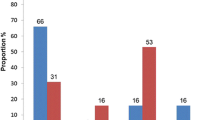
Abstract
Background
Approximately 0.8% of adults aged 18–49 in Myanmar are seropositive for Human Immunodeficiency Virus (HIV). Identifying the demographic, epidemiological and clinical characteristics of people living with HIV (PLHIV) is essential to inform optimal management strategies in this resource-limited country.
Methods
To create a “snapshot” of the PLHIV seeking anti-retroviral therapy (ART) in Myanmar, data were collected from the registration cards of all patients who had been prescribed ART at two large referral hospitals in Yangon, prior to March 18, 2016.
Results and discussion
Anti-retroviral therapy had been prescribed to 2643 patients at the two hospitals. The patients’ median [interquartile range (IQR)] age was 37 (31–44) years; 1494 (57%) were male. At registration, injecting drug use was reported in 22 (0.8%), male-to-male sexual contact in eleven (0.4%) and female sex work in eleven (0.4%), suggesting that patients under-report these risk behaviours, that health care workers are uncomfortable enquiring about them or that the two hospitals are under-servicing these populations. All three explanations appear likely. Most patients were symptomatic at registration with 2027 (77%) presenting with WHO stage 3 or 4 disease. In the 2442 patients with a CD4+ T cell count recorded at registration, the median (IQR) count was 169 (59–328) cells/mm3. After a median (IQR) duration of 359 (185–540) days of ART, 151 (5.7%) patients had died, 111 (4.2%) patients had been lost to follow-up, while 2381 were alive on ART. Tuberculosis (TB) co-infection was common: 1083 (41%) were already on anti-TB treatment at registration, while a further 41 (1.7%) required anti-TB treatment during follow-up. Only 21 (0.8%) patients were prescribed isoniazid prophylaxis therapy (IPT); one of these was lost to follow-up, but none of the remaining 20 patients died or required anti-TB treatment during a median (IQR) follow-up of 275 (235–293) days.
Conclusions
People living with HIV in Yangon, Myanmar are generally presenting late in their disease course, increasing their risk of death, disease and transmitting the virus. A centralised model of ART prescription struggles to deliver care to the key affected populations. TB co-infection is very common in Myanmar, but despite the proven efficacy of IPT, it is frequently not prescribed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Myanmar is a country at a critical point in its history. After five decades of military rule, the newly elected government faces enormous challenges in improving the health, welfare and prosperity of its people [1, 2]. The human immunodeficiency virus (HIV) epidemic is one of the country’s greatest health concerns: in 2015, 0.8% of the country’s adults aged 18–49 were seropositive [3]. Myanmar’s health system remains poorly resourced and under-equipped to achieve the UNAIDS “90–90–90” target or the ambitious Sustainable Development Goal of ending the AIDS epidemic by 2030 [4, 5]. HIV prevalence is highest in people who inject drugs (PWID), men who have sex with men (MSM) and female sex workers (FSW) [6]. However, it is frequently challenging for Myanmar health services to reach these key affected populations (KAPs) and ensure that they are receiving optimal care [6].
One of the key aspects of the country’s response to the HIV epidemic has been a rapid scale up of anti-retroviral therapy (ART). In 2015, 111,563 individuals—representing an estimated 50% of the people living with HIV (PLHIV)—were receiving ART and the rollout has accelerated since [6]. All PLHIV in Myanmar receiving ART have basic demographic, epidemiological and clinical data collected prospectively and entered into the National AIDS Programme’s “Patient HIV Care and Antiretroviral Treatment (ART) Record”, commonly referred to as “The White Card”. The White Card records a patient’s gender, age, address and marital and employment status. It documents their referral origin, their risk factors for infection, the CD4+ count, the WHO disease stage and the presence or history of tuberculosis. It also records the prescribed ART regimen and if a patient dies or is lost to follow up.
These simple data provide a useful insight into the characteristics of the PLHIV receiving ART, and therefore might inform approaches for the optimal delivery of care. Just as importantly, the absence of key populations in these data might highlight PLHIV who are not receiving ART, permitting policy makers to develop strategies to reach under-serviced populations, improving the health of these often vulnerable populations, and also preventing ongoing transmission [6,7,8].
This study examined White Card data collected at two large ART prescribing centres to create a “snapshot” of the HIV epidemic in Myanmar. It was hoped that this might both identify the successes of the current programme and the challenges that remain.
