Abstract
Background
Water intake and hydration status have been suggested to impact cognition; however, longitudinal evidence is limited and often inconsistent. This study aimed to longitudinally assess the association between hydration status and water intake based on current recommendations, with changes in cognition in an older Spanish population at high cardiovascular disease risk.
Methods
A prospective analysis was conducted of a cohort of 1957 adults (aged 55–75) with overweight/obesity (BMI between ≥ 27 and < 40 kg/m2) and metabolic syndrome from the PREDIMED-Plus study. Participants had completed bloodwork and validated, semiquantitative beverage and food frequency questionnaires at baseline, as well as an extensive neuropsychological battery of 8 validated tests at baseline and 2 years of follow-up. Hydration status was determined by serum osmolarity calculation and categorized as < 295 mmol/L (hydrated), 295–299.9 mmol/L (impending dehydration), and ≥ 300 mmol/L (dehydrated). Water intake was assessed as total drinking water intake and total water intake from food and beverages and according to EFSA recommendations. Global cognitive function was determined as a composite z-score summarizing individual participant results from all neuropsychological tests. Multivariable linear regression models were fitted to assess the associations between baseline hydration status and fluid intake, continuously and categorically, with 2-year changes in cognitive performance.
Results
The mean baseline daily total water intake was 2871 ± 676 mL/day (2889 ± 677 mL/day in men; 2854 ± 674 mL/day in women), and 80.2% of participants met the ESFA reference values for an adequate intake. Serum osmolarity (mean 298 ± 24 mmol/L, range 263 to 347 mmol/L) indicated that 56% of participants were physiologically dehydrated. Lower physiological hydration status (i.e., greater serum osmolarity) was associated with a greater decline in global cognitive function z-score over a 2-year period (β: − 0.010; 95% CI − 0.017 to − 0.004, p-value = 0.002). No significant associations were observed between water intake from beverages and/or foods with 2-year changes in global cognitive function.
Conclusions
Reduced physiological hydration status was associated with greater reductions in global cognitive function over a 2-year period in older adults with metabolic syndrome and overweight or obesity. Future research assessing the impact of hydration on cognitive performance over a longer duration is needed.
Trial registration
International Standard Randomized Controlled Trial Registry, ISRCTN89898870. Retrospectively registered on 24 July 2014
Similar content being viewed by others

Background
Cognitive decline is an important public health concern given 55 million people have been diagnosed with dementia and almost 80 million people are projected to be affected by 2030 [1]. Cognitive function is particularly important, especially as the population ages, because it determines the maintenance of our independence, the performance of everyday activities, and the quality of life [1]. Cognitive decline and dementia have a diverse etiology, and effective treatment is still not available [2]. For this reason, prevention strategies targeting modifiable factors, such as nutritional habits and dietary intake to slow the development of cognitive impairment, remain a promising public health approach [2]. Evidence to date assessing individual dietary factors and dietary patterns in relation to cognitive health has been summarized in the Lancet Neurology [3], while there is information related to various macro- and micronutrients and patterns, evidence related to water appears limited.
Water intake is a nutritional habit that is often overlooked, yet it is considered essential for the optimal physiological function of the human body [4]. As the most abundant component of the human body, water intake and optimal hydration are considered key aspects for the proper functioning of organ systems, aiding, among others, in efficient digestion, elimination of toxins, energy production, thermoregulation, and joint lubrication, as well as a multitude of biochemical reactions [5, 6]. Furthermore, proper hydration is thought to be important for optimal cognitive functioning as it plays a vital role in neural conductivity [7].
Dehydration occurs when the body loses more water than is taken in, whereas hypohydration refers to the state of water deficit; these conditions result in less-than-optimal hydration and may elicit adverse physiological consequences [8]. For the purposes of this paper, dehydration will be the term used to encompass the state of improper hydration due to unbalanced water loss or water deficit. In Europe, the percentage of the population reported to have inadequate water intake is estimated to vary from 5 to 35% [9,10,11]. Understanding water intake and hydration status is of particular importance in older adults as this population tends to be less likely to meet recommendations on water intake and is at greater risk for dehydration due to blunted sensitivity to thirst signals, lower body reserves due to reduced muscle mass, reduced ability to deal with heat stress, and use of medications or laxatives with diuretic effects [12,13,14,15]. Multiple health organizations and dietary guidelines have acknowledged that adequate hydration status is associated with the preservation of physical and mental functions and that water intake is the best way to achieve hydration [16,17,18,19].
To maintain adequate hydration status, several countries and public organizations have proposed water intake recommendations for the public [20, 21]. These recommendations have been determined based on data from population studies and accounted for water from beverage and food sources [22]. For instance, the European Food Safety Authority (EFSA), based on data from population studies from 13 European countries, proposed the dietary reference values (DRV) for the adequate intake (AI) of water which increases with age up to 2.5 L and 2.0 L of water daily for men and women (aged 14 to 70 years), respectively [20]. Similarly, the Institute of Medicine (IOM) proposed increasing water intake levels with age up to 3.7 L/day and 2.7 L/day (aged 19 to 70 years) in men and women, respectively. Yet, the World Health Organization and other guidelines related to cognitive health do not currently include recommendations related to water intake or hydration status [23,24,25]. Further understanding of health behaviors, such as cognitive functioning, related to something as fundamental as water intake can have a substantial impact on public health.
Inadequate water intake and dehydration have been associated with existing signs of cognitive impairment among older adults living in long-term care facilities [26,27,28,29]. Moreover, acute studies have shown that dehydration and water supplementation affect mood and cognitive performance [30]. However, fluid and water intake has received limited attention in epidemiological studies, and the literature scarcely examines water intake as a predictor of cognitive performance among older adults. The few studies that have assessed hydration status as a potential predictor of cognitive function among community-dwelling older adults have been inconclusive [31,32,33,34]. Furthermore, to date, few studies have prospectively captured and examined the impact of water and hydration status on cognitive function over a multi-year period. Therefore, the objective of the present analyses was to prospectively investigate the relation between hydration status, water intake, and 2-year changes in cognitive performance in community-dwelling older adults with metabolic syndrome and overweight or obesity.
Methods
Study design
This prospective cohort study is based on data collected during the first 2 years of the PREDIMED-Plus (PREvención con DIeta MEDiterránea Plus) study. Briefly, the PREDIMED-Plus study is an ongoing randomized, parallel-group, 6-year multicenter, controlled trial designed to assess the effect of lifestyle interventions on the primary prevention of cardiovascular disease. The primary aim of the trial is to assess the effects of an intensive weight loss intervention based on an energy-reduced Mediterranean diet (MedDiet), physical activity promotion, and behavioral support (intervention group) compared to usual care and dietary counseling only with an energy-unrestricted MedDiet (control group) on the prevention of cardiovascular events. Details of the design and methods of PREDIMED-Plus have been previously described [35, 36] and are available at https://www.predimedplus.com/.
Ethics, consent, and permissions
The PREDIMED-Plus study protocol and procedures were approved by the Research Ethics Committees from each of the participating centers, and the study was registered with the International Standard Randomized Controlled Trial Registry (ISRCTN; ISRCTN89898870). All participants provided written informed consent.
Study participants
PREDIMED-Plus participants were recruited from 23 centers across Spain between September 2013 and December 2016. A total of 6874 adults met the eligibility criteria and were randomly allocated in a 1:1 ratio to either the intervention or the control group. Couples sharing the same household were randomized together, and the couple was used as a unit of randomization. Eligible participants were community-dwelling adults (aged 55 to 75 for men; 60 to 75 for women) with overweight or obesity (BMI: 27 to 40 kg/m2) who met at least three criteria for metabolic syndrome (35), without previous cardiovascular events or diagnosed neurodegenerative diseases at baseline. All participants provided written informed consent.
