Abstract
Background
The use of the point-of-care lateral flow lipoarabinomannan (LF-LAM) test may expedite tuberculosis (TB) diagnosis in HIV-positive patients. However, the test’s clinical utility is poorly defined outside sub-Saharan Africa.
Methods
The study enrolled consecutive HIV-positive adults at a tertiary referral hospital in Yangon, Myanmar. On enrolment, patients had a LF-LAM test performed according to the manufacturer’s instructions. Clinicians managing the patients were unaware of the LF-LAM result, which was correlated with the patient’s clinical course over the ensuing 6 months.
Results
The study enrolled 54 inpatients and 463 outpatients between July 1 and December 31, 2015. On enrolment, the patients’ median (interquartile range) CD4 T-cell count was 270 (128–443) cells/mm3. The baseline LF-LAM test was positive in 201/517 (39%). TB was confirmed microbiologically during follow-up in 54/517 (10%), with rifampicin resistance present in 8/54 (15%). In the study’s resource-limited setting, extrapulmonary testing for TB was not possible, but after 6 months, 97/201 (48%) with a positive LF-LAM test on enrolment had neither died, required hospitalisation, received a TB diagnosis or received empirical anti-TB therapy, suggesting a high rate of false-positive results. Of the 97 false-positive tests, 89 (92%) were grade 1 positive, suggesting poor test specificity using this cut-off. Only 21/517 (4%) patients were inpatients with TB symptoms and a CD4 T-cell count of < 100 cells/mm3. Five (24%) of these 21 died, three of whom had a positive LF-LAM test on enrolment. However, all three received anti-TB therapy before death — two after diagnosis with Xpert MTB/RIF testing, while the other received empirical treatment. It is unlikely that knowledge of the baseline LF-LAM result would have averted any of the study’s other 11 deaths; eight had a negative test, and of the three patients with a positive test, two received anti-TB therapy before death, while one died from laboratory-confirmed cryptococcal meningitis. The test was no better than a simple, clinical history excluding TB during follow-up (negative predictive value (95% confidence interval): 94% (91–97) vs. 94% (91–96)).
Conclusions
The LF-LAM test had limited clinical utility in the management of HIV-positive patients in this Asian referral hospital setting.
Similar content being viewed by others
Background
Tuberculosis (TB) is the most common cause of death in HIV-infected patients globally [1, 2]. Limited access to laboratory services in low- and middle-income countries means that almost half the fatal cases of TB/HIV co-infection are unrecognised before the patients die [1, 3].
Even where there is access to laboratory services, there are significant challenges in diagnosing TB in HIV-positive patients [4]; mycobacterial culture is resource-intensive and may take weeks to provide results, sputum microscopy is rapid and inexpensive but has a sensitivity of less than 50% in HIV-positive patients [5], and while the Xpert MTB/RIF assay is endorsed by the World Health Organization (WHO), its use has not been shown to improve mortality [6,7,8].
Against this background, the development of the point-of-care urine-based lateral flow lipoarabinomannan (LF-LAM) test has been hailed as a significant advance [9]. The test requires no laboratory infrastructure, is cheaper and simpler to perform than the Xpert MTB/RIF assay, and lacks the infection risks associated with sputum collection [10]. The test has shown greatest utility in facilitating the diagnosis of TB in HIV-positive hospital inpatients with advanced immunodeficiency and symptoms of TB, the population at greatest risk of death [4, 11]. In sub-Saharan Africa, the use of LF-LAM testing to diagnose TB proved cost-effective [12, 13] and reduced 8-week mortality in HIV-positive hospital inpatients [14]. More data are required to support the use of the test in outpatients, although extrapolating from the findings in inpatients, it has been suggested that it may have a role in seriously ill outpatients with more advanced immunodeficiency [10].
The utility of LF-LAM in excluding the diagnosis of TB in HIV-positive patients is incompletely defined, although the expert consensus in current WHO recommendations is that the test should not be used for this purpose [10]. The test’s relatively high negative predictive value (NPV) might be hypothesised to assist in excluding TB before isoniazid prophylaxis therapy (IPT) or antiretroviral therapy (ART) is initiated, but the safety of this approach has not been established. It is also not clear that using the test to exclude TB is better than a simple clinical history, which also has an excellent NPV [15].
