Abstract
Background
In low-incidence countries, most tuberculosis (TB) cases occur among migrants and are caused by reactivation of latent tuberculosis infection (LTBI) acquired in the country of origin. Diagnosis and treatment of LTBI are rarely implemented to reduce the burden of TB in immigrants, partly because the cost-effectiveness profile of this intervention is uncertain.
The objective of this research is to perform a review of the literature to assess the cost-effectiveness of LTBI diagnosis and treatment strategies in migrants.
Methods
Scoping review of economic evaluations on LTBI screening strategies for migrants was carried out in Medline.
Results
Nine studies met the inclusion criteria. LTBI screening was cost-effective according to seven studies. Findings of four studies support interferon gamma release assay as the most cost-effective test for LTBI screening in migrants. Two studies found that LTBI screening is cost-effective only if carried out in immigrants who are contacts of active TB cases.
Discussion and Conclusions
Our findings support the cost-effectiveness of LTBI diagnostic and treatment strategies in migrants especially if they are focused on young subjects from high incidence countries. These strategies could represent and adjunctive and synergistic tool to achieve the ambitious aim of TB elimination.
Similar content being viewed by others
Background
In low incidence countries, the majority of the cases of active tuberculosis (TB) occur among migrants from high incidence countries [1]. In this setting migrants can develop TB following three main mechanisms [2]:
-
1.
TB can already be present at the time they enter in the host country;
-
2.
TB can be the consequence of the reactivation of latent tuberculosis infection (LTBI) acquired in the country of origin, occurring months to years after the settlement in the host country [3, 4];
-
3.
Primary progressive TB can follow a new infection acquired in the host country [4, 5] or during a return travel to the country of origin [6].
Most countries with low TB incidence adopt TB screening policies for migrants from high TB incidence countries. The majority of countries screen migrants for active TB through chest x-ray (CXR) before or soon after arrival, while screening for LTBI is not consistently imple mented [7]. Screening protocols that include CXR as first step are able to identify the majority of migrants with active TB at entry and, occasionally, migrants with radiological alterations suggestive of LTBI. However, most persons with LTBI go undetected as a diagnostic test for this condition is not usually applied. Epidemiological studies based on molecular techniques to genotype the M. tuberculosis isolates showed that 55–90 % of TB cases diagnosed in foreign born patients are due to LTBI reactivation [8, 9].
Screening migrants for LTBI and providing treatment to those with this condition is a plausible strategy to prevent the disease and reduce the risk of spread infection in the native population [10, 11]. Diagnosis and treatment of LTBI was recommended in Europe already more than ten years ago [12, 13], and is now included among the main interventions of the new global post-2015 strategy for TB control [13].
Only 16 of 29 industrialized countries belonging to the Organization for Economic Co-operation and Development, screen immigrants for LTBI, most frequently post-arrival in the host country [7].
In 11 of these 16 countries, the screening is compulsory for legal migrants. Children and young adults (<40 years) are most commonly targeted for LTBI screening. The most common test used for screening is Tuberculin Skin Test (TST), used in 11 out of 16 countries. Patients and physicians compliance to the LTBI screening protocol is essential for the effectiveness [10], but it is reported to be low [11].
Appropriate information on cost-effectiveness of LTBI screening strategies may help the policy makers to decide appropriate interventions. Thus, we performed a review of published economic evaluations (EE) of different LTBI screening strategies.
Methods
Ethical approval was not required for this review study.
Inclusion criteria
In this review we included studies with all the three following criteria: 1) had migrants as study target population; 2) included diagnosis and treatment for LTBI; 3) reported findings of EE analyses. Both model-based EEs and those alongside clinical trials (or in combinations as well) were included.
Search strategy
MEDLINE and the Cochrane Library electronic databases were searched for studies published up to July 2014. The terms used for the search strategy were: (latent tuberculosis OR LTBI OR “latent tuberculosis”[Mesh]) AND (screening OR Mantoux OR IGRA OR “mass screening”[Mesh] OR “tuberculin test”[Mesh] OR “Interferon-gamma Release Tests”[Mesh]) AND (cost-effect* OR cost-bene* OR “Cost- Benefit Analysis”[Mesh]). No language restriction was done. The search was performed also using the term “latent tuberculosis screening in migrants” as free text.
Data extraction and assessment
All references retrieved were collected using the EndNote®, version X5 (Thomson Reuter) program. Identified titles and abstracts were screened for their eligibility for inclusion and the full text of potentially relevant studies was obtained and examined. Two review authors (LZ, GC) independently screened titles and abstracts of each study. Based on the full text revision, the two reviewers independently selected the studies, and inter-reviewer disagreement was solved by discussion. The following information was extracted: setting, study design, participants, EEs data, including type of EE, screening alternatives, cost description, analysis perspective, source of data (literature, clinical studies), modeling (if any, including time horizon and discount rate), key results and authors’ conclusions. The following data were collected for clinical trials used for EE analyses: inclusion criteria, study participants, setting, design and methods, results and authors’ conclusions. Agreement on inclusion was calculated using the Kappa statistics.
Study quality was assessed using an established checklist of criteria for assessing the quality of economic evaluations in health care [14]. This tool includes ten items regarding cost effectiveness analysis: the presence of a clearly stated hypothesis and comparator; the used methods; the medical evidence; appropriate costs and benefits considered; a marginal analysis and a sensitivity analysis have been undertaken; the analysis was appropriate to the local environment.
Results
Search results
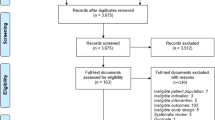
The literature search resulted in 109 titles. A total of 86 duplicates or non-pertinent or non-appropriate references were deleted, resulting in 23 potentially relevant studies. Reviewers agreed on 18 of 23 papers (78.3 %) selected for reliability check (K = 0.697), and disagreements were resolved by consensus. Ten of 23 papers (43.5 %) met inclusion criteria and were therefore included in the final step of review (Fig. 1).
General characteristics of included studies
The 10 research studies included in the review (Table 1) were published in 8 journals, 2 in the Am J Respir Crit Care Med and Thorax, and the others in 6 different journals. The distribution of papers per year of publication ranged from 2000 to 2014.
Four studies were performed in the US, 3 in the United Kingdom, and 3 in Canada.
In four studies age limitations in the study population were used: children 4 to 18 years old [17], migrants >16 year old (median 29 years) [16], migrants or foreign born subjects > 18 year old [18], and migrants aging <35 years [15].
Four studies considered immigrants at the moment of their entrance or application for residence permission [11, 18–20]. Two studies referred to “new immigrants” or foreign born subjects without further specification [17, 21, 22]. Two studies referred to immigrants arrived within 5 years [7, 15]. One study considered both recent migrants and foreign born residents in US for more than 5 years [23].
In 5 studies the screening was carried out according to TB incidence in the country of origin regardless of age [11, 17, 19, 20, 23], whereas 3 studies compared the cost-effectiveness of screening migrants with different thresholds of TB incidence in the country of origin [14, 16, 21]. One study considered migrants from all developing countries [18].
Several LTBI diagnostic strategies (screening with CXR and follow-up of inactive TB, screening with TST, screening with TST followed by confirmation with Interferon gamma release assay - IGRA, screening with single step IGRA) were compared with each other and/or with different non-LTBI screening strategies including no screening, screening for active TB with CXR, and close-contact investigation (Table 1). Isoniazid for 6–9 months was the LTBI treatment regimen used in the majority of studies [11, 17, 19, 20, 22, 23], in one study isoniazid plus rifampicin for 3 months was used [16], in one study either isoniazid for 6 months or isoniazid plus rifampicin for 3 months were used [15], and in one study different LTBI treatment regimens (isoniazid for 9 months, rifampicin for 4 months, rifampicin plus pyrazinamide for 2 months) were compared [18]. In one study the LTBI treatment regimen was not specified [21].
All evaluations complied with the following quality criteria: presence of a clear hypothesis, methodology, selection of comparators, choice of appropriate benefits, and local applicability. Only four evaluations referred to clinical trials [15–17, 21], while medical evidence was derived from published literature in the remaining 6 studies. Appropriate direct and indirect health costs were considered in 8 assessments and sensitivity analysis was performed in seven evaluations. The lower quality study was the oldest [19]. Additional file 1: Table S1 presents different assumptions concerning progression rate and sensitivity as well as specificity of the TST and IGRAs applied in the different papers.
Economic evaluations of included studies
Cost-effectiveness analyses (CEA) were conducted in all EEs, and only one cost-benefit analysis (CBA) [16]. Cost-utility analysis (CUA) was performed in addition to CEA in three EEs [18, 20, 23]. EEs were conducted under the perspective of public health payer. Direct health costs were assessed in all studies while indirect costs were considered in one study [18]. As shown in Additional file 1: Table S2, four EEs were based on the results of specific studies while the remaining 6 assessments were based on available data in literature. Three studies [17, 20, 23] were carried out prospectively for 12 to 31 months. Decision modeling was adopted in 8 EEs with a time horizon up to 20 years [11, 15, 16, 19, 20] or lifetime [18, 23]. Data were collected from studies carried out ad hoc in two EEs [20], while models were fed with data publicly available in the remaining 6 EEs.
Screening for LTBI in migrants was cost-effective according to 8 studies [15–18, 20–23] and cost-saving according the selected scenarios in 3 of these studies [17, 18, 20]. In 5 out of 8 studies in which LTBI screening of migrants was cost-effective, a comparison between single step IGRA and TST or TST plus confirmatory IGRA was carried out [15, 16, 22–24]. In all 5 studies one step IGRA was the most cost-effective strategy (Quantiferon®, QTF in 4 studies [15, 16, 21, 22] and an unspecified IGRA in the remaining study [23]). In the 2 studies in which LTBI screening strategy was not cost-effective (dominated), the dominant strategies were contact tracing investigations in one case [17], and screening for active TB with CXR in the other case [11].
One article found that screening for LTBI with a single step IGRA was cost-effective regardless of time since immigration [23], while in other studies the cost-effectiveness was evaluated in recent migrants only.
One study compared the cost-effectiveness of screening stratified by age and showed that screening was more cost-effective when addressing recent immigrant adults than children and that screening of US foreign-born residents aged less than 45 years was more cost-effective than screening of foreign-born residents aged 45–64 years [23]. In this study patient age affected cost-effectiveness results through its impact on the lifetime risk of reactivation. In 3 other studies, in which LTBI screening was cost-effective, the analysis was limited to children [17], or young adults [15, 16].
Three studies compared the cost-effectiveness of screening at different thresholds of TB incidence in the country of origin. According to one study, the highest cost-effectiveness was reached by screening immigrants from countries with TB incidence above 200/100,000 while for two other studies, the threshold was 250/100,000 [15, 16, 21].
One article provided information on which LTBI treatment regimen (among isoniazid for 9 months, rifampicin for 4 months or rifampicin plus pyrazinamide for 2 months) would be more cost-effective according to migrants country of origin showing that pyrazinamide plus rifampicin for 2 months would be the most cost-effective treatment for migrants from Vietnam, Philippines, and Haiti, while isoniazid would be the most advantageous regimen for other migrants [18].
Discussion
According to the majority of studies included in this review, at least three important elements can be underlined. First, screening programs for detecting and treating LTBI in immigrants lead to substantial health and economic benefits under a societal perspective. Second, a one-step IGRA-protocol is the most cost-effective strategy for LTBI screening in migrants. Third, targeting young migrants from countries at higher incidence of TB increases the cost-effectiveness of screening.
Limitations and possible biases should be considered. First, our review, even if performed in a systematic methodology, did not respect all the criteria for systematic reviews (for example we consulted only two electronic database) so we cannot make strong and quantitative conclusions. Secondly, we don’t know whether the LTBI screening strategy that seems more cost-effective according to the majority of the selected studies (the use of IGRA) would be cost-effective in all context/jurisdiction, as this heavily depends on the country-specific treatment procedures and costs. Furthermore, we don’t have the quality or quantitative weights of each study to be able to make a pooled conclusion on cost-effectiveness. All these issues can potentially be solved in a meta-analysis, that is not attempted in this review given the high inhomogeneities among the different studies included.
Additionally, all the selected studies included in the review were carried out in countries were a CXR at entry is currently performed for active TB screening of newly arrived migrants. Most of the papers aimed at defining cost-effectiveness of adding screening for LTBI to the ongoing CXR-based screening for active TB [15, 18–21, 23]. The cost-effectiveness of LTBI screening of migrants population should also be evaluated in other low endemic countries.
While most of the studies found that screening programs for detecting and treating LTBI lead to substantial health and economic benefits under a societal perspective, other studies reached opposite conclusions. The importance of how methodological differences affect results of EEs has been recently demonstrated in a systematic review on methodological aspects of cost-effective analysis of IGRAs for the diagnosis of LTBI [24]. According to this study, some of the most relevant contributing factors generating different conclusions are the study inputs selection, the inconsistencies in the costing approach, the utility of the QALY (Quality Adjusted Life Year) as the effectiveness outcome, and the manner in which authors choose to present and interpret study results.
Among the studies selected in our review, Oxlade O et al. state that screening for active TB with CXR would be the most cost-effective and QFT the least cost-effective for screening of migrants on arrival [11], while Pareek M et al. concluded that mandatory CXR on arrival could be safely eliminated in order to improve screening cost-effectiveness with single-step IGRA [16]. Opposite conclusions were reached because Oxlade O et al. assumed a very low prevalence of LTBI in arriving immigrants (0.08–2.1 %) [11], while Pareek M et al. assumed a high rate of LTBI treatment completion (85 % in case base scenario) [16].
Studies performed under program conditions have shown that completion of LTBI treatment could be much lower that what was assumed in the study published by Pareek M et al. [16] with a better trend observed when shorter and unsupervised regimen are used [25]. It will be interesting to evaluate the completion rate and cost-effectiveness of shorter regimen such as weekly-administered rifapentine plus isoniazid for three month [26, 27] in migrants.
In migrants the diagnostic delay in cases of active TB is mainly related to the delayed presentation of the patients to the health system [28, 29] and it is responsible for the spread of the disease among other members of their community. Contact investigation should therefore be strengthened in migrants, as well as in the general population [30]. However, as assumed by the majority of economic models reviewed, screening and treatment for LTBI in migrants would prevent active TB cases and solve the problem at its roots.
The reliability of Markov models, where time horizons of 20 years or life time were used, may be matter of concern. In fact, in most cases, modeling was based on published or retrospective data while the prospective trials supporting three EEs were all open label and only one was controlled. The actual epidemiological data shows that the global TB burden is reducing, though at a slow pace, at global level [31], and in industrialized countries, particularly among native populations [32]. In this scenario, while the yield of the screening for LTBI will decrease over time, however the contribution to the reduction of incidence by diagnosis and treatment of LTBI will progressively increase.
Growing consensus indicates that progress in TB control in the low- and middle-income world will require not only investment in strengthening TB control programs, diagnostics, and treatment but also action on the social determinants of the disease [33]. To reduce the incidence of TB, the drivers of the epidemic and social determinants of TB need to be addressed. These include co-morbidities, substance use, the social and economic conditions that determine both the course of the TB epidemic and exposure to these risk factors [34]. This is probably true for control of TB in migrants that often live in disadvantaged socio-economic conditions in the host country with an increased risk of both, to reactivate LTBI or to acquire a new infection. In this perspective LTBI screening and treatment in migrants could represent a synergistic tool to achieve the ambitious aim of TB elimination.
Conclusions
The majority of the studies support the use of LTBI screening strategies in migrants based on their cost-effectiveness findings. When LTBI screening for migrants are implemented they should focus especially on young migrants from high incidence countries and effort should be done to maximize the adherence to LTBI treatment. In this view shorter LTBI treatment regimen are preferred. Based on our review of EE analysis studies, the use of one step IGRA is the best option in this particular setting. These findings should be confirmed by a cost-effectiveness evaluation based on a medium-term prospective study.
LTBI should be well integrated among the TB control program for migrants and must be part of a wider approach with the aim of facilitating access of migrants to the national health system, re-orienting health services, improving the adhesion to anti-tuberculosis treatment in cases of active TB, and promoting early diagnosis of active TB cases by primary care health operators [35].
References
European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2012. 2012.
Klinkenberg E, Manissero D, Semenza JC, Verver S. Migrant tuberculosis screening in the EU/EEA: yield, coverage and limitations. Eur Respir J. 2009;34(5):1180–9.
Codecasa LR, Porretta AD, Gori A, Franzetti F, Degli Esposti A, Lizioli A, et al. Tuberculosis among immigrants from developing countries in the province of Milan, 1993–1996. Int J Tuberc Lung Dis. 1999;3(7):589–95.
Lillebaek T, Andersen AB, Bauer J, Dirksen A, Glismann S, de Haas P, et al. Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. J Clin Microbiol. 2001;39(3):855–61.
Diel R, Rusch-Gerdes S, Niemann S. Molecular epidemiology of tuberculosis among immigrants in Hamburg, Germany. J Clin Microbiol. 2004;42(7):2952–60.
Lobato MN, Hopewell PC. Mycobacterium tuberculosis infection after travel to or contact with visitors from countries with a high prevalence of tuberculosis. Am J Respir Crit Care Med. 1998;158(6):1871–5.
Pareek M, Baussano I, Abubakar I, Dye C, Lalvani A. Evaluation of immigrant tuberculosis screening in industrialized countries. Emerg Infect Dis. 2012;18(9):1422–9.
Franzetti F, Codecasa L, Matteelli A, Degli Esposti A, Bandera A, Lacchini C, et al. Genotyping analyses of tuberculosis transmission among immigrant residents in Italy. Clin Microbiol Infect. 2010;16(8):1149–54.
Hernandez-Garduno E, Kunimoto D, Wang L, Rodrigues M, Elwood RK, Black W, et al. Predictors of clustering of tuberculosis in Greater Vancouver: a molecular epidemiologic study. CMAJ. 2002;167(4):349–52.
Alvarez GG, Gushulak B, Abu Rumman K, Altpeter E, Chemtob D, Douglas P, et al. A comparative examination of tuberculosis immigration medical screening programs from selected countries with high immigration and low tuberculosis incidence rates. BMC Infect Dis. 2011;11:3.
Oxlade O, Schwartzman K, Menzies D. Interferon-gamma release assays and TB screening in high-income countries: a cost-effectiveness analysis. Int J Tuberc Lung Dis. 2007;11(1):16–26.
Broekmans J, Migliori G, Rieder H, Lees J, Ruutu P, Loddenkemper R, et al. European framework for tuberculosis control and elimination in countries with a low incidence. Recommendations of the World Health Organization (WHO), International Union Against Tuberculosis and Lung Disease (IUATLD) and Royal Netherlands Tuberculosis Association (KNCV) Working Group. Eur Respir J. 2002;19(4):765–75.
World Health Organization. Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: World Health Organization; 2014. EB134R4.
Haycox A, Walley T. Pharmacoeconomics: evaluating the evaluators. Br J Clin Pharmacol. 1997;43:451–6.
Pareek M, Watson JP, Ormerod LP, Kon OM, Woltmann G, White PJ, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis. 2011;11(6):435–44.
Pareek M, Bond M, Shorey J, Seneviratne S, Guy M, White P, Lalvani A, Kon OM. Community-based evaluation of immigrant tuberculosis screening using interferon γ release assays and tuberculin skin testing:observational study and economic analysis. Thorax. 2013 Mar;68(3):230-9. doi:10.1136/thoraxjnl-2011-201542. Epub 2012.
Brassard P, Steensma C, Cadieux L, Lands LC. Evaluation of a school-based tuberculosis-screening program and associate investigation targeting recently immigrated children in a low-burden country. Pediatrics. 2006;117(2):e148–56.
Khan K, Muennig P, Behta M, Zivin JG. Global drug-resistance patterns and the management of latent tuberculosis infection in immigrants to the United States. N Engl J Med. 2002;347(23):1850–9.
Dasgupta K, Schwartzman K, Marchand R, Tennenbaum TN, Brassard P, Menzies D. Comparison of cost-effectiveness of tuberculosis screening of close contacts and foreign-born populations. Am J Respir Crit Care Med. 2000;162(6):2079–86.
Porco TC, Lewis B, Marseille E, Grinsdale J, Flood JM, Royce SE. Cost-effectiveness of tuberculosis evaluation and treatment of newly-arrived immigrants. BMC Public Health. 2006;6:157.
Hardy AB, Varma R, Collyns T, Moffitt SJ, Mullarkey C, Watson JP. Cost-effectiveness of the NICE guidelines for screening for latent tuberculosis infection: the QuantiFERON-TB Gold IGRA alone is more cost-effective for immigrants from high burden countries. Thorax. 2010;65(2):178–80.
Iqbal AZ, Leighton J, Anthony J, Knaup RC, Peters EB, Bailey TC. Cost-effectiveness of using Quantiferon Gold (QFT-G)(R) versus tuberculin skin test (TST) among U.S. and foreign born populations at a public health department clinic with a low prevalence of tuberculosis. Public Health Nurs (Boston, Mass). 2014;31(2):144–52.
Linas BP, Wong AY, Freedberg KA, Horsburgh Jr CR. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2011;184(5):590–601.
Oxlade O, Pinto M, Trajman A, Menzies D. How methodologic differences affect results of economic analyses: a systematic review of interferon gamma release assays for the diagnosis of LTBI. PLoS One. 2013;8(3), e56044.
Matteelli A, Casalini C, Raviglione MC, El-Hamad I, Scolari C, Bombana E, et al. Supervised preventive therapy for latent tuberculosis infection in illegal immigrants in Italy. Am J Respir Crit Care Med. 2000;162(5):1653–5.
Sterling T, Munsiff SS, Frieden TR. Management of latent tuberculosis infection in immigrants. N Engl J Med. 2003;348(13):1289–92. author reply 1289–1292.
Shepardson D, Marks SM, Chesson H, Kerrigan A, Holland DP, Scott N, et al. Cost-effectiveness of a 12-dose regimen for treating latent tuberculous infection in the United States. Int J Tuberc Lung Dis. 2013;17(12):1531–7.
Gagliotti C, Resi D, Moro ML. Delay in the treatment of pulmonary TB in a changing demographic scenario. Int J Tuberc Lung Dis. 2006;10(3):305–9.
Mor Z, Kolb H, Lidji M, Migliori G, Leventhal A. Tuberculosis diagnostic delay and therapy outcomes of nonnational migrants in Tel Aviv, 1998-2008. Euro Surveill. 2013;18(12). pii: 20433.
Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41(1):140–56.
World Health Organization. Global tuberculosis control 2011. 2011.
European Centre for Disease Control and Prevention, World Health Organization Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2012. In: Surveillance report. Stockholm: European Centre for Disease Control and Prevention; 2012. p. 73–97.
Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101(4):654–62.
Rasanathan K, Sivasankara Kurup A, Jaramillo E, Lonnroth K. The social determinants of health: key to global tuberculosis control. Int J Tuberc Lung Dis. 2011;15 Suppl 2:S30–6.
Ministero della Salute. Aggiornamento delle raccomandazioni per le attività di controllo della tubercolosi. “Politiche efficaci a contrastare la tubercolosi nella popolazione immigrata”. 2010.
Acknowledgments
Authors thank the COHEMI (COordinating resources to assess and improve HEalth status of MIgrants from Latin America)-project study group that includes: Maurizio Bonatia, Chiara Pandolfinia, Francesca Severinoa, Valeria Confalonieria, Gianni Tognonia, Zeno Bisoffib, Dora Buonfrateb, Andrea Anghebenb, Marco Albonicob,c, Alessandro Bartolonid, Marianne Strohmeyerd, Lorenzo Zammarchid, Filippo Bartalesie, Jose Muñozf, Ana Requena-Mendezf, Maria Rouraf, LaiaVenturaf, Robert Poolg, Christopher Pellg, Anita Hardong, Peter Chiodinij, Juan Moreirak, Mariella Anselmik, Roberto Sempérteguik, Eduardo Gotuzzol, Maria Alejandra Menal, Carola Liendol, Héctor H. Garciam, Javier Bustosm, Saul Santivañezm, Faustino Torricon, Daniel Lozanon, Teresa Hinojosa Cabrerao, Javier Ochoa Moróno, Ignacio AbaporiCuellaro, Jaime Amorós Suarezo, Guido ChumirayRojaso, Alessandra Nicolettip, Elisa Brunop.
a) Department of Public Health, Laboratory for Mother and Child Health, IRCCS–Istituto di RicercheFarmacologiche Mario Negri, Milan, Italy.
b) Centre for Tropical Diseases, SacroCuore-Don Calabria Hospital, Negrar, Verona, Italy.
c) Ivo de Carneri Foundation, Milano, Italy.
d) Infectious Disease Unit, Department of Experimental & Clinical Medicine, University of Florence School of Medicine, Florence, Italy.
e) SOD Malattie Infettive e Tropicali, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy.
f) Servicio de Medicina Tropical y SaludInternacional, Centre de Recerca en SalutInternacional de Barcelona, Hospital Clínic-Universitat de Barcelona, Barcelona,Spain
g) Centre for Social Science and Global Health, University of Amsterdam, The Netherlands.
j) Hospital for Tropical Diseases and London School of Hygiene & Tropical Medicine, London, United Kingdom.
k) Centre for Community Epidemiology and Tropical Medicine, Esmeraldas, Ecuador.
l) Instituto de Medicina Tropical Alexander von Humboldt, Universidad Cayetano Heredia, Lima, Peru.
m) Cysticercosis Unit, Instituto de CienciasNeurologicas, Department of Microbiology, Universidad PeruanaCayetano Heredia, Lima, Peru.
n) Colectivo de EstudiosAplicados y Desarrollo Social, Cochabamba, Bolivia.
o) Taller de Educacion y Comunicacion TEKO-GUARANÌ, Camiri, Bolivia.
p) Dipartimento G.F. Ingrassia Sezione di Neuroscienze, Università di Catania, Catania, Italy.
Availability of data and materials
Not applicable.
Funding source
This work has been supported by the European Commission within the 7th Framework Programme under the COHEMI (COordinating resources to assess and improve HEalth status of MIgrants from Latin America)-project - grant agreement no. FP7-GA-261495.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the study: AB, FB, EG, MB. Performed the study: LZ, GC, MS, CL. Analyzed the data: LZ, GC, MB. Wrote the paper: LZ, GC, MB, AM, AB. All authors read and approved the final manuscript.
Authors' information
Not applicable.
Additional file
Additional file 1: Table S1.
Different assumptions concerning progression rate and sensitivity as well as specificity of the TST and IGRAs applied in the different papers. Table S2. Clinical trials referring to economic evaluations. (DOCX 22 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zammarchi, L., Casadei, G., Strohmeyer, M. et al. A scoping review of cost-effectiveness of screening and treatment for latent tuberculosis infection in migrants from high-incidence countries. BMC Health Serv Res 15, 412 (2015). https://doi.org/10.1186/s12913-015-1045-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-015-1045-3