Abstract
Background
Vaccination-impact studies of the live-attenuated pentavalent oral vaccine Rotateq® have demonstrated that the burden of rotavirus gastroenteritis has been reduced significantly after the introduction of RotaTeq® vaccination, but less is known about the benefit of this vaccination on hospital overcrowding.
Methods
As part of an observational surveillance conducted during the RV seasons 2000/2001 to 2011/2012, we analysed hospital discharge data collected retrospectively from two Finnish hospitals (Oulu and Tampere), concerning ICD 10 codes A00-09 (acute gastroenteritis, AGE) and A08.0 (rotaviral acute gastroenteritis RV AGE). We estimated the reduction in the number of beds occupied and analysed the bed occupancy rate, for RV AGE and all cause AGE, among 0–16 year-old children, before and after the implementation of the RV immunisation program.
Results
The rate of bed days occupied for RV AGE was reduced by 86% (95% CI 66%-94%) in Tampere and 79% (95% CI 47%-92%) in Oulu after RV vaccination implementation. For all cause AGE, reduction was 50% (95% CI 29% to 65%) in Tampere and 70% (95% CI 58% to 79%) in Oulu. Results were similar among 0–2 year-old children. This effect was also observed on overcrowding in both hospitals, with a bed occupancy rate for all cause AGE >25% in only 1% of the time in Tampere and 9% in Oulu after the implementation of the immunisation program, compared to 13% and 48% in the pre-vaccination period respectively. After extrapolation to the whole country, the annual number of prevented hospitalizations for all cause AGE in the post-vaccination period in Finland was estimated at 1,646 and 2,303 admissions for 0–2 and 0–16 year-old children respectively.
Conclusions
This study demonstrated that universal RV vaccination is associated with a clear decrease in the number of bed days and occupancy rates for RV AGE and all cause AGE. Positive consequences include increase in quality of care and a better healthcare management during winter epidemics.
Similar content being viewed by others
Background
In the pre-vaccination era rotavirus (RV) has been estimated to be responsible for two thirds of hospitalizations and emergency department consultations for acute gastroenteritis (AGE) in children [1] and it was estimated that RV infections lead to a yearly average of about 700,000 outpatient visits, more than 87,000 hospitalizations and 231 death in Europe [2].
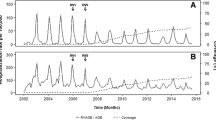
Seasonal peaks of RV gastroenteritis occur yearly from November to May [3] and coincide in many countries with other winter epidemics such as bronchiolitis due to respiratory syncytial virus (RSV) and influenza [4]-[7] (Figure 1), leading potentially to overcrowding with increased risks for nosocomial infections and lessened quality of patient care. In the literature rotavirus has been repeatedly mentioned as a major cause of hospital overcrowding during the winter season [3],[6],[7].
Coincidence of rotavirus, RSV and influenza from national surveillance data in Finland. From THL: http://www3.thl.fi/stat/.
The live-attenuated pentavalent oral vaccine Rotateq® was licensed in 2006. Postlicensure studies have shown high vaccine effectiveness in preventing hospitalization for RV gastroenteritis in the field, ranging from 83% to 98% [8]-[11], and consistent with pre-licensure efficacy results.
Furthermore, vaccination-impact studies have demonstrated that the burden of rotavirus gastroenteritis has been reduced significantly after the introduction of RotaTeq® vaccination. Evidence included reductions in healthcare utilization due to RV AGE (hospitalizations and emergency-department visits reduced by up to 90%), reductions in the magnitude and duration of the RV season as assessed by laboratory testing for RV, and the possible induction of herd immunity [12].
Less is known about the benefit of the implementation of immunization programs with Rotateq® to avoid situations of seasonal hospital overcrowding. Several studies have been conducted to evaluate overcrowding associated with AGE in emergency departments [13],[14], with one study specifically assessing the workload in pediatric emergency units during the epidemic season [15], no study to our knowledge has measured seasonal hospital disruption due to RV gastroenteritis.
Several indicators have been studied to estimate emergency department crowding and two scales for quantifying crowding in emergency departments have been developed: NEDOCS and EDWIN [16]-[19]. However, no consensus and no indicators have been developed to measure hospital disruption. We propose to use bed occupancy rates as a proxy to assess situations of hospital overcrowding.
A prospective surveillance study was established in 2009 at two University hospitals in Finland (Tampere and Oulu) to document the number of cases of pediatric AGE requiring hospitalization, and the role of rotavirus and specific serotypes in those cases in the first three years after the implementation of the universal vaccination program with RotaTeq®. The study showed that after the implementation of universal RotaTeq® vaccination, RV AGE was virtually eliminated among children age-eligible for the vaccination program [11].
In the frame of this study, a retrospective analysis of the discharge database was performed to evaluate the impact of the introduction of RV vaccines into the national vaccination program on the rotavirus infections at hospitals.
The aim of the present analysis was to evaluate the benefit of the implementation of the National Immunization Program with Rotateq® to avoid situations of seasonal hospital overcrowding.
Methods
The study was conducted in accordance with the Declaration of Helsinki, Good Pharmacoepidemiology Practice guidelines and local laws, rules and regulations. The ethics committee of Pirkanmaa Hospital District (Tampere) reviewed and approved the study protocol and amendments. The study was also approved by the head of each hospital.
In the frame of an observational surveillance study published elsewhere [11], hospital discharge data were retrospectively collected from Tampere and Oulu hospitals. We further analyzed the data of this retrospective study part for the RV seasons 2000/2001 to 2011/2012. The following information was extracted from the hospital information systems (discharge registers) and analyzed: ICD 10 codes A00-09 (all codes for intestinal infectious diseases), A08.0 (rotaviral enteritis).
Admission and discharge dates were used to calculate the number of beds occupied daily by infants and children diagnosed with RV AGE and all cause AGE (including RV AGE) during the RV seasons 2000/2001 to 2011/2012. In some cases, e.g. when patients were transferred from one hospital unit to another, or if a second admission occurred less than 14 days after a first discharge and if both admissions were coded with different ICD discharge codes (among A00-A09), one of them being A08.0 (the code for RV AGE), this one was selected as this code was only allocated in case of positive routine laboratory ELISA test result. In addition to clinical diagnostic, some samples are sometimes taken as part of the usual care of patients in both hospitals. In this case, PCR was done to identify precisely the type of RV causing the disease. Consequently, most reliable figures are the all cause AGE which do not depend on local diagnostic and testing practices.
The 0–16 year age group was chosen to follow hospital admissions including age groups not targeted by the vaccination, but who may be subject to herd immunity.
The period of 2000/01 to 2005/06 was considered as the pre-vaccination period, the period of 2006/07 to 2008/09 as a transition period during which both rotavirus vaccines (RotaTeq® and Rotarix®) were available in Finland, but with lower coverage rates (reaching 30-35%) [20], as the vaccines were not included into the national vaccination program. Universal routine vaccination against RV AGE was started in Finland on September 1st, 2009 and the period of 2009/10 to 2011/12 was thus considered as post-vaccination period. Each season started the 1st of September and ended the 31th of August of the following year.
The total number of bed days for RV AGE and for all cause AGE was reported by vaccination periods and seasons for each hospital. To assess the impact of the national RV vaccination program, two statistics were calculated. The first was the relative reduction of the annual number of bed days between the post- and pre-vaccination periods, and the second was the absolute reduction (difference) (95% CI) of the number of bed days, based on a vaccine probe approach [21]. For both estimates, we calculated 95% confidence interval (CI) based on the negative binomial distribution in order to take into account for over dispersion. Rates of bed days per 100,000 children were calculated based on children population figures from Statistics Finland available at http://www.stat.fi/til/vaerak/tau_en.html.
The proportions of RV positive cases among all causes AGE were also described per period and season and trend in these proportions between the post- and pre-vaccination periods was studied using a chi-squared test for trend in proportions.
We compared the number of beds occupied for all cause AGE and available beds per day, by estimating a bed occupancy rate defined as the ratio between the number of beds occupied for all cause AGE and the number of available beds in Tampere (n = 16) and in Oulu (n = 9). The bed occupancy rate was then stratified in 5 classes, 0-25%, 26-50%, 51-75%, 76-100% and >100%. The distribution of days according to the defined bed occupancy classes was compared between the post- and pre-vaccination periods using a chi-squared test.
We extrapolated the results of this study to the whole country by estimating the 2013 annual absolute number of bed days and prevented bed days after the introduction of the national RV program in Finland. For this, we pooled the data of Tampere and Oulu by period (i.e. pre- and post-vaccination) to calculate the rates (and its 95% CI) of number of bed days and number of prevented bed days per 100,000 children aged respectively 0–16 and 0–2. Then, we applied these rates to the whole national population figures of children 0–16 and 0–2 years in 2013. Population figures were extracted from Statistics Finland, available at: http://www.stat.fi/til/vaerak/tau_en.html. Analyses were done using SAS, SPSS and R, and p-values <0.05 were considered as statistically significant.
Results
In Tampere, the overall number of bed days per 100,000 0–2 year-old children for RV AGE decreased from 925 in the pre-vaccination to 70 in the post-vaccination period corresponding to a statistically significant reduction of 93% (95% CI 77%-98%) (Table 1). Similar results were observed when enlarging the age group to children up to 16 years, with the overall number of bed days per 100,000 children for RV AGE decreasing from 220 to 31, corresponding to a reduction of 86% (95% CI 66%-94%). For all cause AGE, a lower decrease from 2,839 bed days per 100,000 children in the pre-vaccination era to 972 bed days per 100,000 children in the post-vaccination era was observed among 0-2y children, corresponding to a reduction of 66% (95% CI 49% to 77%) and from 886 to 440 bed days per 100,000 children, thus a 50% reduction (95% CI 29% to 65%) among 0-16y children. Table 1 also presented the corresponding absolute reduction of the number of bed days per 100,000 children.
In Oulu, the average annual number of bed days for RV AGE per 100,000 0–2 year -old children decreased from 1,017 in the pre-vaccination to 170 in the post-vaccination period corresponding to a statistically significant reduction of 83% (95% CI 35%-96%) (Table 2). For 0–16 year-old children, the average annual number of bed days per 100,000 children for RV AGE decreased from 261 to 54, corresponding to a reduction of 79% (95% CI 47%-92%). For all cause AGE, a lower decrease from 3,850 bed days per 100,000 children in the pre-vaccination era to 757 bed days per 100,000 children in the post-vaccination era was observed among 0-2y children, corresponding to a reduction of 80% (95% CI 68% to 88%) and from 1,233 to 365 bed days per 100,000 children, thus a 70% reduction (95% CI 58% to 79%) among 0-16y children. Table 2 also presented the corresponding absolute reduction of the number of bed days per 100,000 children.
The proportion of RV positive cases among all cause AGE was also significantly reduced in the post-vaccination period compared to the pre-vaccination period in 0-16y children and 0-2y children from Tampere (Table 1) and in 0-16y children from Oulu (Table 2). The proportion of RV positive cases among all cause AGE in 0-2y children from Oulu did not significantly vary between vaccination periods (22.4% vs 26.4%, p = 0.087) and this was mainly due to the high proportion observed during the 2009/10 season.
In both hospitals in Oulu and in Tampere, bed occupancy for all cause AGE was significantly lower in the post-vaccination period than in the pre-vaccination period (Table 3, Figure 2). In Oulu, while bed occupancy for all cause AGE was >25% during 774 (48%) days in the pre-vaccination period, it decreased to 103 (9%) in the post-vaccination period. In Tampere, these figures were 235 (13%) in the pre-vaccination period and 12 (1%) in the post-vaccination period.
Table 4 shows the estimated national absolute number of bed days and number of prevented bed days after the introduction of the national RV immunization program in Finland which prevented 6,219 (95% CI, 5,956-6,483) bed days for all cause AGE for 0–16 year-old children and 4,443 (95% CI, 4,252-4,633) for 0–2 year-old children, leading to a number of bed days for all cause AGE of 3,846 (95% CI, 3,682-4,009) for 0–16 year-old children and of 1,552 (95% CI, 1,451-1,653) for 0–2 year-old children in 2013.
The average length of stay for all cause AGE decreased significantly from 3.1 days in the pre-vaccination to 2.6 days in the post-vaccination period (p < 0.001) in Tampere and from 2.8 days to 2.4 (p < 0.001) in Oulu.
Discussion
This study highlights the substantial impact of the national RV vaccination program on the reduction of the number of bed days for RV AGE and to a lesser extend for all cause AGE in two Finnish hospitals. Reduction in AGE hospitalizations non coded specifically as rotavirus suggest that testing and coding practices may underestimate the true rate of rotavirus hospitalizations as it has been described elsewhere [22]. Additionally, the mean length of hospital stay per episode decreased when comparing pre-vaccination periods with post-vaccination periods. Overall results suggest that universal vaccination with RotaTeq® led to a dramatic decrease of hospital overcrowding reducing the risk for nosocomial infections and situations of hospital disruption with negative adverse effects on the overall quality of patient care. These results are supported by the vaccine-probe design that allowed estimating the vaccine-preventable RV AGE and all AGE incidence, and extrapolate these results at the nationwide scale.
Our study presents some limitations. We cannot exclude that not all patients were tested, as hospitalization time may in some cases have been too short to allow children producing stool during their stay at hospital. When a laboratory result was not available at the time of hospital discharge of a patient, the case could not be recorded as RV AGE. Thus rotavirus as a cause for AGE is likely to be underestimated. We conclude that the reduction of the mean hospital length of stay could be attributable to the rotavirus vaccination program but cannot exclude that a modification of the hospital organization has occurred during the 10-year study period. Variability was observed in the population-based rates of severe rotavirus infections each year and this natural secular variability in rotavirus disease should be considered in the assessment of the impact of the vaccine, as it has been reported elsewhere [9],[23],[24]. This may explain the high rates of RV AGE in the transition period and the first year of the post vaccination period in the Oulu hospital.
In 2007 the Finnish National Institute for Health and Welfare (THL) estimated that without vaccination about 2,400 hospital admissions, 3,600 hospital outpatient visits, 9,000 primary care physician consultations and 0.5 deaths attributable to rotavirus would occur annually among children under 5 years of age in Finland [25]. When extrapolating the results of this study to the whole country, we estimated that 6,129 and 4,443 bed days for all cause AGE were prevented in 2013 by rotavirus vaccination in children 0–16 years and 0–2 years respectively. Assuming a hospital length of stay of 2.7 days (Oulu: 2.6 days and Tampere: 2.8 days) in the post-vaccination period, the annual number of prevented hospitalizations for all cause AGE can be estimated at 1,646 and 2,303 admissions for 0–2 and 0–16 year-old children respectively, thus confirming the assumptions made by Salo et al. in 2007 [25] who estimated that universal rotavirus vaccination could prevent some 2,000 hospital admissions in under five year-old children yearly.
Finland represents a privileged place to study RV disease and the impact of RV vaccination due to the high burden of RV and high vaccine uptake. It was one of the first countries in Europe recommending rotavirus vaccination in December 2007. In September 2009 it introduced universal rotavirus vaccination with exclusive use of the pentavalent human-bovine reassortant rotavirus vaccine RotaTeq® and following a vaccination schedule at age 2, 3 and 5 months reaching coverage rates of about 96% for the vaccines included in the NIP. In Finland, the RV related burden of disease was extensively studied through observational studies [26]-[28] and through data collected during the clinical development of rotavirus vaccines [2],[29]-[32].
The impact of RV vaccination on the number of hospitalization was reported previously [11],[32]-[36]. The present study describes that RV vaccination can also reduce seasonal hospital overcrowding due to RV AGE during winter epidemics by assessing the reduction in the number of occupied beds per day. While RV AGE hospitalization only describes the number of patients presenting at hospital with RV AGE, bed occupancy rather reflects the pressure that hospitals and health care workers face every day and specifically in periods of overlapping winter epidemics. Overcrowding occurs when new admissions for RV AGE exceed discharge of recovered patients, with adverse consequences such as lower quality of care [15], increased risk of nosocomial transmission among patients and healthcare workers (HCWs) [37],[38], work stress [37]-[40], which may lead to hospital disruption with unsolved ethical and organizational issues [3],[41]-[43].
We used bed occupancy to estimate crowding due to all cause AGE in children 0–16 year-old. While several indicators have been studied to estimate emergency department crowding with NEDOCS and EDWIN [16]-[19], no consensus and no indicators have been developed to measure hospital disruption. It may be reasonable to think that high occupancy rates (e.g. >50%) are associated with hospital disruption and impaired care. In addition to that a previous study conducted in a ward for paediatric infectious diseases at the Hospital of Oulu identified room sharing as one of the factors increasing the risk of hospital acquired infections [44].
Conclusions
In conclusion, this study demonstrated that universal RV vaccination is associated with a clear decrease in the number of bed days and occupancy rates for RV AGE and all cause AGE. As a consequence, it is expected that RV vaccination increases quality of care by allowing a better management of overlapping winter epidemics, such as bronchiolitis or influenza and by decreasing associated costs for healthcare institutions.
References
Van Damme P, Giaquinto C, Huet F, Gothefors L, Maxwell M, Van der Wielen M: Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis. 2007, 195 (Suppl 1): S4-S16. 10.1086/516714.
Soriano-Gabarro M, Mrukowicz J, Vesikari T, Verstraeten T: Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J. 2006, 25: S7-S11. 10.1097/01.inf.0000197622.98559.01.
Bruijning-Verhagen P, Sankatsing V, Kunst A, van den Born C, Bleeker E, Thijsen S, Ijzerman EP, van der Velden VH, Bonten MJ: Rotavirus-related hospitalizations are responsible for high seasonal peaks in all-cause pediatric hospitalizations. Pediatr Infect Dis J. 2012, 31: e244-e249. 10.1097/INF.0b013e31826a5ba1.
Armengaud JB, El Hajje MJ, Moulin F, Marc E, Chalumeau M, Lebon P, Gendrel D: [Simultaneous outbreaks of rotavirus and respiratory syncytial virus in Paris: a 12-year survey]. Med Mal Infect. 2007, 37: 262-265. 10.1016/j.medmal.2007.02.005.
Gendrel D, Basse N, Palmer P, Marc E, Taty-Taty R, Ravilly S, