Abstract
Background
Rotavirus causes severe gastroenteritis in infants and young children worldwide. The UK introduced the monovalent rotavirus vaccine (Rotarix®) in July 2013. Vaccination is free of charge to parents, with two doses delivered at 8 and 12 weeks of age. We evaluated vaccine impact across a health system in relation to socioeconomic deprivation.
Methods
We used interrupted time-series analyses to assess changes in monthly health-care attendances in Merseyside, UK, for all ages, from July 2013 to June 2016, compared to predicted counterfactual attendances without vaccination spanning 3–11 years pre-vaccine. Outcome measures included laboratory-confirmed rotavirus gastroenteritis (RVGE) hospitalisations, acute gastroenteritis (AGE) hospitalisations, emergency department (ED) attendances for gastrointestinal conditions and consultations for infectious gastroenteritis at community walk-in centres (WIC) and general practices (GP). All analyses were stratified by age. Hospitalisations were additionally stratified by vaccine uptake and small-area-level socioeconomic deprivation.
Results
The uptake of the first and second doses of rotavirus vaccine was 91.4% (29,108/31,836) and 86.7% (27,594/31,836), respectively. Among children aged < 5 years, the incidence of gastrointestinal disease decreased across all outcomes post-vaccine introduction: 80% (95% confidence interval [CI] 70–87%; p < 0.001) for RVGE hospitalisation, 44% (95% CI 35–53%; p < 0.001) for AGE hospitalisations, 23% (95% CI 11–33%; p < 0.001) for ED, 32% (95% CI 7–50%; p = 0.02) for WIC and 13% (95% CI -3–26%; p = 0.10) for GP. The impact was greatest during the rotavirus season and for vaccine-eligible age groups. In adults aged 65+ years, AGE hospitalisations fell by 25% (95% CI 19–30%; p < 0.001).
The pre-vaccine risk of AGE hospitalisation was highest in the most socioeconomically deprived communities (adjusted incident rate ratio 1.57; 95% CI 1.51–1.64; p < 0.001), as was the risk for non-vaccination (adjusted risk ratio 1.54; 95% CI 1.34–1.75; p < 0.001). The rate of AGE hospitalisations averted per 1,000 first doses of vaccine was higher among infants in the most deprived communities compared to the least deprived in 2014/15 (28; 95% CI 25–31 vs. 15; 95% CI 12–17) and in 2015/16 (26; 95% CI 23–30 vs. 13; 95% CI 11–16).
Conclusions
Following the introduction of rotavirus vaccination, incidence of gastrointestinal disease reduced across the health-care system. Vaccine impact was greatest among the most deprived populations, despite lower vaccine uptake. Prioritising vaccine uptake in socioeconomically deprived communities should give the greatest health benefit in terms of population disease burden.
Similar content being viewed by others
Background
Prior to the introduction of rotavirus vaccination, rotavirus was the leading cause of severe gastroenteritis in children under 5 years of age worldwide, resulting in approximately 453,000 deaths per year and 40% of diarrhoeal hospital admissions [1, 2]. Two orally administered live-attenuated rotavirus vaccines, Rotarix® (GlaxoSmithKline Biologicals, Belgium) and RotaTeq® (Merck Vaccines, USA), have been introduced in over 90 countries worldwide [3]. The global mortality from rotavirus gastroenteritis (RVGE) has subsequently more than halved (recently estimated at between ~120,000 and ~215,000) and the number of all-cause acute gastroenteritis (AGE) hospitalisations is estimated to have reduced by 38% [4,5,6,7].
Although the majority of the severe disease burden is in developing countries, rotavirus was estimated to cause approximately 80,000 general practice (GP) consultations and 750,000 diarrhoea episodes each year in the UK [8]; 45% of hospitalisations and 20% of emergency department (ED) attendances for AGE in children under 5 years of age were attributable to rotavirus [9]. The National Health Service (NHS) in England is free at the point of use for all UK residents, with vaccinations included in the routine immunisation schedule also free of charge. The monovalent rotavirus vaccine (Rotarix®) was introduced into the UK childhood immunisation schedule in July 2013, with two doses delivered at 8 and 12 weeks of age [10]. Vaccine uptake in England increased rapidly, reaching over 91% for one dose by February 2014 and over 94% by mid-2016 [11]. To date, studies in the UK have separately, and for varied populations and time periods, analysed vaccine impact on rotavirus laboratory detections (77% reduction in infants) [12], RVGE hospitalisations (> 80% reduction in infants) [13], all-cause AGE hospitalisations (26% in infants) [12] and GP attendances for diarrhoea related illness (20–30% in those under 5 years old) [14].
This study aimed to assess the effect of rotavirus vaccination on multiple levels of the UK health-care system simultaneously, by examining the trends in hospitalisations, ED attendances, community health consultations and GP consultations for outcomes of gastroenteritis, diarrhoea and rotavirus gastroenteritis in a defined population before and after vaccine introduction. This approach will, for the first time, provide estimates of rotavirus vaccine impact in an entire health economy. Secondly, within the UK, children under 5 years of age are over-represented in the most socioeconomically deprived populations [15, 16], and experience significantly higher incidence of all-cause AGE hospitalisations than more affluent populations [17]. It is known that in the UK, the uptake of routine childhood vaccines (e.g. vaccines for measles, mumps and rubella, human papillomavirus and influenza) is lower in socioeconomically deprived populations [18,19,20]. Thus, we examined the uptake and impact of rotavirus vaccination in Merseyside, an area with a wide variation in socioeconomic deprivation, to assess whether vaccine uptake and impact are equitable.