Importantly, most of the research to define the clinical utility of the LF-LAM test has been performed in sub-Saharan Africa, the region with the world’s highest rates of HIV/TB co-infection. By contrast, relatively few data have been collected in Asia [2, 16,17,18]. This is significant as the performance of a diagnostic test depends on the local prevalence of the condition, and effective management strategies based on the performance of a diagnostic test in one location are not necessarily generalisable to other populations [19].
Furthermore, the LF-LAM test itself has evolved over time [20]. When the faintest bands in an earlier version were used to define a positive result (grade 1 cut-off) as recommended by the manufacturer, the test’s specificity in culture-negative patients was poor (66%, 95% confidence interval (CI), 57–74); however, this improved to 96% (95% CI, 89–100) when the more intense grade 2 cut-off was used [21]. To simplify the test’s interpretation, in January 2014, the manufacturer revised the reference card that ‘scores’ the test to have only four bands; the band intensity for a grade 1 positive test on the new reference card corresponding to the band intensity of the previous grade 2 positive test. However, the performance of this revised version of the test requires validation [4].
Therefore, this study was performed to evaluate the clinical utility of the LF-LAM test in a cohort of HIV-positive patients in Myanmar. It was hypothesised that the test result would correlate well with the patients’ subsequent clinical course, providing justification for its employment in diagnostic and management strategies to reduce mortality in HIV-positive patients in Southeast Asian countries.
Methods
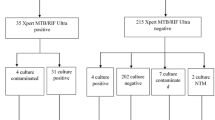
This observational study was performed at Insein General Hospital, a tertiary referral hospital in Yangon, Myanmar’s largest city. All HIV-positive inpatients and outpatients (whether seeking care for symptoms or in routine follow-up) were eligible for inclusion. Patients were enrolled consecutively between July 1, 2015, and December 31, 2015 (Fig. 1). A 6-month period was chosen to assess the everyday applicability of the LF-LAM test in this high caseload hospital.
After obtaining informed consent, study doctors — who were not involved in the patient’s routine care — collected a detailed medical history using a dedicated pro forma, performed a physical examination and recorded available laboratory data. Patients were defined as having symptoms suggestive of TB if they had experienced any cough, fever, night sweats or weight loss in the previous month. A chest X-ray was ordered and sputum was collected for culture (Lowenstein Jensen medium) and Xpert MTB/RIF testing in all patients. If the patient could not produce sputum spontaneously, it was induced using hypertonic saline with appropriate precautions. A separate study doctor, blinded to the clinical details of the case, then collected urine in all patients and performed the LF-LAM test (Alere Determine™ TB LAM Ag) according to the manufacturer’s instructions. Positive tests were graded according to the manufacturer’s new reference card (grade 1, 2, 3 or 4). For the test result to be scored as grade 1, the band on the test-strip had to be at least as intense as the corresponding grade 1 band on the reference card, but not as intense as the grade 2 band. If the study doctor was uncertain of the LF-LAM result, a consensus was reached with another study doctor and one of the authors (SST), both of whom were also unaware of the patient’s clinical presentation. To further reduce the risk of bias, digital photographs were taken of the LF-LAM tests at the time of testing. Another author (JH), unaware of the patient’s clinical presentation, later examined these photographs to grade the tests independently.
The patient’s usual medical team continued the patient’s care without knowledge of the LF-LAM test result, as the test was not approved for use in Myanmar at the time the study was undertaken. This care included the initiation of empirical TB therapy if this was felt to be clinically indicated. The patients’ clinical course was followed prospectively by the study doctors for 6 months after enrolment. The patients were defined as having confirmed TB if they returned either a positive culture for Mycobacterium tuberculosis or a positive Xpert MTB/RIF assay in the ensuing 6 months. Patients were defined as having a complicated course if they had a confirmed TB diagnosis, received empirical anti-TB therapy, required hospitalisation or died during the study.