The present longitudinal analysis involves a sub-study conducted in 10 of the 23 PREDIMED-Plus recruiting centers. Of the participants in the PREDIMED-Plus sub-study who had a completed validated 32-item Spanish fluid intake questionnaire, participants were excluded if they did not have a completed baseline Food Frequency Questionnaire (FFQ) or who reported implausible total energy intakes based on those proposed by Willet (≤ 500 and ≤ 3500 kcal/day in women and ≤ 800 and ≤ 4000 kcal/day in men) [37]. For the water and fluid intake analyses, using the interquartile range method (using a 1.5 multiplier for the first and third quartiles), participants with extreme intakes of fluid (daily fluid intakes for men < 188 mL or > 3862 mL and women < 263 mL or > 3539 mL) were excluded for the assessment of water and fluid intakes. Similarly, participants without blood sample values for urea, sodium, potassium, glucose, and serum osmolarity values < 100 mmol/L were also excluded from the analyses of hydration status and cognition. Furthermore, associations were tested for those participants who had completed the various cognitive tests.
Assessment of water and fluid intake
A validated, semi-quantitative 32-item Beverage Intake Assessment Questionnaire (BIAQ) [10] and a 143-item validated semi-quantitative FFQ (38) specifying usual portion sizes, were administered by trained dietitians to assess habitual fluid and dietary intakes, respectively. These two questionnaires have been validated within populations of older, Spanish individuals, which are analogous to the current study population, and both have been found to be reproducible with relative validity [10, 38]. The BIAQ recorded the frequency of consumption of various beverage types during the month prior to the visit date. The average daily fluid intake from beverages was estimated from the servings of each type of beverage. The questionnaire items on beverages included: tap water, bottled water, natural fruit juices, bottled fruit juices, natural vegetable juices, bottled vegetable juices, whole milk, semi-skimmed milk, skimmed milk, drinking yogurt, milkshakes, vegetable drinks, soups, jellies and sorbets, soda, light/zero soda, espresso, coffee, tea, beer, non-alcoholic beer, wine, spirits, mixed alcoholic drinks, energy drinks, sports drinks, meal replacement shakes, and other beverages. The water and nutrient contents of the beverages were estimated mainly using the CESNID Food Composition Tables [39], complemented with data from the BEDCA Spanish Database of Food Composition [40].
The FFQ collected data on food intake based on the year prior to the visit according to nine possible frequency categories, which ranged from “never or almost never” to “> 6 portions/day” and based on the dietary guidelines for the Spanish population [41]. The information collected was converted into grams per day, multiplying portion sizes by consumption frequency and dividing the result by the period assessed. Ten food groups composed of vegetables, fruits, legumes, cereals, dairy beverages, meat and poultry, fats, nuts, fish/seafood, and other foods were determined to assess the contribution of foods to total water intake. Food groups and energy intake were estimated using Spanish food composition tables [42, 43]. Drinking water intake, water intake from all fluids, total water intake, EFSA total fluid water intake (TFWI), and EFSA total water intake (TWI) were computed (descriptions summarized in Additional file 1: Table S1). Drinking water intake was estimated based on tap and bottled water intakes based on BIAQ responses. Water intake from all fluids was computed from tap and bottled water, plus water from other beverages based on responses to the BIAQ. Total water intake encompassed water intake from all fluids in addition to water present in food sources based on responses to the FFQ. Water intake was further categorized based on established reference values. The EFSA recommendations for total water intake (EFSA TWI) for older adults (2.5 L/day and 2.0 L/day for men and women, respectively) in conditions of moderate environmental temperature and moderate physical activity [20] were used as reference values. Further categorizations were determined based on total water intake from fluids alone, based on EFSA recommendations (EFSA TFWI), where recommended levels for older adults are set to at least 2.0 L/day and 1.6 L/day for men and women, respectively [20].
Assessment of hydration status
Hydration status was estimated based on calculated serum osmolarity (SOSM), which is considered a more reliable biomarker of hydration status than urinary markers in older adults [44]. Fasting serum glucose, urea, sodium, and potassium were measured by standard methods. Blood urea nitrogen was determined from urea values using the conversion factor of 0.357 and reported in mmol/L. With all relevant serum analyte measures presented in mmol/L, SOSM was estimated using the following equation [45]:
Based on this equation, dehydration, impending dehydration, and hydrated statuses were defined as SOSM > 300, 295–300, and < 295 mmol/L, respectively [28, 46].
Assessment of cognitive performance
A battery of 8 neuropsychological tests assessing different cognitive domains was administered at baseline and 2-year follow-up by trained staff to assess cognitive performance. The following tests were assessed: the Mini-Mental State Examination (MMSE), two Verbal Fluency Tests (VFTs), two Digit Span Tests (DSTs) of the Wechsler Adult Intelligence Scale-III (WAIS-III), the Clock Drawing Test (CDT), and two Trail Making Tests (TMTs).
Briefly, a Spanish-validated version of the MMSE questionnaire, a commonly used cognitive screening test, was used in the present analysis [47]. A higher MMSE score indicates better cognitive performance [48]. Verbal ability and executive function were evaluated using the VFTs, which consist of two parts: the semantic verbal fluency task-animal category version (VFT-a) and the phonemic verbal fluency task-letter “p” version (VFT-p) [49]. The DST of the WAIS-III Spanish version assessed attention and memory. The DST Forward Recall (DST-f) is representative of attention and short-term memory capacity, and the DST Backward Recall (DST-b) is considered as a test of working memory capacity [50, 51]. The CDT-validated Spanish version was mainly used to evaluate visuospatial and visuo-constructive capacity [52,53,54]. Lastly, the TMT, another tool often used to assess executive function, consists of two parts. Part A (TMT-A) assessed attention and processing speed capacities, and part B (TMT-B) further examined cognitive flexibility [55]. All instruments included in the cognitive battery have been standardized for the Spanish population in the age range of the study population.
To assess overall cognitive function, a global cognitive function (GCF) score was determined as the main outcome measure, in addition to evaluating the individual neuropsychological tests (supplementary analyses). Raw scores at baseline and scores of changes at 2 years of follow-up for each individual cognitive assessment, as well as GCF, were standardized using the mean and standard deviation from the baseline measurements as normative data, creating z-scores [56]. GCF was calculated as a composite z-score of all 8 assessments, adding or subtracting each individual test value based on whether a higher score indicates higher or lower cognitive performance, respectively, using the formula:
Covariate assessment
The trained staff collected baseline socio-demographic (i.e., sex, age, education level, and civil status) and lifestyle (i.e., physical activity, total energy intake, alcohol intake, caffeine consumption [57], sleeping habits, and smoking status) related variables, as well as information about medication use, in face-to-face interviews using self-reported general questionnaires and a 143-item validated semi-quantitative FFQ for the dietary related variables [38], which were further estimated using Spanish food composition tables [42, 43]. Leisure time physical activity was estimated using the validated Minnesota-REGICOR Short Physical Activity questionnaire [58]. These socio-demographic and lifestyle variables were considered as possible covariates because of reports that younger adults, women, individuals with higher educational attainment, married, more active, greater consumers, and non-smokers tend to consume higher amounts of fluids from beverages and foods and hence more likely to meet recommendations on water intake [59, 60]. Alcohol was accounted for as a potential covariate as it may act as a diuretic at certain levels [61] as well as being associated with an elevated risk of dementia when consumed regularly [62]. Similarly, caffeinated beverages may have a mild diuretic effect [63], as well as may affect attention and alertness [64] and could be associated with reduced cognitive decline and dementia risk [65]. Sleeping habits have also been associated with cognitive health [66]. Anthropometric measures, including weight and height were measured by trained staff using calibrated scales and wall-mounted stadiometers, respectively. Body mass index (BMI), which may modify the relationship between water intake and hydration status [67], was calculated as weight in kilograms divided by height in meters squared. History of chronic disease (i.e., type 2 diabetes, hypertension, and hypercholesterolemia) was self-reported or collected from patient medical records and included as these conditions may cause fluid imbalance, cause dehydration, and have been associated with cognitive performance possibly leading to mild cognitive impairment [68,69,70]. Depressive symptomatology was evaluated using the Beck Depression Inventory-II (BDI-II), given the association observed with cognitive health [71] where depressive symptomatology risk was established as a score ≥ 14 [72].