Methods
Study setting
The study population was the metropolitan area of Merseyside, England, with an estimated resident population of 1.4 million and an annual birth cohort of approximately 16,000. In 2016, 80,000 of the population were under 5 years of age [16]. Merseyside contains five local authorities (Knowsley, Liverpool, Sefton, St Helens and Wirral), containing multiple NHS trusts and organisations. Health-care for the population is provided in the community by GP practices and walk-in centres (WICs), offering both primary and urgent care. There are five hospitals with emergency and secondary-care facilities, including a large paediatric hospital (Alder Hey Children’s NHS Foundation Trust). The organisations and facilities have been previously described [21].
Data sources and case definitions for outcome measures
Data sources and full case definitions have been previously published [21]. Table 1 summarises these details, and amends any discrepancies. Notably, data on GP consultations were obtained through the NHS clinical commissioning groups (CCGs). Coding for non-infectious gastroenteritis (ICD-10 K52.9) was included in the all-cause AGE hospitalisation outcome measure, since unspecified gastroenteritis was classified under this code until April 2012 [22].
Area of residence and socioeconomic deprivation
In each of the health data sets accessed, an indicator for neighbourhood area of residence (lower super output area [LSOA]) was included. English LSOAs are small statistical boundaries defined following the 2001 and 2011 censuses and consist of approximately 1,500 people. A standardised measure of socioeconomic deprivation was assigned to each participant, using the LSOA of their residence and the English indices of deprivation 2015, the Index of Multiple Deprivation (IMD) [15]. The English indices of deprivation are produced and quality controlled using national census and other administrative data [15]. They are constructed from 37 robust indicators in seven domains: education skills and training, employment, income, living environment, crime, and barriers to housing and other services [15]. These domains are combined and weighted to calculate one of the most robust and commonly used measures of deprivation in England, the IMD [15, 18].
Uptake of rotavirus vaccination
Pseudo-anonymised vaccine status data were extracted from the Child Health Information Service (CHIS) [23, 24], which is managed locally by NHS trusts and holds a unique record for each child born in these areas until the age of 18 years. We obtained a CHIS data extract on children eligible for rotavirus vaccination born from May 2013 to June 2016. The extract included a unique identifier, year and month of birth, year and month of first and second doses of rotavirus vaccine, and LSOA of residence. CHIS could be accessed for four out of the five local authorities in Merseyside. Data for Wirral could not be extracted due to the lack of access to the CHIS database during the study period, which was related to organisational restructuring. We used codes in the CHIS data set to exclude from the analysis deaths, stillbirths and children who were born in Merseyside during the study period but subsequently moved out.
Statistical analyses
Impact
We examined monthly hospitalisations and attendances to health-care providers using an interrupted time-series design. Firstly, to predict counterfactual numbers of hospitalisations and attendances that would have been expected in the absence of vaccination for the vaccine period, we fitted generalised linear models with Poisson or negative binomial distributions (to account for over-dispersion in the data) to pre-vaccine introduction monthly counts, offset for a data-set-specific denominator (Table 1). We adjusted for seasonal trends by including a categorical term for calendar month and secular trends by including a linear term for surveillance year (July to June) as explanatory variables in the models. Secondly, to quantify the percentage reduction in monthly attendances and hospitalisations, we included all data pre- and post-vaccine introduction in a second model with a binary indicator variable denoting the post-vaccine period. This second model also included the same terms to adjust for seasonal and secular trends and allowed the calculation of incidence rate ratios (IRRs). The percentage reduction was calculated as \( 100\times \left(1-\mathrm{IRR}\right) \). The RVGE season in the UK in the pre-vaccine period was consistently between the months of January and May with the peak occurring in early to mid-March in most years [25]. For the sensitivity analysis, we examined the specificity of the end point by stratifying by events that occurred in-season (January to May) and out-of-season (June to December). To investigate vaccine impact by age, the analysis was stratified by age group (< 12 months, 12–23 months, 24–59 months, 5–14 years, 15–64 years, 65+ years and 0–59 months).
Socioeconomic deprivation, vaccine uptake and hospitalisations
Firstly, we wished to assess whether the incidence of all-cause AGE hospitalisations varied by level of socioeconomic deprivation. To achieve this, we fitted negative binomial generalised linear models with the number of hospitalisations as the dependent variable and the quintile of deprivation as the independent variable, offset for population denominator and adjusting again for seasonal and secular trends. The quintile of deprivation was calculated using the IMD scores for LSOAs nationally, whereby quintile 5 is the least deprived and quintile 1 the most deprived. Since the population of Merseyside is skewed towards the most deprived national quintiles (45% of the population are in the most deprived quintile and 8% in the least deprived), we combined the two least deprived quintiles into category 4/5 (least deprived). All-cause AGE hospitalisations were included in the model for the time period July 2004 to June 2016 because LSOA information was not available prior to April 2004. The models allowed the calculation of IRR for socioeconomic deprivation groups by comparing the 4/5 least deprived category to the other quintiles, stratified by age group.
Secondly, we describe the uptake of the first and second doses of rotavirus vaccine by month of birth for children born between May 2013 and December 2015. December 2015 was selected as the cut-off to allow all children in the cohort to reach 25 weeks of age, the upper time limit for rotavirus vaccination [26]. To investigate associations between socioeconomic deprivation and vaccine uptake, we fitted logistic regression models where the dependent variable was vaccine status and the independent variable was the national quintile of IMD and adjusted for gender and year and month of birth. The models allowed the calculation of risk ratios (RRs) for socioeconomic deprivation group by comparing the 4/5 least deprived category to the other quintiles.
