD, Clark C, Gutierrez G. Pulmonary lactate release in patients with sepsis and the adult respiratory distress syndrome. J Crit Care. 1996;11:2–8.
Zapol WM, Rimar S, Gillis N, Marletta M, Bosken CH. Nitric oxide and the lung. Am J Respir Crit Care Med. 1994;149:1375–80.
Gow AJ, Luchsinger BP, Pawloski JR, Singel DJ, Stamler JS. The oxyhemoglobin reaction of nitric oxide. Proc Natl Acad Sci U S A. 1999;96:9027–32.
Naples R, Laskowski D, McCarthy K, Mattox E, Comhair SA, Erzurum SC. Carboxyhemoglobin and methemoglobin in asthma. Lung. 2015;193:183–7.
Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–94.
Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61.
Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–8.
Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99–107.
Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000;86:1224–9.
Yasuda H, Sasaki T, Yamaya M, Ebihara S, Maruyama M, Kanda A, Sasaki H. Increased arteriovenous carboxyhemoglobin differences in patients with inflammatory pulmonary diseases. Chest. 2004;125:2160–8.
Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, Beltran M, Guerrero ML. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation. 1994;89:1733–44.
Wensel R, Opitz CF, Anker SD, Winkler J, Hoffken G, Kleber FX, Sharma R, Hummel M, Hetzer R, Ewert R. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002;106:319–24.
Khirfan G, Naal T, Abuhalimeh B, Newman J, Heresi GA, Dweik RA, Tonelli AR. Hypoxemia in patients with idiopathic or heritable pulmonary arterial hypertension. PLoS One. 2018;13:e0191869.
Wright JL, Levy RD, Churg A. Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatment. Thorax. 2005;60:605–9.
Wright JL, Churg A. Effect of long-term cigarette smoke exposure on pulmonary vascular structure and function in the Guinea pig. Exp Lung Res. 1991;17:997–1009.
Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD. Am J Respir Crit Care Med. 2008;134:808–14.
Ferrer E, Peinado VI, Diez M, Carrasco JL, Musri MM, Martinez A, Rodriguez-Roisin R, Barbera JA. Effects of cigarette smoke on endothelial function of pulmonary arteries in the Guinea pig. Respir Res. 2009;10:76.
Barbera JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21:892–905.
Funding
A.R.T is supported by NIH grant # R01HL130307. M.K.A was supported by the Egyptian Ministry of Higher Education and Scientific Research Scholarship program grant.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Disclosures
The authors have no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Author information
Authors and Affiliations
Contributions
GK Participated in the design of the study, data collection, statistical analysis, interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. MA Participated in the design of the study, data collection, interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. MDF Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. WD Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. RAD Participated in the design of the study, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. ART Participated in the design of the study, data collection, statistical analysis, interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. ART is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Cleveland Clinic institutional review board (study number: 06–245 and 14–1069). Written informed consent was waived given the retrospective nature of the study.
Competing interests
Ghaleb Khirfan MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Mostafa K. Ahmed MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Michael D. Faulx MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Wael Dakkak MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Raed A. Dweik MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Adriano R. Tonelli MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Khirfan, G., Ahmed, M.K., Faulx, M.D. et al. Gasometric gradients between blood obtained from the pulmonary artery wedge and pulmonary artery positions in pulmonary arterial hypertension. Respir Res 20, 6 (2019). https://doi.org/10.1186/s12931-018-0969-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-018-0969-7