Statistical analyses
For the present analyses, a prospective cohort study was conducted within the framework of the PREDIMED-Plus study using the database updated to December 22, 2020. Participants were categorized into quantiles based on baseline water intake (drinking water, water intake from all fluids, total water intake), recommended categories of water intake (EFSA TWI, EFSA, TFWI), and hydration status according to serum osmolarity. Baseline characteristics of participants for each category and quantile of water intake and hydration status were presented as numbers and percentages using Pearson’s chi-square test for categorical variables and means ± standard deviations or median (interquartile range [P25–P75]) using one-way ANOVA or Kruskal Wallis test for continuous variables, as appropriate.
Multivariable linear regression models were fitted to assess longitudinal associations comparing the 2-year change in cognitive function across baseline variables of hydration status and water intake and for meeting the EFSA recommendations for TWI and TFWI [20]. When analyses were performed with categorical variables, p for trend was calculated. The p for linear trend was calculated by assigning the median value of each category and modeling it as a continuous variable.
Multivariable linear regression models were adjusted for several potential confounders. Model 1 adjusted for age (years), sex (man or woman), intervention group, participating center size (< 100, 100 to < 150, 150 to < 200, ≥ 200 participants for hydration status and < 100, 100 to < 200, 200 to < 300, ≥ 300 participants for fluid-related analyses), respective baseline cognitive function score, and corrected for clusters (to account for couples living in the same household being randomized as a single unit). Model 2 was additionally adjusted for BMI (kg/m2), educational level (primary, secondary, or college), civil status (single, divorced or separated, married, widower), smoking status (current, former, or never), and physical activity (METs/min/day). Model 3 was additionally adjusted for sleeping habits (hours of nighttime sleep), depressive symptomatology (yes/no), diabetes prevalence (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), total energy intake (kJ per day), alcohol consumption in g/day (and adding the quadratic term), and caffeine intake (g/day). To assess the linear trend, the median value of each category of exposure variables (hydration status and various assessments of water and fluid intake) was assigned to each participant and was modeled as continuous variables in linear regression models. The Bonferroni correction was used to correct for multiple comparisons and reduce the risk of a type 1 error.
Several stratified and sensitivity analyses were additionally performed to test the robustness of the findings. First, sex-stratified regression approaches were employed to examine the relationships between hydration status and these water and fluid intake categories and 2-year changes in global cognitive function. Sensitivity analyses were additionally performed, testing the addition of estimated glomerular filtration rate (eGFR), an indicator of renal function derived based on serum creatinine level, age, and sex [73], or dietary intake covariates (amount [g/day] of vegetables, fruits, legumes, grains, non-fluid dairy, meat, oils, fish, nuts, and pastries determined via the validated 143-item semi-quantitative FFQ [19]) to the multivariable models, but also after removal of participants with baseline MMSE < 24 (mild dementia and poorer) [74], or the removal of participants with extreme GCF z-scores at baseline (< 5% and > 95%).
The data were analyzed using the Stata 14 software program (StataCorp LP, TX, USA), and the results were originally considered statistically significant at a p-value (2-tailed) < 0.05, and after the Bonferroni correction, statistical significance was considered at a p-value (2-tailed) < 0.005.
Results
A total of 1957 participants (mean age 65.0 ± 4.9 years and 50.5% women) were available for the assessment of water and fluid intake and 1192 participants for the assessment of hydration status after excluding missing values or implausible data (Additional file 1: Fig. S1). Table 1 presents the baseline characteristics of the study population according to sex, water (tap and bottle) intake amount, and hydration status. The median (range) consumption of drinking water intake in men and women was 900 (0 to 3100) and 900 (0 to 2700) mL/day, respectively. Compared with participants in the group with the lowest drinking water intake (< 500 mL/day), those with the highest drinking water intake (1.8 to 3.1 L/day) were more likely to be younger (p < 0.001), have a higher BMI (p = 0.001), and have a lower alcohol intake (p < 0.001). Compared to participants considered to be hydrated according to serum osmolarity status, participants considered to be dehydrated tended to be older (p = 0.008), women (p = 0.008), have type 2 diabetes (p < 0.001), have depressive symptoms (p = 0.025), and less likely to drink alcohol (p = 0.002). Participants involved in the present analyses did not differ from the rest of the participants enrolled in the PREDIMED-Plus trial in terms of age, sex, BMI, and prevalence of obesity and type 2 diabetes (p > 0.05 for all comparisons). Furthermore, 80% of participants (69% of men and 90% of women) met the EFSA fluid intake recommendations based on questionnaire responses, yet serum osmolarity values indicated over 50% of participants were physiologically dehydrated with only 10% of participants being considered physiologically hydrated based on serum osmolarity levels.
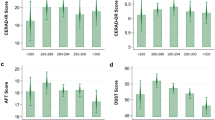
Figures 1 and 2 summarize the multivariable-adjusted β-coefficients (95% CIs) of water intake and hydration status categorically and continuously, respectively, with 2-year changes in GCF z-scores (full details from all models are presented in Additional file 1: Tables S2-S4). Categorical analyses showed a non-significant trend towards participants considered to have a dehydrated status (β: − 0.11; 95% CI: − 0.24 to 0.02; p for trend = 0.058) to have a greater decline in global cognitive function compared to those who were considered hydrated (Fig. 1). No significant associations were observed between the various classifications of water intake (i.e., drinking water, all fluids, water from beverage and food sources, and based on ESFA water and fluid recommendations) and 2-year changes in GCF in the multivariable-adjusted models. The results of the continuous linear regression analyses suggest the presence of a significant association between hydration status and global cognitive decline over a 2-year period (β: − 0.10; 95% CI: − 0.02 to − 0.004; p = 0.002) (Fig. 2).