Finally, we estimated the all-cause AGE hospitalisations averted per 1,000 vaccine first doses delivered in the 2014/15 and 2015/16 seasons for vaccine-eligible cohorts aged < 12 months and 12–23 months. We define the rate of hospitalisations averted per 1,000 vaccine first doses delivered as:
where RDA is the rate of hospitalisations averted per 1,000 vaccine first doses delivered. X is the model-predicted counterfactual number of hospitalisations that would have been expected in the absence of vaccination for the vaccine period. Y is the observed number of hospitalisations in the vaccine period, P the population denominator, V the proportion of the population vaccinated with one dose of rotavirus vaccine, i the deprivation group, j the age group and k the surveillance year.
We used the RDA in the Merseyside population in this study to provide an estimate of the number of all-cause AGE hospitalisations averted at a national level if the 95% vaccine uptake targets set by the World Health Organization (WHO) were achieved across all deprivation strata [27, 28]. We define the total number of all-cause AGE hospitalisations averted at a national level in 2015/16 at uniform 95% uptake as:
where NDA is the number of all-cause AGE hospitalisations averted. RDA is the rate of hospitalisations averted per 1,000 vaccine first doses delivered in the Merseyside population. N is the national population denominator, derived from mid-year LSOA population estimates 2015/16 [16]. i is the deprivation group, j is the age group and k is the surveillance year.
Data handling and analysis were conducted in R version 3.3 (R Development Core Team, Vienna, Austria).
Results
Vaccine uptake
Rotavirus vaccine uptake (at least one dose of vaccine) in children born between May 2013 and December 2015 was 91.4% (29,108/31,836) and completion of the full rotavirus vaccine schedule (i.e. two doses) was 86.7% (27,594/31,836). In the least deprived population, vaccine uptake for at least one dose was 93.6% (4,135/4,420) and 90.2% (3,989/4,420) for completion of the two-dose schedule; in the most deprived population uptake was 90.6% (16,550/18,259) and 84.9% (15,505/18,259), respectively (Fig. 1). The most deprived populations had a 54% increased risk of non-vaccination compared to the least deprived populations (RR 1.54; 95% CI 1.34–1.75). Furthermore, the most deprived populations had almost twice the risk (RR 1.97; 95% CI 1.62–2.41) of non-completion of the two-dose schedule compared to the least deprived.
Vaccine impact by age
Impact in those under 5 years old
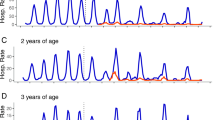
In children less than 5 years of age, a clearly defined rotavirus season was observed prior to vaccine introduction, with the peak predominately occurring in March across all outcome measures for all years prior to vaccine introduction (Fig. 2). The incidence of gastrointestinal disease fell across all health outcomes following vaccine introduction (Fig. 2 and Table 2). The greatest proportional reduction, 80% (95% CI 70–87%), was for RVGE hospitalisation. All-cause AGE hospitalisations fell by 44% (95% CI 35–53%), ED attendances for gastrointestinal conditions by 23% (95% CI 11–33%), and WIC and GP consultations for infectious gastroenteritis by 32% (95% CI 7–50%) and 13% (95% CI -3–26%), respectively. Reductions were greatest in the rotavirus season for all outcomes. All-cause AGE hospitalisations fell by 58% (95% CI 45–67%) and GP consultations by 29% (95% CI 8–45%).
Trends in five study outcome measures for children aged 0–14 years in Merseyside, UK, July 2008 to June 2016. Each analysis examines trends, including a comparison of observed incidence (blue line) after rotavirus vaccination (July 2013 to June 2016) in the UK with expected incidence (red line) and associated 95% confidence intervals (red shaded area) in the absence of vaccination. Expected incidence and 95% confidence intervals are based on predictions from regression models fitted to available historic data for each outcome measure. The black hashed line represents the introduction of rotavirus vaccine in the UK in July 2013. CI confidence interval, ED emergency department, GP general practice, WIC walk-in centre
Disease reductions were highest in vaccine-eligible age groups. RVGE hospitalisation fell by 87% (95% CI 78–93%) in infants aged < 12 months and 84% (95% CI 73–91%) in children 12–23 months. All-cause AGE hospitalisations fell by 46% (95% CI 36–54%) in infants < 12 months and 50% (95% CI 40–59%) in children 12–23 months. For GPs, infectious gastroenteritis consultations fell by 19% (95% CI 4–33%) in infants, averting 136 consultations per 10,000 registered population. There were also significant reductions in gastrointestinal disease outcomes for vaccine-ineligible children aged 24–59 months. RVGE hospitalisations decreased in this age group by 66% (95% CI 44–81%) and all-cause AGE hospitalisations decreased by 26% (95% CI 11–39%). However, in the 2014/15 season, a peak of incidence was detected in May across all primary outcome measures, which was comparable in magnitude to the pre-vaccine rotavirus peak observed in March. Disease rates by surveillance year and pre- and post-vaccine introduction are provided in Additional file 1: Table S1.
Impact in children aged 5 to 14 years
In the pre-vaccine period, children aged 5–14 years had the lowest yearly rates of hospitalisation for all-cause AGE (18 per 10,000 population) (Table 2). Rotavirus seasonality in children aged 5–14 years was less pronounced and inconsistent across all outcome measures in the pre-vaccine period (Fig. 2). In this vaccine-ineligible age group, between July 2013 and June 2016 there were only two laboratory-confirmed detections of RVGE at Alder Hey Children’s Hospital. Furthermore, all-cause AGE hospitalisations and ED attendances for gastrointestinal conditions also fell (Table 2). GP consultations (-3%, 95% CI -21–12%) and WIC attendances (0%, 95% CI -52–34%) for infectious gastroenteritis remained similar to pre-vaccine levels. There were no differences between changes in incidence in the rotavirus season and out of the rotavirus season.
Impact in persons aged 15 to 64 years
Data were available for four out of five of the primary outcomes. There was no clearly identified seasonality in the pre-vaccine period for the non-specific outcome measures in this age group (Fig. 3). Moderate reductions were seen in persons aged 15–64 years across all outcome measures (Table 2). In the post-vaccine period, hospitalisations for all-cause AGE fell by 8% (95% CI 2–14%), ED attendances for gastrointestinal conditions by 29% (95% CI 16–40), and WIC and GP consultations for infectious gastroenteritis by 24% (95% CI 7–38%) and 26% (95% CI 18–33%), respectively. There were no significant differences in the level of percentage change on comparing the in-season and out-of-season periods.
Trends in four study outcome measures for older children and adults aged 15+ years in Merseyside, UK, July 2008 to June 2016. Each analysis examines trends, including comparison of observed incidence (blue line) after rotavirus vaccination (July 2013 to June 2016) in the UK with expected incidence (red line) and associated 95% confidence intervals (red shaded area) in the absence of vaccination. Expected incidence and 95% confidence intervals are based on predictions from regression models fitted to available historic data for each outcome measure. The black hashed line represents the introduction of rotavirus vaccine in the UK in July 2013. CI confidence interval, ED emergency department, GP general practice, WIC walk-in centre
Impact in 65+ year olds
There were significant moderate reductions in all-cause AGE hospitalisations, ED attendances for gastrointestinal conditions and GP consultations for infectious gastroenteritis (Fig. 3 and Table 2). The reduction in attendance at WICs for infectious gastroenteritis was non-significant (47%; 95% CI -15–75%). The absolute rate of consultations averted was 19 per 10,000 registered population for GPs and 34 per 10,000 for WICs (Table 2). During the rotavirus season, proportional reductions were slightly higher than out-of-season, although the difference was not significant.
Vaccine impact by socioeconomic deprivation status
Burden of gastrointestinal infection prior to vaccine introduction
Prior to vaccine introduction, the risk of being admitted to hospital for all-cause AGE was 57% higher (IRR = 1.57; 95% CI 1.51–1.64) in the most socioeconomically deprived populations of Merseyside compared to the least (Fig. 4). Age-group-stratified analyses showed that in all age groups apart from those 5–14 years of age (IRR = 1.08; 95% CI 0.96–1.21), the risk of hospitalisation with all-cause AGE was significantly greater in the most socioeconomically deprived populations of Merseyside compared to the least. Children < 12 months of age in the most socioeconomically deprived quintile had the highest rate of hospitalisation (47 per 1,000 person years), compared with 36 per 1,000 person years in the least deprived (IRR = 1.31; 95% CI 1.16–1.47). Among 12–23-month-olds, the age group with the second highest rates of hospitalisation, the difference between the most deprived (30 per 1,000 person years) and least deprived (26 per 1,000 person years) was less pronounced (IRR = 1.15; 95% CI 1.01–1.31).
Hospitalisations averted per child vaccinated
We estimated the number of all-cause AGE hospitalisations potentially averted in Merseyside due to rotavirus vaccination in two vaccine-eligible age cohorts, < 12 months and 12–23 months of age. In children aged < 12 months living in the most deprived populations, it was estimated that in 2014/15 and 2015/16, 28 (95% CI 25–31) and 26 (95% CI 23–30) all-cause AGE hospitalisations were averted per 1,000 first-dose rotavirus vaccines delivered, respectively. In the least deprived populations, 15 (95% CI 12–17) and 13 (95% CI 11–16) all-cause AGE hospitalisations were averted per 1,000 first-dose rotavirus vaccines delivered in 2014/15 and 2015/16, respectively (Fig. 5). For the cohort aged 12–23 months, it was estimated that there were 18 (95% CI 15–20) all-cause AGE hospitalisations averted per 1,000 persons vaccinated with at least one dose of rotavirus vaccine in 2015/16 in the most deprived populations, and 13 all-cause AGE hospitalisations averted (95% CI 11–16) in the least deprived populations.
If the WHO target for primary childhood immunisations of 95% uptake was attained in each deprivation stratum nationally (England), 10,811 all-cause AGE hospitalisations of infants would have been averted in 2015/16, with 41% (4,395; 95% CI 3,898–4,925) of those averted in the most deprived population (Table 3). Among 12–23-month-olds, 9,472 all-cause AGE hospitalisations would be expected to have been averted, with 31% (2,940; 95% CI 2,570–3,330) of those averted in the most deprived population.
Discussion
In this study, which is one of the few studies to evaluate the impact of rotavirus vaccine introduction simultaneously across all levels of the health-care system in a defined geographic area, we have demonstrated reductions in gastrointestinal disease burden across all levels of health-care and across all ages. Reductions were greatest for the most specific and severe disease outcomes (rotavirus hospitalisations and AGE hospitalisations) during the rotavirus season and for the youngest children who were in vaccine-eligible age groups. Smaller reductions among older unvaccinated populations suggest herd protection. The impact of vaccination was also greater in the most socioeconomically deprived populations, despite lower vaccine coverage.
Most previous studies that evaluated rotavirus vaccine impact in high-income countries focussed on severe disease outcomes, with the magnitude of reductions similar to those described here for children in both vaccine-eligible and ineligible age groups [12, 29,30,31,32,33,34,35,36,37,38,39,40,41,42]. The reduction in all-cause AGE of 46% (36–55%) for infants and 50% (38–60%) for children 12–23 months of age was also similar to that reported in earlier UK studies, as was the indication of herd protective effects in older adults and children [12, 36].