Extrapulmonary samples were not collected, as facilities to process these samples were not available in Myanmar’s public health system at the time of the study. However, it was reasoned that, after 6 months of follow-up, if a patient had not had a confirmed TB diagnosis and had not died, required hospitalisation or received empirical anti-TB therapy, a positive baseline LF-LAM test was likely to be a false-positive test.
Statistical analysis
All data were de-identified and entered into an electronic database (Microsoft Excel). Statistical analysis was performed using statistical software (Stata). Groups were analysed using the Kruskal–Wallis test and the χ2 test. Inter-rater reliability was tested using Cohen’s kappa. The LF-LAM test’s performance was evaluated using the original test results reported by the study doctors rather than the results of the validation photographs.
Results
Patient characteristics
The study enrolled 517 consecutive patients (Fig. 1), 463 (89.6%) inpatients (Fig. 2) and 54 (10.4%) outpatients (Fig. 3). The patients’ median age was 34 (interquartile range (IQR), 30–41) years; 259 (50.1%) were male and 258 (49.9%) were female. The median (IQR) CD4 T-cell count on enrolment was 270 (128–443) cells/mm3; 360/517 (70%) patients were receiving ART and 14/517 (3%) were receiving IPT. The patients’ other characteristics are presented in Table 1.
Clinical course of patients enrolled as outpatients. aOne case of suspected miliary TB, one case of suspected TB meningitis. bOne case of confirmed TB meningitis (rifampicin resistant on Xpert MTB/RIF assay). cTwo cases of suspected TB meningitis, one case of suspected miliary TB, one case of suspected toxoplasmosis, one case of HIV cachexia. dOne case of confirmed cryptococcal meningitis, one case of suspected toxoplasmosis
Clinical course
No patient was lost to follow-up (Fig. 1). After 6 months, 54/517 (10%) had a confirmed TB diagnosis including eight (15%) with rifampicin resistance on Xpert MTB/RIF testing. All 54 patients with a confirmed TB diagnosis received anti-TB therapy; 123 (27%) of the remaining 463 patients received empirical anti-TB therapy during the study in the absence of a confirmed TB diagnosis. Sixteen (3%) of the 517 patients died during the study — 6/54 (11%) enrolled as inpatients and 10/463 (2%) enrolled as outpatients. Only 4/54 (7%) inpatients and 2/463 (0.4%) outpatients died within 8 weeks of enrolment. Of the 463 enrolled as outpatients, 23 (5%) were hospitalised during follow-up.
LF-LAM test results and inter-rater reliability
All 517 patients had a LF-LAM test performed on enrolment — 201 (39%) had a positive test (≥ grade 1), 43 (8.3%) had ≥ grade 2 positive test and 20 (3.9%) had a ≥ grade 3 positive test. The photograph of the LF-LAM test could be read with confidence in 503/517 (97%). The qualitative result was the same as the initial assessment in 459/503 (91%, kappa coefficient 0.82) using a grade 1 cut-off to define a positive test.
Characteristics of patients with a positive LF-LAM test
The patients with a positive LF-LAM test were more likely to be inpatients at enrolment (P < 0.001) or have TB symptoms (P = 0.01). They were more likely to have advanced immunodeficiency (CD4 T-cell count < 100 cells/mm3) (P = 0.003), a history of hazardous alcohol use (P < 0.001), a body mass index < 18 kg/m2 (P = 0.03) or a haemoglobin level < 10 g/dL (P = 0.02) (Table 1).
Correlation between a positive LF-LAM test result and the subsequent clinical course
Patients with a positive LF-LAM test were more likely to have a confirmed TB diagnosis during follow-up than patients with a negative test (36/201 (18%) vs. 18/316 (6%), P < 0.001) and were more likely to have a complicated course (104/201 (52%) vs. 101/316 (32%), P < 0.001) (Table 1, Additional file 1: Table S1 and Additional file 2: Table S2). The 43/517 (8.3%) patients with a more strongly positive test (≥ grade 2) were more likely than patients with a negative or grade 1 result to have a confirmed TB diagnosis during follow-up (17/43 (40%) vs. 37/474 (8%), P < 0.001) and to have a complicated course (35/43 (81%) vs. 170/474 (36%), P < 0.001). There were 97/517 (19%) patients in the entire cohort with a false-positive LF-LAM test. Of these 97 patients, 89 (92%) had a grade 1 positive test (Table 1).