Hydration status, water and fluid intakes categorically with 2-year changes in global cognitive function (z-scores). Data are presented as beta-coefficients and 95% CI. Multivariable linear regression models were adjusted for baseline covariates, including baseline GCF score, age (years), sex, intervention PREDIMED-Plus randomized group, and participating center (for hydration status: ≤ 100, 100 to < 150, 150 to < 200, > 200 participants; for fluid-related exposures: ≤ 100, 100 to < 200, 200 to < 300, > 300 participants), body mass index (kg/m2), educational level (primary, secondary, or college), civil status (single, divorced or separated, married, widower), smoking habit (current, former, or never), physical activity (METs/min/day), sleep status (hours per day), depressive symptomatology (yes/no), diabetes prevalence (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), energy intake (kcal/day), alcohol consumption in g/day (and adding the quadratic term), and caffeine intake (mg/day). aHydration status refers to serum osmolarity, where dehydration, impending dehydration, and hydrated statuses were defined as SOSM > 300, 295–300, and < 295 mmol/L, respectively. bDrinking water refers to tap and bottled water intakes. cWater, all fluids refers to tap and bottled water, plus water from other beverages and fluid food sources, such as soups and smoothies. dTotal water refers to water from all fluids in addition to water present in food sources. eEFSA TFWI refers to the recommended levels of total fluid water intake for older adults at 2.0 L/day and 1.6 L/day for men and women, respectively. fEFSA TWI refers to the recommended levels of total water intake, from fluids and foods, for older adults at 2.5 L/day and 2.0 L/day for men and women, respectively. Abbreviations: EFSA, European Food Safety Authority; TFWI, total fluid water intake; TWI, total water intake; SOSM, serum osmolarity
Hydration status, water and fluid intakes continuously with 2-year changes in global cognitive function (z-scores). Data are presented as beta-coefficients and 95% CI. Multivariable linear regression models were adjusted for baseline covariates, including baseline GCF score, age (years), sex, intervention PREDIMED-Plus randomized group, and participating center (≤ 100, 100 to < 150, 150 to < 200, > 200 participants), body mass index (kg/m2), educational level (primary, secondary, or college), civil status (single, divorced or separated, married, widower), smoking habit (current, former, or never), physical activity (METs/min/day), sleep status (hours per day), depressive symptomatology (yes/no), diabetes prevalence (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), energy intake (kcal/day), alcohol consumption in grams/day (and adding the quadratic term), and caffeine intake (mg/day). Beta represents the changes in global cognitive function, expressed as z-scores, with each hydration or fluid intake component continuously. Bonferroni correction analyses have been run to correct for multiple comparisons and indicate a p < 0.005 is statistically significant
Additional file 1: Tables S2-S4 show the unadjusted and multivariable-adjusted β-coefficients (95% CIs) of water and fluid intake (from both beverage and food sources, assessed individually and combined), as well as hydration status with changes in the global and individual assessments of cognitive function over a 2-year period. When each neuropsychological test was investigated separately, participants with the highest category of intake of drinking water (1.0 to 1.5 L/day) non-statistically significantly presented with a 0.17-point increase (β: 0.17; 95% CI: 0.02 to 0.32; p for trend = 0.021) in DST-f z-score compared to those with the lowest water intake (< 0.5 L/day) over a 2-year period (Additional file 1: Table S2). Total fluid intake showed similar findings where participants in the highest category of intake of total fluid water (2.5 L, range 2.2 to 3.4 L/day) presented with a 0.12-point increase (β: 0.12; 95% CI: 0 to 0.24; p for trend = 0.041) in DST-f z-score compared to those with the lowest water intake (1.1 L, range 0.4 to 1.4 L/day) over a 2-year period (Additional file 1: Table S2). No other associations in the multivariable-adjusted categorical or continuous analyses were observed. Furthermore, post hoc analyses of available data showed no significant differences in changes over time in the various fluid intake assessment variables (over a 2-year duration) or hydration status (over a 1-year duration) (p > 0.005) among the participants.
Stratified analyses
When the analyses were stratified by sex, no changes in significance were observed with the associations between water and fluid intakes, in either categorical or continuous investigations, and global cognitive function. However, when hydration-related analyses were restricted to men or women participants, findings were attenuated when women only were assessed both categorically and continuously (p > 0.005), whereas in men those with a SOSM ≥ 300 mmol/L, indicating a dehydrated status, showed a higher cognitive decline over the 2-year period compared to those hydrated (β: − 0.20, 95% CI: − 0.36 to − 0.04, p = 0.012 and p-trend = 0.002), and the significance remained in the continuous analyses (β: − 0.013, 95% CI: − 0.022 to − 0.004, p = 0.004) (Additional file 1: Figs. S2-S3).
Sensitivity analyses
Regarding the sensitivity analyses, associations additionally adjusted for eGFR did not significantly modify the findings (Additional file 1: Tables S5-S7). Moreover, associations additionally adjusted for intake of dietary variables, where applicable, including the amount (g/day) of vegetables, fruits, legumes, grains, non-fluid dairy, meat, oils, fish, nuts, and pastries did not significantly modify the findings (Additional file 1: Tables S5-S7). Furthermore, the main results did not substantially change after the removal of extreme GCF baseline z-scores (< 5% and > 95%). Whereas following the removal of participants with a baseline MMSE score < 24 (n = 51), the categorical association of hydration status with GCF became greater showing a more dehydrated status related to greater 2-year global cognitive decline compared to hydrated participants (p-trend = 0.046), and the association of the continuous variables remained significant (p = 0.002). The findings related to water and fluid intake did not substantially change with the removal of participants with a baseline MMSE score < 24 (Additional file 1: Tables S5-S7).
Discussion
To the best of our knowledge, this is the first multi-year prospective cohort study to assess the association between water intake (from fluid and food sources) and hydration status, with subsequent changes in cognitive performance in older Spanish adults with metabolic syndrome and overweight or obesity. In this large sample of older Spanish adults, poorer hydration status was associated with greater global cognitive decline over a 2-year period, particularly in men, whereas water intake, from fluid and/or food sources, and meeting related EFSA recommendations, was not associated with global cognitive function. Nonetheless, when results of cognitive function tests were considered independently, water intake (> 1.5 L/day compared to < 0.5 L/day from tap or bottled water and 2.2 to 4.4 L/day compared to < 1.4 L/day of water from all fluid sources) was related to better attention and short-term memory, as assessed by DST-f, over a 2-year period.
Despite the general acknowledgment that an appropriate level of fluid intake and hydration status is important for health, there have been limited investigations to date assessing the relationship between fluid intake or hydration status and cognitive function. Existing evidence suggests that good hydration status may be associated with better cognitive test results and that mild, induced dehydration can impair cognitive abilities [75], but findings are not consistent and there are only a few studies exploring the relationship of hydration status and hardly any assessing amount of water intake, with cognitive performance in older community-dwelling adults.
Of the few relevant and recent studies that have been conducted, one cross-sectional analysis of 2506 community-dwelling older American adults (aged ≥ 60 years) from the Nutrition and Health Examination Survey (NHANES) 2011–2014 cycles assessed both hydration status and water intake in relation to cognitive performance [34]. In comparison with the present findings, this study found women (but not men) considered to be hydrated, based on a SOSM level between 285 and 289 mmol/L, had better attention and processing speeds, based on a Digit Symbol Substitution test (DSST), than women not at optimal hydration [34]. These cross-sectional findings differ from the present observations where global cognitive function, but not individual tests related to attention and processing, was associated with hydration status. Whereas, correspondingly, water intake (but not hydration status) was positively associated with DST-f, which is similar to the DSST in that it is an indicator of attention as well as short-term memory capacity, and this was seen across all older adults (both men and women). Additionally, in the NHANES study, cognitive test scores were significantly lower among adults who failed to meet EFSA recommendations on adequate intake (AI) of water in bivariate analyses, yet this significance was attenuated in the multivariable analyses among both women and men. Yet, using the alternative AI of daily water intake of 1500 mL or more, which is comparable to the highest drinking water intake group in the present study, women scored higher on the Animal Fluency Test, a measure of verbal fluency and hence executive function, and DSST than women with intake levels below this amount, and findings among men trended in the same direction [34].
Similarly, hydration status has been associated with cognitive function in two cross-sectional studies of older community-dwelling adults by Suhr and colleagues [32, 33]. First, Suhr et al. showed that in 28 healthy community-dwelling older adults (aged 50 to 82 years), a lower hydration status, determined in this study via total body water measured using the bioelectrical impedance method, was related to a decreased psychomotor processing speed, poorer attention, and memory [33]. A second cross-sectional study by Suhr et al. [32] conducted in 21 postmenopausal women (aged 50 to 78 years) reported a positive association between total body water, also measured by the bioelectrical impedance method, and working memory or memory skills.
Moreover, the possible relation between hydration and cognitive function shown by the present findings and demonstrated by the abovementioned cross-sectional studies in older adults align with acute heat- and exercise-induced dehydration studies in younger populations, as illustrated by a meta-analysis of 33 trials (pre-post or crossover design) of 413 adults free of disease (aged < 45 years). It should be noted that these studies assessed dehydration over a period of less than 72 h with 27 (81%) of the studies including only men participants, and 25 (76%) studies involving recreationally or highly athletic individuals. The authors concluded that despite variability among the included studies, dehydration impaired cognitive performance, particularly for tasks involving attention, executive function, and motor coordination when water deficits exceeded 2% body mass loss [76].