For less severe disease outcomes (people with disease presenting to GPs and WICs), we demonstrated smaller relative reductions compared to more specific or severe disease outcomes. However, these reductions constitute a substantial contribution to the absolute number of health-care contacts averted through vaccination. The impact on non-specific outcome measures was consistently highest during the rotavirus season for children under 5 years, suggesting that the observed reduction in incidence of AGE is likely to be due to a real reduction in incidence of rotavirus disease. The smaller reductions seen in consultations in primary care (WICs and GPs) are likely explained by the non-specific gastroenteritis outcome measure and also because of the presumed lower effectiveness of rotavirus vaccine against milder disease [43, 44]. Furthermore, the reductions in GP consultations for infectious gastroenteritis observed for children in vaccine-eligible age groups (19% for infants and 13% for 12–23 months) are epidemiologically plausible, since a study from the pre-vaccine period estimated that rotavirus was detected by enzyme-linked immunosorbent assay (ELISA) in 14% and by ELISA and/or PCR in approximately 19% of infectious intestinal disease cases seen in GP consultations for UK children under 5 [45, 46], with this estimate likely to be higher in infants. Furthermore, the estimated reductions in WIC attendances and GP consultations are comparable to that reported from an analysis of UK syndromic surveillance of GP consultations for gastroenteritis, diarrhoea and vomiting (26% reduction for infants) [12]. They are also comparable with reductions in AGE outpatient attendances reported in Finland (13% reduction in infants) [33, 47] and all-cause AGE community clinic visits in Israel (19% reduction in infants and 16% for 12–23-month-olds) [48].
We have shown that the most deprived populations were at the greatest risk of all-cause AGE prior to vaccine introduction, with the highest rates of disease occurring in infants in the most deprived populations. This supports previous findings from a lower-resolution national study, which showed that the rate of hospitalisation with all-cause AGE increased with increasing deprivation [17]. The uptake of rotavirus vaccination in our study population was also associated with neighbourhood-level deprivation, with a significantly lower uptake of the first dose of the vaccine and lower completion of the full two-dose schedule in the most deprived populations. Similar findings have been shown in Merseyside for measles, mumps and rubella vaccination, and locally and nationally for childhood influenza vaccination [18, 19].
We were able to overlay a combination of small-area-level deprivation status, vaccine uptake and all-cause AGE hospitalisations to estimate the disease averted per first vaccine dose delivered for different deprivation strata. In infants, disease averted by vaccination was higher in the most deprived areas, suggesting that even with lower vaccine uptake, the most deprived populations benefit the most from the vaccination programme. The higher rates of disease averted in infants < 12 months of age living in the most deprived populations is likely to reflect the higher baseline burden of disease in this group and the relative inequity of hospitalisation rates prior to vaccine introduction. However, for 12–23-month-olds, there is a smaller difference in incidence of disease averted between the least deprived and the most deprived areas, reflecting the lower baseline inequity in disease burden between the deprivation strata.
Nationally, there are disproportionately more infants and young children living in the most deprived quintile (26%) compared to the least deprived (15%) [15, 16]. With individual-level vaccine effectiveness known to be lower in persons with a lower socioeconomic status from studies conducted in high-income settings [49, 50], improving vaccine uptake in the most deprived populations will have the biggest impact towards reducing rotavirus-associated disease. We estimate that over 41% of all-cause AGE hospitalisations averted in infants due to rotavirus vaccination would be averted in the most deprived populations if vaccine uptake was equitable across deprivation strata at the WHO vaccine uptake target of 95% [27, 28].
Strengths and limitations
This ecological study using routine health service data is subject to a number of limitations. There is an inherent problem with clinical coding of rotavirus gastroenteritis in UK hospitals. A quality analysis at Alder Hey Children’s Hospital showed that only 39% of laboratory-confirmed rotavirus hospitalisations were coded as ICD-10 rotaviral enteritis (A08.0), and this figure is lower in other UK hospitals [51]. Therefore, in this study, for the RVGE hospitalisation outcome measure, we used hospitalisations that were laboratory-confirmed rotavirus from Alder Hey rather than ICD-10 codes.
In the context of this outcome measure, it is important to acknowledge the change in rotavirus diagnostic testing methods that occurred at Alder Hey during the study period (Table 1). An enzyme immunoassay was used for 10 of the 14 study years, whilst immuno-chromatography was utilised between 2005 and 2008. The immuno-chromatographic method used (VIKIA®, Rota-Adeno) has a slightly lower diagnostic accuracy compared to enzyme immunoassay methods [52, 53]. However, the pre-vaccine introduction time series spanned 11 years, and since the change in testing practices was not accompanied by a clear non-secular variation in RVGE hospitalisation rates, we would not expect this change to have impacted significantly on effect estimates.
Since rotavirus detection is not routinely undertaken in community settings, such as GP and WICs, syndromic and non-specific outcomes related to gastroenteritis were used, and we were, therefore, unable to account for the contribution of other pathogens causing AGE. However, the predictable seasonality of rotavirus infection allowed the analysis to focus on the rotavirus season, which should improve the robustness of reduction estimates in age-eligible children. In older children and adults, the estimates are more uncertain because there is limited laboratory testing and surveillance data on rotavirus seasonality and disease burden in these age groups in the pre-vaccine period. The lack of routine testing is evidenced by the recommendation in the Standards for Microbiology Investigation S7: gastroenteritis and diarrhoea that rotavirus testing is only standard for sporadic cases of gastroenteritis under the age of 5 years and immunocompromised cases [54].