Correlation between a negative LF-LAM test result and the subsequent clinical course
Patients with a negative LF-LAM test were less likely to have a confirmed TB diagnosis (NPV (95% CI), 94% (91–97)) or complicated course in the ensuing 6 months (NPV (95% CI), 68% (63–73)). However, the absence of TB symptoms on clinical history had a similar ability to exclude both endpoints (NPV (95% CI), 94% (91–96) for a confirmed TB diagnosis and 71% (66–76) for a complicated course, respectively).
Utility of the LF-LAM test in symptomatic inpatients
There were 41 (7.9%) inpatients in the cohort with symptoms of TB on enrolment (median CD4 T-cell count (IQR), 96 (37–277) cells/mm3) (Fig. 4). These patients were less likely to be receiving ART than the rest of the cohort (13/41 (32%) vs. 347/476 (73%), P < 0.001). Six (15%) of these 41 patients died; four had a positive LF-LAM test, three of whom received anti-TB therapy prior to death. Two of these three had TB diagnosed with Xpert on sputum, while the third had empirical anti-TB therapy for presumed TB meningitis. The one patient with a positive LF-LAM test who received no anti-TB therapy prior to death, died from laboratory-confirmed (India Ink positive) cryptococcal meningitis (Table 2). The LF-LAM test’s PPV in these 41 patients for either death, confirmed TB or empirical anti-TB therapy in the subsequent 6 months was 63% (95% CI, 42–81).
Ability of the LF-LAM test to predict important clinical endpoints in inpatients with TB symptoms. aIncludes the 6 patients with confirmed TB and 10 patients who had empirical anti-TB therapy started. bAll 7 patients had empirical anti-TB therapy started. PPV positive predictive value, NPV negative predictive value, both presented as percentage (95% confidence interval)
Only 21/517 (4.1%) were inpatients with TB symptoms and a CD4 T-cell count of < 100 cells/mm3. These patients were less likely to be receiving ART than the rest of the cohort (7/21 (33%) vs. 353/496 (71%), P < 0.001). Five (24%) of the 21 patients died; three of whom had a positive LF-LAM test, but all received anti-TB therapy prior to death. Two of the three had TB diagnosed with Xpert on sputum, while the third had empirical anti-TB treatment for presumed TB meningitis (Table 2). The LF-LAM test’s PPV in these 21 patients for either death, confirmed TB or empirical anti-TB therapy in the subsequent 6 months was 83% (95% CI, 52–98).
Ability of the LF-LAM test to avert mortality
Of the 16 deaths in the study, six occurred in inpatients and are described above. The other ten occurred in outpatients, eight of whom had a negative LF-LAM test. The remaining two patients both received empirical anti-TB therapy prior to death (Table 2).
Discussion
In this observational study of consecutively enrolled HIV-positive Southeast Asian patients at a tertiary referral hospital, the LF-LAM test had limited clinical utility. The test had poor specificity and would have been unlikely to avert any deaths in the 6 months of the study. The test is also unable to identify drug resistance, which was present in almost 15% of the confirmed infections. In this Southeast Asian referral hospital setting at least, the LF-LAM test appears to add little to existing management strategies.
Most of the studies assessing the LF-LAM test have focussed on its sensitivity and specificity in diagnosing TB in HIV-positive patients in sub-Saharan Africa [4, 10]. However, the test’s PPV and NPV, which take into account the local prevalence of the disease, have more relevance to clinical decision-making [19]. The burdens of HIV and TB in Myanmar are among the highest in Southeast Asia: approximately 0.8% of adults in the country aged 18–49 are HIV-positive and TB kills an estimated 4800 HIV-positive patients annually [2, 22]. However, the incidence of HIV-TB co-infection in Myanmar is approximately 15 times lower than in South Africa, where much of the LF-LAM test’s validation has occurred [2, 23]. This is important to remember when considering LF-LAM-based diagnostic and management algorithms developed in Africa for use in other regions, as the test’s PPV for TB infection will necessarily be lower in countries with a lower TB prevalence [19].
In this series, the PPV of the LF-LAM test was poor — if clinicians had used the test to guide treatment, many patients would have received anti-TB therapy they did not require. For individual patients, this unnecessary therapy would have increased the risk of adverse effects and drug-drug interactions, and may have influenced adherence to ART [24, 25]. At a hospital level, there would have been the logistic and financial challenges of delivering this additional therapy [25]. While the test’s NPV was better, it was not superior to a focussed history in excluding the diagnosis, replicating the findings in another Southeast Asian cohort where a simple history was very helpful in excluding active TB [15].
Meanwhile, few, if any, of the deaths in the study would have been averted if the patients’ clinicians had been aware of the LF-LAM result. Only six of the patients who died in the study had a positive LF-LAM test, five of whom received anti-TB therapy before their death. The one patient with a positive LF-LAM result, who did not receive anti-TB therapy before death, died of laboratory-confirmed cryptococcal meningitis almost 3 months after enrolment. Despite the cohort’s median CD4 T-cell count of 270 cells/mm3, there were only six deaths in the first 8 weeks of the study, two of which occurred in outpatients. The low mortality rates seen in this study are similar to those seen from other studies performed in HIV-positive patients in Myanmar [26,27,28] and much lower than those seen in locations where the LF-LAM-based management algorithms have a mortality benefit [14].
WHO recommendations suggest that the LF-LAM test has greatest utility in hospital inpatients with signs and symptoms of TB and advanced immunodeficiency (CD4 T-cell count < 100 cells/mm3) [4]. Certainly, the test’s PPV for both a confirmed TB diagnosis and a complicated clinical course was greater in this group in this study (Additional file 1: Table S1 and Additional file 2: Table S2). However, the pre-test probability of a TB diagnosis in the ensuing 6 months in this population is already so high that any diagnostic test must have an excellent performance to affect the post-test probability and influence clinical management; unfortunately, in this series, that was not the case.
The poor specificity of the LF-LAM test in this cohort is quite different to the excellent specificity reported in some South African series with older versions of the test [4, 29]. However, we used the test according to the manufacturer’s instructions and we used the manufacturer’s reference card to define all positive tests. Independent review of photographs of the test strips excluded the possibility of significant test misinterpretation. The fact that over 90% of the false-positive tests were grade 1 positive results suggests a problem with using this cut-off to define a positive test. However, if the cut-off were raised to grade 2, the test’s sensitivity would fall and the false-positive rate would still be a concerning 19%.
It is unclear whether the poor specificity seen in this series is an issue with the test itself, the batch of tests that were used in this study, or the study doctors’ ability to read the tests. Even with the reference card, the fact that a consensus was sometimes required to interpret the test highlights some of the issues with its use in guiding clinical management and is reminiscent of the poor interobserver agreement when the faintest band was used to define a positive result in the older version of the test [21]. Cross-reactivity with non-tuberculous mycobacteria, including M. kansasii, M. avium and M. fortuitum, has also been proposed as an explanation for reduced specificity in different locations, and the prevalence of these organisms has substantial geographic variation [20, 30, 31].
Whatever the explanation for the disappointing results, they suggest that this novel diagnostic test is unlikely to have a major impact on TB-related mortality in HIV-positive patients in Myanmar. Meanwhile, established interventions that unequivocally reduce TB-related mortality remain poorly implemented in the country [28, 32]. There has been a significant recent increase in ART prescription in Myanmar [33]; however, even at this teaching hospital in the country’s largest city, almost one third of patients were not receiving ART at enrolment. Additionally, less than 3% of the patients in this cohort were receiving IPT despite its proven efficacy and recommendation in WHO guidelines [34,35,36].
Our results require validation. However, even if other studies in Asia showed the LF-LAM test to be more reliable, it is likely to have very limited everyday applicability in Myanmar [37]. The study took place at a high caseload referral hospital, but only 4% of the consecutively enrolled cohort were inpatients with symptoms of TB and advanced immunodeficiency, the population in whom the test is most helpful. With improving ART coverage in the country, the number of patients with advanced immunodeficiency is expected to fall, further reducing the test’s utility. Another under-reported limitation of the LF-LAM test is its inability to assist in the diagnosis of drug-resistant TB, present in 15% of this series and a significant and growing issue in Myanmar [2]. In any patient with a positive LF-LAM test, exclusion of drug-resistant TB would require supplementary testing.
The study’s interpretation is complicated by the frequent prescription of empirical anti-TB therapy [38]. However, while empirical anti-TB therapy has many limitations, in the absence of reliable diagnostic testing, its judicious administration in high-risk patients may sometimes be justified [25, 39]. Indeed, in a country where TB is the most common cause of death in HIV-positive patients, the mortality rate was low compared to other series from similar settings [40,41,42,43]. This does not necessarily suggest that empirical anti-TB therapy should be prescribed at the inflated rate seen here. However, the risk–benefit ratio of such a strategy can be optimised by targeting the patients at highest risk of disease and death, such as those with advanced immunodeficiency [44], while excluding patients at lower risk of disease such as those with recent treatment [45] or those with co-morbidities that might be exacerbated by drug toxicity [25]. Hopefully, future improvements in diagnostic testing for TB will eventually obviate the need for this relatively crude therapeutic approach.
The main limitation of the study is the fact that only sputum specimens were collected in the patients, a low-quality reference standard when extrapulmonary TB is common in HIV-positive patients [4]. Performing simultaneous mycobacterial culture of blood, urine and tissue specimens would have undoubtedly increased the number of confirmed TB diagnoses, but this requires substantial laboratory capacity that is not available in the public health systems of Myanmar and other Southeast Asian low- and middle-income countries. However, notwithstanding this fact, every patient was followed for 6 months after enrolment, permitting complete documentation of the important clinical endpoints of death, hospitalisation and the prescription of anti-TB therapy, and there were no patients lost to follow-up, a significant strength of the study. While the reference standard was imperfect, if a patient was alive without hospitalisation or receipt of anti-TB therapy in the 6 months of follow-up, it is reasonable to assume that the positive LF-LAM tests at enrolment in these patients were false positives. Indeed, our estimation of the false-positive rate is likely to be conservative as it is almost certainly the case that not all patients who were hospitalised or who received empirical anti-TB therapy had active TB.
Conclusions
In this Southeast Asian tertiary referral hospital setting, the LF-LAM test generated a large number of false-positive tests, which, if used to guide therapy, would have resulted in many patients receiving unnecessary treatment. Although the test’s NPV was better, it was not superior to a simple, clinical history. Knowledge of the LF-LAM test result would have been unlikely to avert any of the deaths in the study and would not have recognised the significant number of drug-resistant cases. Even in the target population of inpatients with TB symptoms, a relatively small proportion of patients in even this high caseload hospital, the test had limited practical and clinical utility. Meanwhile, almost a third of patients at this tertiary referral hospital were not receiving ART at enrolment and 97% were not receiving IPT. While future studies may demonstrate that the LF-LAM test has utility in more nuanced, geographically specific, diagnostic algorithms, particularly in inpatients with advanced immunodeficiency, as a stand-alone test in this study, it added little to existing everyday diagnostic and management strategies. In the Southeast Asian setting, overcoming barriers to the rollout of ART and uptake of IPT are likely to have a far greater impact on reducing TB-related morbidity and mortality.
Abbreviations
- ART:
-
antiretroviral therapy
- IPT:
-
isoniazid prophylaxis therapy
- LF-LAM:
-
lateral flow lipoarabinomannan
- NPV:
-
negative predictive value
- PPV:
-
positive predictive value
- TB:
-
tuberculosis
- WHO:
-
World Health Organization
References
Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002.
World Health Organization. Global Tuberculosis Report 2016. Geneva: WHO; 2016.
Manosuthi W, Wiboonchutikul S, Sungkanuparph S. Integrated therapy for HIV and tuberculosis. AIDS Res Ther. 2016;13:22.
Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, Steingart KR. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev. 2016;5:CD011420.
Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis. 2003;3(5):288–96.
Churchyard GJ, Stevens WS, Mametja LD, McCarthy KM, Chihota V, Nicol MP, Erasmus LK, Ndjeka NO, Mvusi L, Vassall A, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450–457.
Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, Bara W, Mungofa S, Pai M, Hoelscher M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(9915):424–35.
World Health Organization. Xpert MTB/RIF implementation manual: technical and operational ‘how-to’; practical considerations. Geneva: WHO; 2014.
Kerkhoff AD, Lawn SD. A breakthrough urine-based diagnostic test for HIV-associated tuberculosis. Lancet. 2016;387(10024):1139–41.
World Health Organization. The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV. Geneva: WHO; 2015.
Lawn SD, Kerkhoff AD, Vogt M, Wood R. HIV-associated tuberculosis: relationship between disease severity and the sensitivity of new sputum-based and urine-based diagnostic assays. BMC Med. 2013;11:231.
Shah M, Dowdy D, Joloba M, Ssengooba W, Manabe YC, Ellner J, Dorman SE. Cost-effectiveness of novel algorithms for rapid diagnosis of tuberculosis in HIV-infected individuals in Uganda. AIDS. 2013;27(18):2883–92.
Sun D, Dorman S, Shah M, Manabe YC, Moodley VM, Nicol MP, Dowdy DW. Cost utility of lateral-flow urine lipoarabinomannan for tuberculosis diagnosis in HIV-infected African adults. Int J Tuberc Lung Dis. 2013;17(4):552–8.
Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, Mehta N, Calligaro G, Lombard CJ, Kadzirange G, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet. 2016;387(10024):1187–97.
Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, Kimerling ME, Chheng P, Thai S, Sar B, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362(8):707–16.
Swaminathan S, Rekha VV. Antigen detection as a point-of-care test for TB: the case of lipoarabinomannan. Future Microbiol. 2012;7(5):559–64.
Singh L, Grover N. Detection of TB antigen by rapid test kit. Med J Armed Forces India. 2011;67(2):196–7.
Gupta-Wright A, Peters JA, Flach C, Lawn SD. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Med. 2016;14:53.
Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2010;8(12 Suppl):S17–29.
Lawn SD. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis. 2012;12:103.
Peter JG, Theron G, van Zyl-Smit R, Haripersad A, Mottay L, Kraus S, Binder A, Meldau R, Hardy A, Dheda K. Diagnostic accuracy of a urine lipoarabinomannan strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J. 2012;40(5):1211–20.
HIV and AIDS Estimates, Myanmar. http://www.unaids.org/en/regionscountries/countries/myanmar. Accessed 18 Jan 2017.
Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15(2):143–52.
Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472–7.
Lawn SD, Ayles H, Egwaga S, Williams B, Mukadi YD, Santos Filho ED, Godfrey-Faussett P, Granich RM, Harries AD. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis. 2011;15(3):287–95.
Thi AM, Shewade HD, Kyaw NT, Oo MM, Aung TK, Aung ST, Oo HN, Win T, Harries AD. Timing of antiretroviral therapy and TB treatment outcomes in patients with TB-HIV in Myanmar. Public Health Action. 2016;6(2):111–7.
Thida A, Tun ST, Zaw SK, Lover AA, Cavailler P, Chunn J, Aye MM, Par P, Naing KW, Zan KN, et al. Retention and risk factors for attrition in a large public health ART program in Myanmar: a retrospective cohort analysis. PLoS One. 2014;9(9):e108615.
Aung NM, Hanson J, Kyi TT, Htet ZW, Cooper DA, Boyd MA, Kyi MM, Saw HA. HIV care in Yangon, Myanmar; successes, challenges and implications for policy. AIDS Res Ther. 2017;14(1):10.
Lawn SD, Kerkhoff AD, Burton R, Schutz C, Boulle A, Vogt M, Gupta-Wright A, Nicol MP, Meintjes G. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med. 2017;15:67.
Kadival GV, Mazarelo TB, Chaparas SD. Sensitivity and specificity of enzyme-linked immunosorbent assay in the detection of antigen in tuberculous meningitis cerebrospinal fluids. J Clin Microbiol. 1986;23(5):901–4.
Nel JS, Lippincott CK, Berhanu R, Spencer DC, Sanne IM, Ive P. Does disseminated nontuberculous mycobacterial disease cause false-positive Determine B-LAM lateral flow assay results? A retrospective review. Clin Infect Dis. 2017. doi:10.1093/cid/cix513.
Getahun H, Granich R, Sculier D, Gunneberg C, Blanc L, Nunn P, Raviglione M. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS. 2010;24 Suppl 5:S57–65.
National AIDS Programme, Ministry of Health: Global AIDS Response Progress Report Myanmar. Nay Pyi Taw: Republic of the Union of Myanmar: National AIDS Programme, Ministry of Health; 2015.
Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, Mosimaneotsile B, Motsamai OI, Bozeman L, Davis MK, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9777):1588–98.
Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, Ouattara E, Anzian A, Ntakpe JB, Minga A, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22.
World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: WHO; 2011.
Scott L, da Silva P, Boehme CC, Stevens W, Gilpin CM. Diagnosis of opportunistic infections: HIV co-infections - tuberculosis. Curr Opin HIV AIDS. 2017;12(2):129–38.
Lawn SD, Nicol MP, Corbett EL. Effect of empirical treatment on outcomes of clinical trials of diagnostic assays for tuberculosis. Lancet Infect Dis. 2015;15(1):17–8.
Kumar R. Empirical use of antituberculosis drugs should not be equated to their inappropriate and indiscriminate use. Indian J Pharmacol. 2011;43(3):363–4.
Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–908.
Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, Dabis F, Pascoe M, Egger M. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6(4):e1000066.
Bachani D, Garg R, Rewari BB, Hegg L, Rajasekaran S, Deshpande A, Emmanuel KV, Chan P, Rao KS. Two-year treatment outcomes of patients enrolled in India’s national first-line antiretroviral therapy programme. Natl Med J India. 2010;23(1):7–12.
Fregonese F, Collins IJ, Jourdain G, Lecoeur S, Cressey TR, Ngo-Giang-Houng N, Banchongkit S, Chutanunta A, Techapornroong M, Lallemant M. Predictors of 5-year mortality in HIV-infected adults starting highly active antiretroviral therapy in Thailand. J Acquir Immune Defic Syndr. 2012;60(1):91–8.
Lawn SD, Little F, Bekker LG, Kaplan R, Campbel E, Orrell C, Wood R. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23(3):335–42.
Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20(12):1605–12.
Acknowledgements
We thank the doctors and nursing staff of the medical wards at Insein General Hospital who cared for the patients. We particularly note the contribution of the study doctors, Dr Pyae Phyo Maung, who performed the LF-LAM testing, and Dr Htet Lwin Oo and Dr Kaung Chit San for patient assessment and follow-up. We also thank Dr Thu Ya Htut for logistical support.
Funding
This work was supported by the National Health and Medical Research Council of Australia through fellowships (to JH [1054195] and NMA (Australia) [1042072]) and through a programme grant [1037304]. This body played no role in the design of the study, the collection, analysis, and interpretation of data or in the writing of the manuscript.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
SST, NMA (Myanmar), ZWH, TTK and MMK managed the patients, recorded the data and revised the manuscript. HAS helped devise the study and revised the manuscript. MAB, NMA (Australia) and DAC revised the manuscript. JH devised the study, performed the statistical analysis and wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval for the study was granted by the Human Ethics Review Committee of the University of Medicine 2 in Yangon, Myanmar, and the Human Research Ethics Committee of the Menzies School of Health Research in Darwin, Australia (HREC 2015-2450). All participants provided informed consent prior to study enrolment.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
Performance of LF-LAM test in predicting a confirmed diagnosis of TB in sputum during 6 months of follow-up stratified by patient characteristics and the cut-off used to define a positive test. (DOCX 20 kb)
Additional file 2: Table S2.
Performance of LF-LAM test in predicting a complicated course during 6 months of follow-up stratified by patient characteristics and the cut-off used to define a positive test. (DOCX 17 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Thit, S.S., Aung, N.M., Htet, Z.W. et al. The clinical utility of the urine-based lateral flow lipoarabinomannan assay in HIV-infected adults in Myanmar: an observational study. BMC Med 15, 145 (2017). https://doi.org/10.1186/s12916-017-0888-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-017-0888-3