Conversely, a cross-sectional study conducted in Poland among 60 community-dwelling older adults (aged 60 to 93 years) found no significant relationship between cognitive performance, as assessed using the MMSE, TMT, and the Babcock Story Recall Test, and hydration status as assessed by urine specific gravity [31]. The discrepancy between the findings from this cross-sectional study and the present PREDIMED-Plus analyses might be because all participants in the cross-sectional study from Poland were considered to be adequately hydrated and hence the authors of that study could not assess the impact of a dehydrated state on cognition.
A noteworthy consideration when interpreting the literature and the main findings of the current study for practical use and in the determination of potential mechanisms of action is the distinction between water intake and water balance (related to hydration status) within the body. When homeostasis of fluids within the body is disrupted, modifying water intake may impact cognitive function, yet due to the dynamic complexity of body water regulation impacting hydration status may be dependent on individualized physiological water intake needs [8]. Thus, while the biological mechanism by which water intake and a hydrated status may reduce the risk of cognitive decline is unclear, evidence suggests that aspects related to hydration and fluid homeostasis or a lack thereof, such as hormone regulation and changes in brain structure, could be a key underlying factor.
Several mechanisms regulate water intake and output to maintain serum osmolarity, and hence hydration status, within a narrow range. Elevated blood osmolarity resulting in the secretion of antidiuretic hormone (ADH), also known as vasopressin or arginine vasopressin, a peptide hormone which acts primarily in the kidneys to increase water reabsorption, is one such mechanism that works to return osmolarity to baseline and preserve fluid balance [77]. In addition to its role in mediating the physiological functions related to water reabsorption and homeostasis, evidence has suggested that ADH participates in cognitive functioning [78] and that the associated cognitive modulations may further interplay with sex hormones [79]. Antidiuretic hormone may be influenced by the androgen sex hormone, which is generally more abundant in the brains of males than in females [80]. As a result, the impact of ADH on cognition could be greater in males [80].
Exercise- and heat-induced acute dehydration studies implicate possible modifications to the brain structure as another potential mechanism of action for an association between water intake, hydration status, and cognitive function. Evidence has proposed that acute dehydration can lead to a reduction in brain volume and subtle regional changes in brain morphology such as ventricular expansion, effects that may be reversed following acute rehydration [81, 82]. Acute dehydration studies have further implicated hydration status in affecting cerebral hemodynamics and metabolism resulting in declines in cerebral blood flow and oxygen supply [83, 84]. A lower vascular and neuronal oxygenation could potentially compromise the cerebral metabolic rate for oxygen, thereby contributing to reductions in cognitive performance [81, 85,86,87,88]. Nonetheless, other potential unknown mechanisms cannot be disregarded.
There are several limitations and strengths of the present analyses that need to be acknowledged. The first notable limitation is that the results may not be generalizable to other populations since the participants are older Spanish individuals with metabolic syndrome and overweight or obesity. Second, measurement error and recall bias are possibilities given the use of questionnaires to estimate water and fluid intake and that these rely on responders’ memory which is a component of cognitive function. However, these questionnaires have been validated and determined as reliable methods of assessing long-term intake in the present study population [37, 38]. Third, despite its longitudinal design, water and fluid intake and hydration status were only considered at baseline; however, as the questionnaires measure habitual beverage and food intake, and older adults are considered to have reasonably stable dietary habits [37, 38], this is not expected to significantly impact the findings. Along these lines, the possible effect of seasonality on water intake and osmolarity was not considered a concern in the present analyses as the validation of the fluid questionnaire measurements included assessments at various points throughout the year (baseline vs. 6 months vs 1 year) with no significant differences observed in fluid consumption across the different time points assessed [10]. Hence, the finding of no difference between 6-month intervals, suggests no significant differences between opposing seasons (e.g., winter vs. summer; spring vs. fall). Furthermore, SOSM determination may not necessarily detect acute dehydration or rehydration immediately prior to the cognitive testing, and it is unknown whether observed elevated SOSMs were due to inadequate water intake, ADH abnormality, or other factors. While it is possible that the hydration status of some individuals was misclassified because serum osmolarity was estimated as opposed to being directly measured, the equation has been shown to predict directly measured serum osmolarity well in older adult men and women with and without diabetes or renal issues with a good diagnostic accuracy of dehydration and has been considered a gold standard for the identification of impending and current water-loss dehydration in older adults [44, 45, 89,90,91]. Lastly, a discrepancy was observed between the percentage of individuals that were considered to have met EFSA fluid intake recommendations and those considered to be dehydrated based on calculated osmolarity. This may have been due to the fact that the EFSA fluid intake recommendations are meant for individuals in good health [20]; whereas the present study population had overweight or obesity, and it has been shown that individuals with higher BMIs have higher water needs related to metabolic rate, body surface area, body weight, and water turnover rates related to higher energy requirements, greater food consumption, and higher metabolic production [92]. Strengths of the present analyses include the longitudinal, prospective design, the large sample size, the use of an extensive cognitive test battery, the use of validated questionnaires, and the robustness of the current findings due to the adjustment of relevant covariates.
Conclusions
Findings suggest that hydration status, specifically poorer hydration status, may be associated with a greater decline in global cognitive function in older adults with metabolic syndrome and overweight or obesity, particularly in men. Further prospective cohort studies and randomized clinical trials are required to confirm these results and to better understand the link between water and fluid intake, hydration status, and changes in cognitive performance to provide guidance for guidelines and public health.
Availability of data and materials
The dataset supporting the conclusions of this article is available upon request pending application and approval of the PREDIMED-Plus Steering Committee. There are restrictions on the availability of data for the PREDIMED-Plus trial, due to the signed consent agreements around data sharing, which only allow access to external researchers for studies following the project purposes. Requestors wishing to access the PREDIMED-Plus trial data used in this study can make a request to the PREDIMED-Plus trial Steering Committee chair: jordi.salas@urv.cat. The request will then be passed to members of the PREDIMED-Plus Steering Committee for deliberation.
Abbreviations
- ADH:
-
Antidiuretic hormone
- AI:
-
Adequate intake
- BDI-II:
-
Beck Depression Inventory-II
- BEDCA:
-
Base de Datos Española de Composición de Alimentos
- BIAQ:
-
Beverage Intake Assessment Questionnaire
- BMI:
-
Body mass index
- CESNID:
-
El Centro de Enseñanza Superior de Nutrición y Dietética
- CDT:
-
Clock Drawing Test
- CI:
-
Confidence interval
- DRV:
-
Dietary reference value
- DSST:
-
Digit Symbol Substitution Test
- DST-b:
-
DST Backward Recall
- DST-f:
-
DST Forward Recall
- EFSA:
-
European Food Safety Authority
- eGFR:
-
Estimated glomerular filtration rate
- En:
-
Energy
- FFQ:
-
Food Frequency Questionnaire
- GCF:
-
Global cognitive function
- IOM:
-
Institute of Medicine
- ISRCTN:
-
International Standard Randomized Controlled Trial Registry
- MedDiet:
-
Mediterranean diet
- METs:
-
Metabolic equivalents
- MMSE:
-
Mini-Mental State Examination
- NHANES:
-
Nutrition and Health Examination Survey
- PREDIMED:
-
PREvención con DIeta MEDiterránea
- Q:
-
Quartile
- SOSM:
-
Serum osmolarity
- TFWI:
-
Total fluid water intake
- TMT-A:
-
Trail Making Test Section A
- TMT-B:
-
Trail Making Test Section B
- TWI:
-
Total water intake
- VFT-a:
-
Verbal Fluency Test Animals Category
- VFT-p:
-
Verbal Fluency Test Letter “p” Category
- WAIS-III:
-
Wechsler Adult Intelligence Scale-III
References
Gauthier S, Rosa-Neto P, Morais JA, Webster C. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London: Alzheimer´s Disease International; 2021.
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment report of the guideline development, dissemination, and implementation. Neurology. 2018;90:126–35.
Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006–15.
Johnson EC, Adams WM. Water intake, body water regulation and health. Nutrients. 2020;12:702.
Lorenzo I, Serra-Prat M, Carlos YJ. The role of water homeostasis in muscle function and frailty: a review. Nutrients. 2019;11:1857–72.
Jéquier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64:115–23.
Kleiner SM. Water. J Am Diet Assoc. 1999;99:200–6.
Armstrong L, Johnson E. Water intake, water balance, and the elusive daily water requirement. Nutrients. 2018;10:1928.
Nissensohn M, Sánchez-Villegas A, Ortega RM, Aranceta-Bartrina J, Gil Á, González-Gross M, et al. Beverage consumption habits and association with total water and energy intakes in the Spanish population: findings of the ANIBES study. Nutrients. 2016;8:232–50.
Ferreira-Pêgo C, Nissensohn M, Kavouras SA, Babio N, Serra-Majem L, Águila AM, et al. Relative validity and repeatability in a Spanish population with metabolic syndrome from the PREDIMED-PLUS study. Nutrients. 2016;8:457–70.
Nissensohn M, Sánchez-Villegas A, Galan P, Turrini A, Arnault N, Mistura L, et al. Beverage consumption habits among the European population: association with total water and energy intakes. Nutrients. 2017;9:383–96.
Rolls BJ, Phillips PA. Aging and disturbances of thirst and fluid balance. Nutr Rev. 2009;48:137–44.
Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL. Age and hypohydration independently influence the peripheral vascular response to heat stress. J Appl Physiol. 1990;68:1902–8.
Begg DP. Disturbances of thirst and fluid balance associated with aging. Physiol Behav. 2017;178:28–34.
Hooper L, Bunn D, Jimoh FO, Fairweather-Tait SJ. Water-loss dehydration and aging. Mech Ageing Dev. 2014;136–137:50–8.
Lieberman HR. Hydration and cognition: a critical review and recommendations for future research. J Am Coll Nutr. 2007;26:555S–61S.
Murray B. Hydration and physical performance. J Am Coll Nutr. 2007;26:542S–8S.
AESAN Scienitific Committee, Alfredo Martínez Hernández J, Cámara Hurtado M, Maria Giner Pons R, González Fandos E, López García E, et al. Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) de revisión y actualización de las Recomen-daciones Dietéticas Para la población española. Revista del Comité Científico de la AESAN. 2020;32:11–58.
U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020-2025. 9th ed; 2020.
EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for water. EFSA J. 2010;8:1459–507.
Institute of Medicine. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, D.C.: National Academies Press; 2005.
Gandy J. Water intake: validity of population assessment and recommendations. Eur J Nutr. 2015;54 Suppl 2:S11–6.
Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.
Volkert D, Chourdakis M, Faxen-Irving G, Frühwald T, Landi F, Suominen MH, et al. ESPEN guidelines on nutrition in dementia. Clin Nutr. 2015;34:1052–73.
National Institute for Health and Care Excellence (NICE). Dementia, disability and frailty in later life-mid-life approaches to delay or prevent onset. NICE guideline; 2015. p. 1–56. www.nice.org.uk/guidance/ng16
Armstrong-Esther CA, Browne KD, Armstrong-Esther DC, Sander L. The institutionalized elderly: dry to the bone! Int J Nurs Stud. 1996;33:619–28.
Marra MV, Simmons SF, Shotwell MS, Hudson A, Hollingsworth EK, Long E, et al. Elevated serum osmolality and total water deficit indicate impaired hydration status in residents of long-term care facilities regardless of low or high body mass index. J Acad Nutr Diet. 2016;116:828–36.
Hooper L, Bunn DK, Downing A, Jimoh FO, Groves J, Free C, et al. Which frail older people are dehydrated? The UK DRIE study. J Gerontol Ser A Biol Sci Med Sci. 2016;71:1341–7.
Lauriola M, Mangiacotti A, D’Onofrio G, Cascavilla L, Paris F, Paroni G, et al. Neurocognitive disorders and dehydration in older patients: clinical experience supports the hydromolecular hypothesis of dementia. Nutrients. 2018;10:562–76.
Majdi M, Hosseini F, Naghshi S, Djafarian K, Shab-Bidar S. Total and drinking water intake and risk of all-cause and cardiovascular mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Int J Clin Pract. 2021;75:e14878.
Bialecka-Dębek A, Pietruszka B. The association between hydration status and cognitive function among free-living elderly volunteers. Aging Clin Exp Res. 2019;31:695–703.
Suhr JA, Patterson SM, Austin AW, Heffner KL. The relation of hydration status to declarative memory and working memory in older adults. J Nutr Health Aging. 2010;14:840–3.
Suhr JA, Hall J, Patterson SM, Niinistö RT. The relation of hydration status to cognitive performance in healthy older adults. Int J Psychophysiol. 2004;53:121–5.
Bethancourt HJ, Kenney WL, Almeida DM, Rosinger AY. Cognitive performance in relation to hydration status and water intake among older adults, NHANES 2011–2014. Eur J Nutr. 2020;59:3133–48.
Martínez-González MA, Buil-Cosiales P, Corella D, Bulló M, Fitó M, Vioque J, et al. Cohort profile: design and methods of the PREDIMED-plus randomized trial. Int J Epidemiol. 2019;48:387–388o.
Salas-Salvadó J, Díaz-López A, Ruiz-Canela M, Basora J, Fitó M, Corella D, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-plus trial. Diabetes Care. 2019;42:777–88.
Willett W. Nutritional epidemiology, 3rd edn. Chapter 11. 3rd ed. New York: Oxford University Press; 2013. p. 306.
Fernández-Ballart JD, Piñol JL, Zazpe I, Corella D, Carrasco P, Toledo E, et al. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr. 2010;103:1808–16.
Palma I, Farra A, Cantós D. Tablas de composición de alimentos por medidas caseras de consumo habitual en España. Madrid: S.A. McGraw-Hill/Interamericana de España; 2008.
RedBEDCA, AESAN. Base de Datos Española de Composición de Alimentos; 2006.
Grupo Colaborativo de la Sociedad Española de Nutrición Comunitaria (SENC), Aranceta Bartrina J, Arija Val V, Maíz Aldalur E, Martínez de la Victoria Muñoz E, Ortega Anta RM, et al. Dietary guidelines for the Spanish population (SENC, December 2016); the new graphic icon of healthy nutrition. Nutr Hosp. 2016;33(Suppl 8):1–48.
Moreiras O, Carbajal A, Cabrera L, Cuadrado C. Tablas de composición de alimentos Guía de prácticas [Food Composition Tables. Practice Guide]. 19th ed. Madrid: Piramide; 2018. p. 496.
Mataix Verdú J, García Diz L, Mañas Almendros M, Martinez de Vitoria E, Llopis González J. Tablas de composición de alimentos [Food composition tables]. 5th ed. Granada; Universidad de Granada; 2013.
Hooper L, Abdelhamid A, Attreed NJ, Campbell WW, Channell AM, Chassagne P, et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD009647.pub2.
Khajuria A, Krahn J. Osmolality revisited - deriving and validating the best formula for calculated osmolality. Clin Biochem. 2005;38:514–9.
Hooper L, Bunn DK, Abdelhamid A, Gillings R, Jennings A, Maas K, et al. Water-loss (intracellular) dehydration assessed using urinary tests: how well do they work? Diagnostic accuracy in older people. Am J Clin Nutr. 2016;104:121–31.