Because of these limitations, the model fit was less good for older populations due to the less seasonal and more random incidence of gastroenteritis disease, and in these situations the analysis may have overestimated the impact of vaccination. Furthermore, we used a non-dynamic regression fit and so we did not account for changes in the force of the infection due to a reduction in the number of cases. We were, therefore, not able to adjust the predicted incidence to account for current levels of infection. A full transmission model would be required to describe fully the reduction in the transmission rate and associated case reduction due to vaccination. Despite these limitations, studies in the UK, Australia, Europe and the US also show an impact in older populations [12, 36, 37, 42, 55,56,57]. The number of hospitalisations averted nationally under a uniform 95% vaccine uptake was made using two main assumptions. Firstly, that the population of Merseyside is representative of the national population and secondly, that the relationship between vaccine uptake and the herd protective effect of vaccination is linear. Therefore, the estimates are likely to be conservative as a consequence of assuming a linear relationship, particularly if the level of rotavirus vaccine uptake required for population protection is reached before 95% uptake.
Finally, the novelty of measuring vaccine impact on multiple levels of a health system simultaneously in a defined population provides robustness that any detected changes are due to rotavirus vaccination rather than idiosyncrasies of one particular data set. For example, we detected delayed peak activity (April/May) in children aged 24–59 months across all outcome measures in season 2014/15, strengthening the evidence that the data sets used in this study were useful in detecting rotavirus activity in non-specific outcomes. This delayed peak is also observed in laboratory-confirmed rotavirus detections nationally.
Conclusion
This analysis identified the effect of rotavirus vaccination on health-care utilisation for acute gastroenteritis in the four major levels of the UK health system for five outcomes of varying specificity. The study strongly indicates that rotavirus vaccination has reduced the incidence of acute gastroenteritis across the health-care system in both vaccine-eligible and ineligible populations. Rotavirus vaccination will, therefore, contribute to alleviating the increasing pressures on acute services across a health system. With an impact greater than that predicted through cost-effective modelling in the UK [58], these data strongly support the sustained use of the vaccine in the UK and continued expansion to other European countries.
We have also shown that prioritising vaccine uptake in the most socioeconomically deprived communities is likely to give the greatest health benefit in terms of population disease burden and can contribute to reducing health inequalities. Further studies are required to disentangle which factors related to socioeconomic deprivation have the greatest influence on vaccine acceptance, so that interventions to improve vaccine uptake can be targeted effectively.
Abbreviations
- AGE:
-
Acute gastroenteritis
- CCG:
-
Clinical commissioning group
- CHIS:
-
Child Health Information Service
- CI:
-
Confidence interval
- ED:
-
Emergency department
- ELISA:
-
enzyme-linked immunosorbent assay
- GP:
-
General practice
- ICD:
-
International Classification of Diseases
- IMD:
-
Index of Multiple Deprivation
- IRR:
-
Incidence rate ratio
- LSOA:
-
Lower super output area
- NHS:
-
National Health Service
- RR:
-
risk ratio
- RVGE:
-
Rotavirus gastroenteritis
- WHO:
-
World Health Organisation
- WIC:
-
walk-in centre
References
Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41.
World Health Organization. Global networks of rotavirus gastroenteritis, 2001–2008. Wkly Epidemiol Rec. 2008;83:421–8.
ROTA Council. Rotavirus deaths & rotavirus vaccine introduction maps – ROTA Council. Global Introduction Status. http://rotacouncil.org/vaccine-introduction/global-introduction-status/. Accessed 16 Nov 2016.
Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788.
Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization–coordinated global rotavirus surveillance network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62 Suppl 2:S96–105.
Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215:1666–72.
Clark A, Black R, Tate J, Roose A, Kotloff K, Lam D, et al. Estimating global, regional and national rotavirus deaths in children aged <5 years: current approaches, new analyses and proposed improvements. PLoS One. 2017;12:e0183392.
Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69–77.
Harris JP, Jit M, Cooper D, Edmunds WJ. Evaluating rotavirus vaccination in England and Wales. Part I. Estimating the burden of disease. Vaccine. 2007;25:3962–70.
Iturriza-Gómara M, Cunliffe N. Rotavirus vaccine: a welcome addition to the immunisation schedule in the UK. BMJ. 2013;346:f2347.
Public Health England. National rotavirus immunisation programme: preliminary data for England, February 2016 to July 2016. Health Prot Rep Wkly Rep. 2016;10:1–6.
Atchison CJ, Stowe J, Andrews N, Collins S, Allen DJ, Nawaz S, et al. Rapid declines in age group–specific rotavirus infection and acute gastroenteritis among vaccinated and unvaccinated individuals within 1 year of rotavirus vaccine introduction in England and Wales. J Infect Dis. 2016;13(2):243–9. doi:https://doi.org/10.1093/infdis/jiv398.
Hungerford D, Read JM, Cooke RPD, Vivancos R, Iturriza-Gómara M, Allen DJ, et al. Early impact of rotavirus vaccination in a large paediatric hospital in the UK. J Hosp Infect. 2016;93:117–20.
Bawa Z, Elliot AJ, Morbey RA, Ladhani S, Cunliffe NA, O’Brien SJ, et al. Assessing the likely impact of a rotavirus vaccination program in England: the contribution of syndromic surveillance. Clin Infect Dis. 2015;61(1):77–85. doi:https://doi.org/10.1093/cid/civ264.
English indices of deprivation – GOV.UK. https://www.gov.uk/government/collections/english-indices-of-deprivation. Accessed 17 Apr 2014.
Office for National Statistics. Lower super output area mid-year population estimates. 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/lowersuperoutputareamidyearpopulationestimates. Accessed 5 Jul 2017.
Pockett RD, Adlard N, Carroll S, Rajoriya F. Paediatric hospital admissions for rotavirus gastroenteritis and infectious gastroenteritis of all causes in England: an analysis of correlation with deprivation. Curr Med Res Opin. 2011;27:777–84.