Methods
The White Cards of all PLHIV commencing ART between the 6th June 2001 and the 18th March 2016 at two Yangon hospitals—Waibargi Hospital (a specialist infectious disease hospital) and Insein General Hospital (a tertiary referral general hospital)—were reviewed. These data were de-identified and transferred from the hospital databases into an electronic database (Microsoft Excel); statistical analysis was performed using statistical software (Stata, version 10). Death was either witnessed in hospital or reported by the next of kin. If there was no contact for 3 months after the last outpatient visit, the patient was defined as lost to follow up. Groups were analysed using the Kruskal–Wallis and Chi squared tests. Multivariate analysis was performed using backwards stepwise logistic regression, with only variables that were significant in univariate analysis at a significance level of <0.05 selected for multivariate analysis. Ethical approval for the study was granted by the Human Research Ethics Committees of the University of Medicine 2, Yangon and the Menzies School of Health Research, Darwin, Australia. The Committees waived the requirement for informed consent as the publication of retrospective, aggregated, de-identified data was felt to pose negligible risk to the participants.
Results
There were 2643 patients with a completed white card at the two hospitals, 1674 at Waibargi Hospital and 969 at Insein General Hospital; their characteristics are presented in Tables 1 and 2. The median [interquartile range (IQR)] age of the patients was 37 (31–44) years; 1494 (57%) patients were male. Most patients were referred after having presented with symptoms to the outpatient department (1959 patients, 74%) or after an inpatient admission (449 patients, 17%); 68 patients (2.6%) presented for voluntary testing while only two patients (0.1%) were referred from a drug treatment service.
All patients had data collected on risk factors for infection: 2271 (86%) were recorded as having acquired the infection heterosexually, 82 (3.1%) were recorded as having acquired the infection from a blood transfusion. Only 22 (0.8%) were documented to have acquired the infection through injecting drug use, while male-to-male sexual contact was reported in eleven (0.4%) and eleven (0.4%) reported that they were female sex workers. Of the 2643 patients, 1545 (58%) were married; 664 (43%) had an HIV positive spouse, while in 207 (13%) cases the spouse was negative and in 674 (44%) the HIV status of the spouse was unknown. Waibargi Hospital looked after a greater number of patients from outside the Yangon region: 30% versus 4.5% at Insein General Hospital (p < 0.001).
Patients generally presented late in the course of the disease: 2027 (77%) presented with WHO stage 3 or 4 disease. In the 2442 patients with a CD4+ T-cell count recorded at registration, the median (IQR) CD4+ count was 169 (59–328) cells/mm3. Tuberculosis was common in the cohort: 1083 (41%) were already on treatment for tuberculosis at the time of registration, while a further 41 (1.7%) required treatment for tuberculosis after registration. Despite the high rate of tuberculosis, only 21 patients in the cohort were prescribed isoniazid prophylaxis therapy (IPT) during the study period. There were 2627 patients with data available regarding co-trimoxazole prophylaxis (CPT), 2125 (81%) of whom received this therapy during the study period, including 1158 (85%) of the 1358 patients with a CD4+ count of <200 cells/mm3.
In the 2450 patients for whom the exact date of ART commencement was available, it was initiated at a median (IQR) of 21 (9–42) days after the HIV diagnosis was recorded. One hundred and seven (4.4%) PLHIV started ART on the same day that the diagnosis was recorded. The most commonly prescribed antiretroviral (ART) regimen was tenofovir, emtricitabine and efavirenz, with 2280 (86%) receiving this combination. In the 1247 patients with a recorded follow up CD4+ T-cell count at least 90 days after registration, there was a median increase in the CD4 count of 150 (47–263) cells/mm3; 192 (15.1%) had a fall in their CD4+ count.
At the time of data analysis, a median (IQR) of 359 (185–540) days after patient registration, 151 (5.7%) patients had died, 111 (4.2%) had been lost to follow up and 2381 (90.1%) were alive and taking ART (Table 1). In multivariate analysis, the independent predictors of death were a CD4 + count at registration of <200 cells/mm3 (odds ratio (OR) 6.4, 95% confidence interval (CI) 3.5–11.6, p < 0.0001); treatment at Waibargi Hospital (OR (95% CI) 1.9 (1.2–2.8), p = 0.004); unemployment (OR (95% CI) 1.8 (1.3–2.7) p = 0.002); age >40 years (OR (95% CI) 1.6 (1.1–2.4) p = 0.008) and a requirement for TB therapy (OR (95% CI) 1.6 (1.1–2.4) p = 0.02). The only variable associated with loss to follow up in either univariate or multivariate analysis was treatment at Waibargi Hospital (OR (95% CI) 2.4 (1.5–3.9), p < 0.001). All 21 patients receiving IPT were being treated at IGH. Of these 21, one was subsequently lost to follow up but none of the remaining 20 died or required TB treatment during the follow up period.
Discussion
People living with human immunodeficiency virus at these two large treatment centres in Myanmar are presenting late in their disease course. The resulting delay in initiating ART increases their risk of disease and death and the likelihood of transmitting the virus [8, 9]. Tuberculosis co-infection is very common, but despite the proven efficacy of IPT [10]—and a strong recommendation for its prescription in WHO guidelines [11]—it was prescribed rarely in this cohort. The greatest burden of HIV in Myanmar is among PWID, MSM and FSW [6], however, very few patients in this cohort reported these risk behaviours at registration. This suggests that either these large ART prescribing centres are not servicing these KAPs, patients avoid acknowledging these risk behaviours or that health care workers are uncomfortable enquiring about them. All three explanations appear likely.
The late presentation of Myanmar PLHIV in this series resembles the findings in studies in other resource-limited settings [12,13,14,15,16]. Late presentation is not restricted to patients with HIV infection in a country whose public health care system is critically under-resourced [2]. In a 2012 WHO report, Myanmar was the country with the lowest government spending per person per year on health [17] and it is sobering to reflect that these data were collected in Yangon, one of the better resourced regions in the country [18]. Meanwhile, 70% of the population reside in rural areas, where resources and access to skilled health personnel are far more limited [2] and it is these areas—particularly the North and East of the country—where the HIV burden is greatest and programmes for PLHIV less developed [19,20,21,22].
The high prevalence of TB in this cohort emphasises the importance of integrating TB and HIV services [23] and brings into sharp relief the very low rates of IPT prescription, although the fact that co-trimoxazole was prescribed commonly demonstrates that there is no reluctance to prescribe prophylaxis per se [24]. Anecdotally, many Myanmar clinicians express concerns about the possibility of IPT leading to the development of resistance or adverse drug reactions, but there is no reason to think that this would occur at a higher rate than in other countries with a high TB prevalence where IPT has shown unequivocal benefit [25, 26]. While the number of patients prescribed IPT in our sample is too small to draw any broad conclusions, it may reassure sceptical Myanmar clinicians that no patients receiving IPT died or required TB treatment during follow-up. In a country where PLHIV are still presenting with advanced immunodeficiency, it is hoped that there is less resistance to the rollout of expanded prophylaxis regimens which have recently been shown to reduce mortality and hospitalisation without increased serious adverse events [27].
The majority of new HIV infections in Myanmar are in PWID (HIV prevalence in this population in 2014: 23.1%), MSM (HIV prevalence in 2014: 6.6%), and FSW (HIV prevalence in 2014: 6.3%) [6], but stigma and discrimination against these populations in Myanmar impose substantial barriers to their access to HIV prevention and care [28]. Both Waibargi and IGH are large hospitals running extremely busy, open-plan outpatient departments. While it appears likely that patients are under-reporting these risk behaviours and/or clinicians are under-documenting them, neither hospital provides an ideal setting for their disclosure. Unfortunately, this is a situation that is replicated around the country; it is estimated that a significant proportion of the KAPs are not being reached by the National AIDS Programme (NAP) and its partners, and an even smaller proportion are being tested for HIV [28].
People who inject drugs are a particularly challenging group to reach, reflected by the fact that this group accounted for 39% of all new infections in 2014 [6]. The fact that the rates of injecting drug use are highest in remote—and sometimes politically unstable—areas in the North of the country adds another layer of complexity to service delivery. However, there are positives: there has been a significant scaling-up of harm reduction activities, including the provision of methadone maintenance therapy and the possession of hypodermic needles is no longer illegal. Meanwhile, supportive drop-in-centres (DICs) continue to be the mainstay of prevention efforts targeting MSM and FSW, providing education, free condoms and outreach programmes. While support groups have been shown to reduce mortality and improve clinical outcomes [29], they undoubtedly improve the quality of life of many MSM and FSW who would otherwise feel ostracised in what remains a conservative country. Some of these DICs also offer onsite health care services and ART access.
The finding that outcomes were worse at Waibargi Hospital—a specialist infectious disease hospital—than IGH—a general hospital—is likely explained by the fact that Waibargi Hospital manages cases that are more complex than IGH. Indeed IGH refers its complicated patients with multiple comorbidities to Waibargi Hospital. Waibargi Hospital also receives more referrals from outside the Yangon region, which hinders optimal follow up care and almost certainly contributes to that hospital’s higher rate of loss to follow up. The association between unemployment—a marker of socioeconomic disadvantage—and death is unsurprising, although the high rate of unemployment in the cohort emphasises the bidirectional relationship between HIV and poverty [30].
There are positives in these data: almost 85% of patients who were prescribed ART had an increase in their CD4+ count and even with high rates of advanced symptomatic disease, overall survival and retention in care was relatively good when compared to other series from low and middle income countries [13, 15, 31, 32]. However, the reality is that Myanmar’s HIV program is grossly underfunded [33]. Total spending for HIV in Myanmar was approximately USD 84.1 million in 2015—an increase from USD 68.9 million in 2014—but this programme is heavily reliant on the Global Fund, and current funding levels are insufficient to reach Myanmar’s stated objectives [34].
One of the greatest issues in the management of HIV in Myanmar is the profoundly under-developed state of laboratory services [35]. The clinical management of other common diseases in Myanmar—such as malaria—requires less laboratory support [36, 37]; however, this is assuredly not the case with HIV. The absence of viral load testing in the public health system precludes early recognition of poor adherence, drug malabsorption and viral resistance, but despite data to support its cost-effectiveness [38], there are enormous challenges in implementing viral load testing in resource-limited settings when even basic laboratory services are lacking [39, 40].
Our study has limitations. Although the data are collected from two of the largest ART prescribing centres in Myanmar’s biggest city, they are not necessarily generalisable to the entire country. There are NGOs that target—and undoubtedly reach—KAPs more effectively and IPT is a key component of the care provided by these services. The data presented here are retrospective and relatively basic; to guide policy in the future, it will be important to collect detailed data prospectively and to link these data with the delivery of services and clinical outcomes. It will be essential to have robust quality assurance mechanisms to ensure that the collected data are complete, timely and accurate if they are to inform optimal policy.
The Myanmar National AIDS Programme (NAP) plans to increase access to public sector ART. It aims to achieve this through further decentralisation of HIV testing and ART prescription, transitioning to a “Test-and-Treat” strategy and expanded co-operation with non-government organisations [6]. There is a particular focus on improving the prevention of mother-to-child transmission, and the integration of TB and HIV services. There is the recognition that the strengthening of laboratory capacity is essential if these ambitious plans are to be to implemented [34]. The NAP acknowledges a failure to reach KAPs and it is hoped that the strengthening of community-based support groups and the integration of harm reduction strategies into community-based settings may improve the delivery of care to these vulnerable populations.
Conclusions
People living with human immunodeficiency virus at two of the largest ART prescription centres in Myanmar are presenting late in their disease course, increasing their risk of death and disease and the likelihood of viral transmission. Although retention rates and survival were relatively good at these centres, a centralised model of ART prescription is struggling to deliver care to the KAPs that bear the greatest burden of disease in the country. While TB co-infection is very common in Myanmar, IPT is frequently not prescribed despite its proven efficacy. Sustained political and financial commitment, health system strengthening, reliable data collection and flexible, evidence-based health policies are required to build on recent progress in the care of PLHIV in the country.
Abbreviations
- ART:
-
anti-retroviral therapy
- DIC:
-
drop in centre
- EFV:
-
efavirenz
- FSW:
-
female sex workers
- FTC:
-
emtricitabine
- HIV:
-
human immunodeficiency virus
- IGH:
-
Insein General Hospital
- IPT:
-
isoniazid prophylaxis therapy
- IQR:
-
interquartile range
- KAP:
-
key affected populations
- MSM:
-
men who have sex with men
- NAP:
-
National AIDS Programme
- NGO:
-
non-government organisation
- PLHIV:
-
people living with human immunodeficiency virus
- PWID:
-
people who inject drugs
- TB:
-
tuberculosis
- TDF:
-
tenofovir disoproxil fumarate
- USD:
-
United States dollars
- WHO:
-
World Health Organization
References
http://hdr.undp.org/en/countries/profiles/MMR. Accessed 15 Dec 2016.
Saw YM, Win KL, Shiao LW, Thandar MM, Amiya RM, Shibanuma A, Tun S, Jimba M. Taking stock of Myanmar’s progress toward the health-related Millennium Development Goals: current roadblocks, paths ahead. Int J Equity Health. 2013;12:78.
HIV and AIDS estimates, Myanmar http://www.unaids.org/en/regionscountries/countries/myanmar. Accessed 15 Dec 2016.
Murray CJ. Shifting to sustainable development goals—implications for Global Health. N Engl J Med. 2015;373(15):1390–3.
Joint United Nations Programme on HIV/AIDS, Joint United Nations Programme on HIV/Aids. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014.
Global AIDS Response Progress Report Myanmar. National AIDS Programme, Ministry of Health, Republic of the Union of Myanmar; 2015.
McKinney MM, Marconi KM, Cleary PD, Kates J, Young SR, O’Neill JF. Delivering HIV services to vulnerable populations: an evaluation and research agenda. Public Health Rep. 2002;117(2):114–22.
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–9.
Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, Wang L, Ou SS, Anderson M, McCauley M, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–90.
Briggs MA, Emerson C, Modi S, Taylor NK, Date A. Use of isoniazid preventive therapy for tuberculosis prophylaxis among people living with HIV/AIDS: a review of the literature. J Acquir Immune Defic Syndr. 2015;68(Suppl 3):S297–305.
Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. World Health Organization; 2011.
Gelaw YA, Senbete GH, Adane AA, Alene KA. Determinants of late presentation to HIV/AIDS care in Southern Tigray Zone, Northern Ethiopia: an institution based case-control study. AIDS Res Ther. 2015;12:40.
Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. Aids. 2008;22(15):1897–908.
Lahuerta M, Ue F, Hoffman S, Elul B, Kulkarni SG, Wu Y, Nuwagaba-Biribonwoha H, Remien RH, El Sadr W, Nash D. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24(1):359–83.
Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, Dabis F, Pascoe M, Egger M. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6(4):e1000066.
Kiertiburanakul S, Boettiger D, Lee MP, Omar SF, Tanuma J, Ng OT, Durier N, Phanuphak P, Ditangco R, Chaiwarith R, et al. Trends of CD4 cell count levels at the initiation of antiretroviral therapy over time and factors associated with late initiation of antiretroviral therapy among Asian HIV-positive patients. J Int AIDS Soc. 2014;17:18804.
WHO Global Health Expediture Atlas. In.: World Health Organization; 2012.
Yangon Region Profile [http://www.unicef.org/myanmar/Yangon_Region_Profile_30-07-15.pdf]. Accessed 15 Dec 2016.
Shan State Profile [http://www.unicef.org/myanmar/Shan_State_Profile_30-07-15.pdf]. Accessed 15 Dec 2016.
Kachin State Profile [http://www.unicef.org/myanmar/Kachin_State_Profile_30-07-15.pdf]. Accessed 15 Dec 2016.
Beyrer C, Razak MH, Labrique A, Brookmeyer R. Assessing the magnitude of the HIV/AIDS epidemic in Burma. J Acquir Immune Defic Syndr. 2003;32(3):311–7.
Williams B, Baker D, Buhler M, Petrie C. Increase coverage of HIV and AIDS services in Myanmar. Confl Health. 2008;2:3.
Lawn SD, Meintjes G, McIlleron H, Harries AD, Wood R. Management of HIV-associated tuberculosis in resource-limited settings: a state-of-the-art review. BMC Med. 2013;11:253.
Suthar AB, Vitoria MA, Nagata JM, Anglaret X, Mbori-Ngacha D, Sued O, Kaplan JE, Doherty MC. Co-trimoxazole prophylaxis in adults, including pregnant women, with HIV: a systematic review and meta-analysis. Lancet HIV. 2015;2(4):e137–50.
Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G, Wilkinson KA, Goliath R, Mathee S, Goemaere E, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384(9944):682–90.
Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, Ouattara E, Anzian A, Ntakpe JB, Minga A, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22.
Hakim VM, Szubert AJ, Siika A, Mallewa J, Agutu C, Pett SL, Bwakura-Dangarembizi M, Lugemwa A, Kaunda S, Karoney M, Maitland K, Griffiths A, Kityo C, Mugyenyi P, Prendergast AJ, Walker AS, Gibb DM, REALITY Trial Team Zimbabwe. Ministry of Health and Child Welfare.: Enhanced infection prophylaxis reduces mortality in severely immunosuppressed HIV-infected adults and older children initiating antiretroviral therapy in Kenya, Malawi, Uganda and Zimbabwe: the REALITY trial. In: 21st International AIDS Conference (AIDS 2016): Durban; 2016.
National Guidelines. A Core Package for HIV Prevention Amongst Key Populations in Myanmar. Department of Health, Republic of the Union of Myanmar, National AIDS Control Programme; 2014.
Bateganya MH, Amanyeiwe U, Roxo U, Dong M. Impact of support groups for people living with HIV on clinical outcomes: a systematic review of the literature. J Acquir Immune Defic Syndr. 2015;68(Suppl 3):S368–74.
Rodrigo C, Rajapakse S. Current Status of HIV/AIDS in South Asia. J Glob Infect Dis. 2009;1(2):93–101.
Bachani D, Garg R, Rewari BB, Hegg L, Rajasekaran S, Deshpande A, Emmanuel KV, Chan P, Rao KS. Two-year treatment outcomes of patients enrolled in India’s national first-line antiretroviral therapy programme. Natl Med J India. 2010;23(1):7–12.
Fregonese F, Collins IJ, Jourdain G, Lecoeur S, Cressey TR, Ngo-Giang-Houng N, Banchongkit S, Chutanunta A, Techapornroong M, Lallemant M. Predictors of 5-year mortality in HIV-infected adults starting highly active antiretroviral therapy in Thailand. J Acquir Immune Defic Syndr. 2012;60(1):91–8.
Piot P, Abdool Karim SS, Hecht R, Legido-Quigley H, Buse K, Stover J, Resch S, Ryckman T, Mogedal S, Dybul M, et al. Defeating AIDS–advancing global health. Lancet. 2015;386(9989):171–218.
Burma. Country Operational Plan. Strategic Direction Summary. http://www.pepfar.gov/documents/organization/257661.pdf. Accessed 15 Dec 2016.
Kyaw LL, Nozaki I, Wada K, Oo KY, Tin HH, Yoshihara N. Ensuring accurate testing for human immunodeficiency virus in Myanmar. Bull World Health Organ. 2015;93(1):42–6.
Kaung M, Kyi TT, Aung NM, Kyaw MP, Min M, Htet ZW, Anstey NM, Kyi MM, Hanson J. The prognostic utility of bedside assessment of adults hospitalised with malaria in Myanmar: a retrospective analysis. Malar J. 2015;14:63.
Aung NM, Kaung M, Kyi TT, Kyaw MP, Min M, Htet ZW, Anstey NM, Kyi MM, Hanson J. The safety of a conservative fluid replacement strategy in adults hospitalised with malaria. PLoS ONE. 2015;10(11):e0143062.
Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, Bonner K, Rousseau C, Garnett G, Cambiano V, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528(7580):S68–76.
Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis. 2016;62(8):1043–8.
Boyd MA, Cooper DA. Optimisation of HIV care and service delivery: doing more with less. Lancet. 2012;380(9856):1860–6.
Authors’ contributions
NMA oversaw the data collection, data entry and was primarily responsible for coordinating the study in Myanmar. JH performed the statistical analysis and wrote the first draft. TTK supervised patient care and data entry. ZWH assisted with data entry and statistical analysis. MMK and HAS supervised patient care and data entry. DAC MAB and HAS revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Dr. Pyae Phyo Maung, Dr. Htet Lwin Oo, Dr. Kaung Chit San, Dr. Kyaw Zin Aung, Dr. Kaung Myat and Dr. Si Hlaing Moe who assisted with data collection.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author NMA. The data are not publicly available due to the fact that they contain information that could compromise research participant privacy.
Ethics approval and consent to participate
Ethical approval for the study was granted by the Human Research Ethics Committees of the University of Medicine 2, Yangon and the Menzies School of Health Research, Darwin, Australia. The Committees waived the requirement for informed consent as the publication of retrospective, aggregated, de-identified data was felt to pose negligible risk to the participants.
Funding
JH (fellowship 1054195) is supported by the National Health and Medical Research Council of Australia, but this body did not provide any direct funding for the study. It played no role in the collection, analysis and interpretation of data, the writing of the manuscript, or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ne Myo Aung and Josh Hanson contributed equally to this work
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Aung, N.M., Hanson, J., Kyi, T.T. et al. HIV care in Yangon, Myanmar; successes, challenges and implications for policy. AIDS Res Ther 14, 10 (2017). https://doi.org/10.1186/s12981-017-0137-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-017-0137-z