Blesa R, Pujol M, Aguilar M, Santacruz P, Bertran-Serra I, Hernández G, et al. Clinical validity of the “mini-mental state” for Spanish speaking communities. Neuropsychologia. 2001;39:1150–7.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. 3rd ed. Iowa: AJA Associates; 1994.
Wechsler D. Wechsler adult intelligence scale-III. San Antonio: Psychological Corporation; 1997.
Rossi L, Neer C-R, Lopetegui S. Escala de inteligencia Para adultos de WECHSLER. WAIS-III Índice de comprensión verbal. Normas Para los subtests: Vocabulario, analogías e información, Para la ciudad de La Plata Edades: 16 a 24 AñosRevista de Psicología; 2008. p. 223–36.
Aprahamian I, Martinelli JE, Neri AL, Yassuda MS. O Teste do Desenho do Relógio: Revisão da acurácia no rastreamento de demência. Dementia e Neuropsychologia. 2009;3:74–80.
Paganini-Hill A, Clark LJ. Longitudinal assessment of cognitive function by clock drawing in older adults. Dement Geriatr Cogn Dis Extra. 2011;1:75–83.
Del Ser QT, García De Yébenes MJ, Sánchez Sánchez F, Frades Payo B, Rodríguez Laso Á, Bartolomé Martínez MP, et al. Evaluación cognitiva del anciano. Datos normativos de una muestra poblacional española de más de 70 años. Med Clin (Barc). 2004;122:727–40.
Llinàs-Reglà J, Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, Torrents Rodas D, Garre-Olmo J. The trail making test: association with other neuropsychological measures and normative values for adults aged 55 years and older from a Spanish-speaking population-based sample. Assessment. 2017;24:183–96.
Brouwer-Brolsma EM, Dhonukshe-Rutten RAM, van Wijngaarden JP, van de Zwaluw NL, In’t Veld PH, Wins S, et al. Cognitive performance: a cross-sectional study on serum vitamin D and its interplay with glucose homeostasis in Dutch older adults. J Am Med Dir Assoc. 2015;16:621–7.
Paz-Graniel I, Babio N, Becerra-Tomás N, Toledo E, Camacho-Barcia L, Corella D, et al. Association between coffee consumption and total dietary caffeine intake with cognitive functioning: cross-sectional assessment in an elderly Mediterranean population. Eur J Nutr. 2021;60:2381–96.
Molina L, Sarmiento M, Peñafiel J, Donaire D, Garcia-Aymerich J, Gomez M, et al. Validation of the REGICOR short physical activity questionnaire for the adult population. PLoS One. 2017;12:e0168148.
Paz-Graniel I, Babio N, Serra-Majem L, Vioque J, Zomeño MD, Corella D, et al. Fluid and total water intake in a senior Mediterranean population at high cardiovascular risk: demographic and lifestyle determinants in the PREDIMED-plus study. Eur J Nutr. 2020;59:1595–606.
Drewnowski A, Rehm CD, Constant F. Water and beverage consumption among adults in the United States: cross-sectional study using data from NHANES 2005–2010. BMC Public Health. 2013;13:1068.
Polhuis KCMM, Wijnen AHC, Sierksma A, Calame W, Tieland M. The diuretic action of weak and strong alcoholic beverages in elderly men: a randomized diet-controlled crossover trial. Nutrients. 2017;9:660–73.
Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, et al. Alcohol consumption and dementia risk: a dose–response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:31–42.
Zhang Y, Coca A, Casa DJ, Antonio J, Green JM, Bishop PA. Caffeine and diuresis during rest and exercise: a meta-analysis. J Sci Med Sport. 2015;18:569–74.
van den Berg B, de Jong M, Woldorff MG, Lorist MM. Caffeine boosts preparatory attention for reward-related stimulus information. J Cogn Neurosci. 2020;33:104–18.
Chen JQA, Scheltens P, Groot C, Ossenkoppele R. Associations between caffeine consumption, cognitive decline, and dementia: a systematic review. J Alzheimers Dis. 2020;78:1519–46.
Xu W, Tan CC, Zou JJ, Cao XP, Tan L. Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2020;91:236–44.
Rosinger AY, Lawman HG, Akinbami LJ, Ogden CL. The role of obesity in the relation between total water intake and urine osmolality in US adults, 2009-2012. Am J Clin Nutr. 2016;104:1554–61.
You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 2021;58:671–85.
Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension: epidemiology, pathobiology, and treatment. Circ Res. 2019;124:1025–44.
An Y, Zhang X, Wang Y, Wang Y, Liu W, Wang T, et al. Longitudinal and nonlinear relations of dietary and serum cholesterol in midlife with cognitive decline: results from EMCOA study. Mol Neurodegener. 2019;14:51–70.
John A, Patel U, Rusted J, Richards M, Gaysina D. Affective problems and decline in cognitive state in older adults: a systematic review and meta-analysis. Psychol Med. 2019;49:353–65.
Sanz J, Luis A, Carmelo P, Resumen V. Adaptación española del Inventario Para la Depresión de Beck-II (BDI-II): 2. Propiedades psicométricas en población general the Spanish adaptation of Beck’s depression inventory-II (BDI-II): 2. Psychometric properties in the general population. Clínica y Salud. 2003;14:249–80.
National Institute of Diabetes and Digestive and Kidney Diseases. Estimating glomerular filtration rate. https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate/estimating. Accessed 1 Jun 2022.
Dementia Care Central, National Institute on Aging. Mini-Mental State Exam (MMSE) Alzheimer’s/Dementia Test: administration, accuracy and scoring. 2020. https://www.dementiacarecentral.com/mini-mental-state-exam/. Accessed 5 Jul 2021.
Masento NA, Golightly M, Field DT, Butler LT, van Reekum CM. Effects of hydration status on cognitive performance and mood. Br J Nutr. 2014;111:1841–52.
Wittbrodt MT, Millard-Stafford M. Dehydration impairs cognitive performance: a meta-analysis. Med Sci Sports Exerc. 2018;50:2360–8.
Kanbay M, Yilmaz S, Dincer N, Ortiz A, Sag AA, Covic A, et al. Antidiuretic hormone and serum osmolarity physiology and related outcomes: what is old, what is new, and what is unknown? J Clin Endocrinol Metab. 2019;104:5406–20.
Insel TR. Translating oxytocin neuroscience to the clinic: a National Institute of Mental Health perspective. Biol Psychiatry. 2016;79:153–4.
Lu Q, Lai J, Du Y, Huang T, Prukpitikul P, Xu Y, et al. Sexual dimorphism of oxytocin and vasopressin in social cognition and behavior. Psychol Res Behav Manag. 2019;12:337–49.
Bluthe R, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18:323–35.
Kempton MJ, Ettinger U, Schmechtlg A, Winter EM, Smith L, McMorris T, et al. Effects of acute dehydration on brain morphology in healthy humans. Hum Brain Mapp. 2009;30:291–8.
Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64:548–50.
Trangmar S, Chiesa S, Kalsi K, Secher N, González-Alonso J. Hydration and the human brain circulation and metabolism. Nutr Hosp. 2015;1:10261.
Rasmussen P, Nybo L, Volianitis S, Møller K, Secher NH, Gjedde A. Cerebral oxygenation is reduced during hyperthermic exercise in humans. Acta Physiol. 2010;199:63–70.
Piil JF, Lundbye-Jensen J, Trangmar SJ, Nybo L. Performance in complex motor tasks deteriorates in hyperthermic humans. Temperature. 2017;4:420–8.
Ogoh S. Relationship between cognitive function and regulation of cerebral blood flow. J Physiol Sci. 2017;67:345–51.
Ogoh S, Tsukamoto H, Hirasawa A, Hasegawa H, Hirose N, Hashimoto T. The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol Rep. 2014;2(9):e12163.
Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–559.
Hooper L, Abdelhamid A, Ali A, Bunn DK, Jennings A, John WG, et al. Diagnostic accuracy of calculated serum osmolarity to predict dehydration in older people: adding value to pathology laboratory reports. BMJ Open. 2015;5:e008846.
Lacey J, Corbett J, Forni L, Hooper L, Hughes F, Minto G, et al. A multidisciplinary consensus on dehydration: definitions, diagnostic methods and clinical implications. Ann Med. 2019;51:232–51.
Siervo M, Bunn D, Prado CM, Hooper L. Accuracy of prediction equations for serum osmolarity in frail older people with and without diabetes. Am J Clin Nutr. 2014;100:867–76.
Chang T, Ravi N, Plegue MA, Sonneville KR, Davis MM. Inadequate hydration, BMI, and obesity among US adults: NHANES 2009-2012. Ann Fam Med. 2016;14:320–4.
Acknowledgements
We thank all PREDIMED-Plus participants and investigators. CIBEROBN, CIBERESP, and CIBERDEM are initiatives of the Instituto de Salud Carlos III (ISCIII), Madrid, Spain. The Hojiblanca (Lucena, Spain) and Patrimonio Comunal Olivarero (Madrid, Spain) food companies donated extra virgin olive oil. The Almond Board of California (Modesto, CA), American Pistachio Growers (Fresno, CA), and Paramount Farms (Wonderful Company, LLC, Los Angeles, CA) donated nuts for the PREDIMED-Plus pilot study.
Funding
This work was supported by the official Spanish Institutions for funding scientific biomedical research, CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and Instituto de Salud Carlos III (ISCIII), through the Fondo de Investigación para la Salud (FIS), which is co-funded by the European Regional Development Fund (six coordinated FIS projects leaded by JS-S and JVi, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, PI20/01158); the Especial Action Project entitled: Implementación y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus grant to JS-S; the European Research Council (Advanced Research Grant 2014–2019; agreement #340918) granted to MÁM-G.; the Recercaixa (number 2013ACUP00194) grant to JS-S; grants from the Consejería de Salud de la Junta de Andalucía (PI0458/2013, PS0358/2016, PI0137/2018); the PROMETEO 17/2017 and PROMETEO 21/2021 grant from the Generalitat Valenciana; the SEMERGEN grant; and the Juan de la Cierva-Incorporación research grant (IJC2019-042420-I) of the Spanish Ministry of Economy, Industry and Competitiveness and European Social Funds. This research was also partially funded by EU-H2020 Grants (Eat2beNICE/H2020-SFS-2016-2) and the Horizon 2020 PRIME study (Prevention and Remediation of Insulin Multimorbidity in Europe; grant agreement #847879). S.K.N. is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR, MFE-171207). CG-M is supported by a predoctoral grant from the University of Rovira I Virgili (2020PMF-PIPF-37). IP-G is supported by a predoctoral grant from Ministerio de Ciencia, Innovación y Universidades (FPU 17/01925). AA-S has received a post-doctoral grant (APOSTD/2020/164) from the Consellería de Innovación, Ciencia y Sociedad Digital, Generalitat Valenciana, Fondo Social Europeo-FSE. CB is supported by a Juan de la Cierva postdoctoral grant from Ministerio de Ciencia, Innovación y Universidades. JS-S, the senior author of this paper, was partially supported by ICREA under the ICREA Academia program. Publication of this work is supported by a conference award grant from Hydration for Health presented by the Danone Global Research & Innovation Center (#2022-08514) awarded to S.K.N. for being the conference attendee voted recipient of the Early Career Researcher Award. None of the funding sources took part in the design, collection, analysis, interpretation of the data; writing of the report; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
LSM, JV, MF, DC, XP, ABC, JAT, JAM, and JSS contributed to the study concept and design and data extraction from the participants from the PREDIMED-Plus study which provides the framework for the present prospective cohort analysis. SKN, NB, IPG, CGM, and JSS made substantial contributions to the conception of the present study. SKN performed the statistical analyses and initial interpretation of the data. NB, IPG, CGM, and JSS contributed to the review of the statistical analyses and interpretation of the data. SKN drafted the manuscript. All authors substantively reviewed the manuscript for important intellectual content and approved the final version to be published. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The PREDIMED-Plus study protocol and procedures were approved by the Research Ethics Committees from each of the participating centers, and the study was registered with the International Standard Randomized Controlled Trial registry (ISRCTN; ISRCTN89898870). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
S.K.N. is a volunteer member of the not-for-profit group Plant-Based Canada and received an Early Career Researcher Award conference grant from Hydration for Health presented by Danone Global Research & Innovation Center. D.C. reported receiving grants from Instituto de Salud Carlos III. R.E. reported receiving grants from Instituto de Salud Carlos III, Uriach Laboratories, and Grand-Fountain Laboratories for clinical trial and personal fees from Brewers of Europe, Fundación Cerveza y Salud; Instituto Cervantes in Albuquerque, Milano, and Tokyo; Fundación Bosch y Gimpera; non-financial support from Wine and Culinary International Forum, ERAB (Belgium), and Sociedad Española de Nutrición; and fees of educational conferences from Pernaud Richart (Mexico) and Fundación Dieta Mediterránea (Spain). R.C. reported receiving fees of educational conferences from Fundación para la investigación del Vino y la Nutrición (Spain). J.L.S. has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, American Society for Nutrition (ASN), International Nut and Dried Fruit Council (INC) Foundation, National Honey Board (US Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS; formerly ILSI North America), Pulse Canada, Quaker Oats Center of Excellence, The United Soybean Board (USDA soy “Checkoff” program), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from IFF), and The Nutrition Trialists Network Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, Nutrartis, and Dairy Farmers of Canada. He has received travel support, speaker fees, and/or honoraria from ASN, Danone, Dairy Farmers of Canada, FoodMinds LLC, Nestlé, Abbott, General Mills, Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council, International Sweeteners Association, and International Glutamate Technical Committee. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Phynova, and Inquis Clinical Research. He is a former member of the European Fruit Juice Association Scientific Expert Panel and a former member of the Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid member of the Board of Trustees and an unpaid scientific advisor for the Carbohydrates Committee of IAFNS. He is a member of the International Carbohydrate Quality Consortium (ICQC), an Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev. J.S.-S. reported receiving research support from the Instituto de Salud Carlos III, Ministerio de Educación y Ciencia, the European Commission, and the USA National Institutes of Health; receiving consulting fees or travel expenses from Instituto Danone and Abbott Laboratories; receiving nonfinancial support from Patrimonio Comunal Olivarero, the Almond Board of California, and Pistachio Growers and Borges S.A; serving on the board of and receiving grant support through his institution from the International Nut and Dried Foundation and personal fees from Instituto Danone; and serving in the Board of Danone Institute International. The rest of the authors declared that they have no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Hydration and water intake definitions. Table S2. Associations between cognitive assessments and water and fluid intake exposures. Table S3. Associations between cognitive assessments and EFSA fluid intake related guidelines. Table S4. Associations between cognitive assessments and hydration status. Table S5. Sensitivity analysis in global cognitive function according to water and fluid intake related exposures Table S6. Sensitivity analysis in global cognitive function according to EFSA fluid intake related guidelines. Table S7. Sensitivity analysis in global cognitive function according to hydration status. Fig. S1. Flow diagram of participants. Fig. S2. Continuous sensitivity analysis by sex. Fig. S3. Categorical sensitivity analysis by sex.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nishi, S.K., Babio, N., Paz-Graniel, I. et al. Water intake, hydration status and 2-year changes in cognitive performance: a prospective cohort study. BMC Med 21, 82 (2023). https://doi.org/10.1186/s12916-023-02771-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02771-4