Hungerford D, Macpherson P, Farmer S, Ghebrehewet S, Seddon D, Vivancos R, et al. Effect of socioeconomic deprivation on uptake of measles, mumps and rubella vaccination in Liverpool, UK over 16 years: a longitudinal ecological study. Epidemiol Infect. 2016;144(6):1201–11. doi:https://doi.org/10.1017/S0950268815002599.
Green HK, Andrews N, Letley L, Sunderland A, White J, Pebody R. Phased introduction of a universal childhood influenza vaccination programme in England: population-level factors predicting variation in national uptake during the first year, 2013/14. Vaccine. 2015;33:2620–8.
Spencer AM, Roberts SA, Brabin L, Patnick J, Verma A. Sociodemographic factors predicting mother’s cervical screening and daughter’s HPV vaccination uptake. J Epidemiol Community Health. 2014;68:571–7.
Hungerford D, Vivancos R, French N, Iturriza-Gomara M, Cunliffe N. Ecological assessment of the direct and indirect effects of routine rotavirus vaccination in Merseyside, UK using data from multiple health systems: a study protocol. BMJ Open. 2014;4:e006161.
Wilson SE, Deeks SL, Rosella LC. Importance of ICD-10 coding directive change for acute gastroenteritis (unspecified) for rotavirus vaccine impact studies: illustration from a population-based cohort study from Ontario, Canada. BMC Res Notes. 2015;8:439.
Department of Health. Information requirements for child health information systems – publications. 2012. https://www.gov.uk/government/publications/information-requirements-for-child-health-information-systems.
Public Health England. Public health functions to be exercised by NHS England Service specification No. 28 Child Health Information Systems (CHIS). London: Department of Health; 2013.
Hungerford D, Vivancos R, EuroRotaNet network members, Read JM, Pitzer VE, Cunliffe N, et al. In-season and out-of-season variation of rotavirus genotype distribution and age of infection across 12 European countries before the introduction of routine vaccination, 2007/08 to 2012/13. Euro Surveill. 2016;21(2). https://doi.org/10.2807/1560-7917.ES.2016.21.2.30106.
Public Health England. Rotavirus: the green book, chapter 27b. 2013. Available from: https://www.gov.uk/government/publications/rotavirus-the-green-book-chapter-27b. Accessed 4 Jul 2017.
World Health Organization Regional Office for Europe. Health 21: the health for all policy framework for the WHO European Region. Report No: 6. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 1999. p. 1–230.
World Health Organization Regional Office for Europe. European vaccine action plan 2015–2020 (2014). Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2014. p. 1–108. http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2014/european-vaccine-action-plan-20152020–2014.
Zeller M, Rahman M, Heylen E, De Coster S, De Vos S, Arijs I, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine. 2010;28:7507–13.
Braeckman T, Van Herck K, Raes M, Vergison A, Sabbe M, Van Damme P. Rotavirus vaccines in Belgium: policy and impact. Pediatr Infect Dis J. 2011;30:S21–4.
Vesikari T, Uhari M, Renko M, Hemming M, Salminen M, Torcel-Pagnon L, et al. Impact and effectiveness of RotaTeq® vaccine based on 3 years of surveillance following introduction of a rotavirus immunization program in Finland. Pediatr Infect Dis J. 2013;32:1365–73.
Paulke-Korinek M, Kollaritsch H, Aberle S, Zwazl I, Schmidle-Loss B, Vecsei A, et al. Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine. 2013;31:2686–91.
Hemming M, Räsänen S, Huhti L, Paloniemi M, Salminen M, Vesikari T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr. 2013;172:739–46.
Leshem E, Moritz RE, Curns AT, Zhou F, Tate JE, Lopman BA, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007–2011). Pediatrics. 2014;134:15–23.
Uhlig U, Kostev K, Schuster V, Koletzko S, Uhlig HH. Impact of rotavirus vaccination in Germany: rotavirus surveillance, hospitalization, side effects and comparison of vaccines. Pediatr Infect Dis J. 2014;33:e299–304.
Marlow R, Muir P, Vipond B, Lyttle M, Trotter C, Finn A. Assessing the impacts of the first year of rotavirus vaccination in the United Kingdom. Euro Surveill. 2015;20:30077.
Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006–2014. Vaccine. 2015;33:2097–107.
Clark HF, Lawley D, Mallette LA, DiNubile MJ, Hodinka RL. Decline in cases of rotavirus gastroenteritis presenting to the Children’s Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin Vaccine Immunol. 2009;16:382–6.
Clark HF, Lawley D, Matthijnssens J, DiNubile MJ, Hodinka RL. Sustained decline in cases of rotavirus gastroenteritis presenting to the Children’s Hospital of Philadelphia in the new rotavirus vaccine era. Pediatr Infect Dis J. 2010;29:699–702.
Rha B, Tate JE, Payne DC, Cortese MM, Lopman BA, Curns AT, et al. Effectiveness and impact of rotavirus vaccines in the United States – 2006–2012. Expert Rev Vaccines. 2014;13:365–76.
Tate JE, Cortese MM, Payne DC, Curns AT, Yen C, Esposito DH, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30:S56–60.
Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 2011;204:980–6.
Bresee JS, Parashar UD, Widdowson M-A, Gentsch JR, Steele AD, Glass RI. Update on rotavirus vaccines. Pediatr Infect Dis J. 2005;24:947–52.
Hungerford D, Smith K, Tucker A, Iturriza-Gómara M, Vivancos R, McLeonard C, et al. Population effectiveness of the pentavalent and monovalent rotavirus vaccines: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2017;17:569.
Tam CC, Viviani L, Adak B, Bolton E, Dodds JP, Cowden JM, et al. The second study of infectious intestinal disease in the community (IID2 study). Manchester, UK: University of Manchester; 2012. https://www.food.gov.uk/science/research/foodborneillness/b14programme/b14projlist/b18021.
Tam CC, O’Brien SJ, Tompkins DS, Bolton FJ, Berry L, Dodds J, et al. Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clin Infect Dis. 2012;54:1275–86.
Leino T, Gren J, Salo H, Tiihonen P, Kilpi T. First year experience of rotavirus immunisation programme in Finland. Vaccine. 2012;31:176–82.
Muhsen K, Chodick G, Goren S, Anis E, Ziv-Baran T, Shalev V, et al. Change in incidence of clinic visits for all-cause and rotavirus gastroenteritis in young children following the introduction of universal rotavirus vaccination in Israel. Euro Surveill. 2015;20(42). https://doi.org/10.2807/1560-7917.ES.2015.20.42.30045.
Muhsen K, Chodick G, Goren S, Shalev V, Cohen D. The uptake of rotavirus vaccine and its effectiveness in preventing acute gastroenteritis in the community. Vaccine. 2011;29:91–4.
Gosselin V, Généreux M, Gagneur A, Petit G. Effectiveness of rotavirus vaccine in preventing severe gastroenteritis in young children according to socioeconomic status. Hum Vaccines Immunother. 2016;12:2572–9.
Riordan FAI, Quigley T. Estimating hospital admissions due to rotavirus gastroenteritis from hospital episode statistics. J Infect. 2004;49:13–6.
de Rougemont A, Kaplon J, Billaud G, Lina B, Pinchinat S, Derrough T, et al. Sensitivity and specificity of the VIKIA Rota-Adeno immuno-chromatographic test (bioMérieux) and the ELISA IDEIA Rotavirus kit (Dako) compared to genotyping. Pathol Biol. 2009;57:86–9.
Lagare A, Moumouni A, Kaplon J, Langendorf C, Pothier P, Grais RF, et al. Diagnostic accuracy of VIKIA® Rota-Adeno and PremierTM Rotaclone® tests for the detection of rotavirus in Niger. BMC Res Notes. 2017;10:505.
Public Health England. UK standards for microbiology investigations S7: gastroenteritis and diarrhoea. Report No.: 7:1. London: Public Health England; 2013. p. 1–20. https://www.gov.uk/government/publications/smi-s-7-gastroenteritis-and-diarrhoea.
Lambert SB, Faux CE, Hall L, Birrell FA, Peterson KV, Selvey CE, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust. 2009;191:157–60.
Paulke-Korinek M, Kundi M, Rendi-Wagner P, de Martin A, Eder G, Schmidle-Loss B, et al. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine. 2011;29:2791–6.
Anderson EJ, Shippee DB, Weinrobe MH, Davila MD, Katz BZ, Reddy S, et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis. 2013;56:755–60.
Jit M, Edmunds WJ. Evaluating rotavirus vaccination in England and Wales. Part II. The potential cost-effectiveness of vaccination. Vaccine. 2007;25:3971–9.
Acknowledgements
The authors acknowledge the contributions of Fiona Hardiman, all staff in the microbiology department, Karl Edwardson from Alder Hey Children's NHS Foundation Trust and Sacha Wyke from Public Health England. The authors acknowledge the contributions of GPs and NHS CCGs in Liverpool, South Sefton, Wirral, Southport and Formby, and St Helens; Liverpool Community Health NHS Trust; Knowsley and St Helens Health Informatics; Wirral Community Health Trust and the Five Boroughs Community Health Trust.
Funding
This study is supported by GlaxoSmithKline Biologicals SA (EPI-Rota-048; study number 201424) and the University of Liverpool. GlaxoSmithKline Biologicals SA was provided with the opportunity to review a preliminary version of this abstract for factual accuracy but the authors are solely responsible for the final content and interpretation. The authors received no financial support or other form of compensation related to the development of the abstract. RV receives a salary contribution from the Health Protection Research Unit in Emerging and Zoonotic Infections of the National Institute for Health Research (NIHR) and RV and MIG also receive salary contributions from the NIHR Health Protection Research Unit in Gastrointestinal Infections.
Availability of data and materials
The data that support the findings of this study are available from a number of local National Health Service Trusts, NHS CCGs and Public Health England but restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Aggregated data are, however, available from the authors upon reasonable request and with the permission of local National Health Service Trusts, NHS CCGs and Public Health England.
Author information
Authors and Affiliations
Contributions
NC, MIG, RV, DH and NF conceived of and designed the study. DH and NC acquired the data. DH, NF, RV and JMR analysed the data and all authors interpreted the data. DH wrote the first draft of the report. All authors reviewed the draft and final manuscript.
Corresponding author
Ethics declarations
Ethical approval was provided by NHS Research Ethics Committee, South Central-Berkshire (reference 14/SC/1140). Signed data-sharing agreements were obtained between NHS organisations and the University of Liverpool.
Consent for publication
Not applicable
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: NC, NF, MIG, RV and DH are in receipt of research grant support from GlaxoSmithKline (GSK) Biologicals. Outside of this study, MIG and DH are in receipt of research grant support from Sanofi Pasteur-MSD (SPMSD) and NC has received honoraria for participation in GSK Rotavirus Vaccine Advisory Board Meetings.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1.
Yearly rates of hospitalisation/attendance for different levels of the health system pre- and post-rotavirus vaccine introduction in Merseyside, UK. (DOCX 25 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hungerford, D., Vivancos, R., Read, J.M. et al. Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Med 16, 10 (2018). https://doi.org/10.1186/s12916-017-0989-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-017-0989-z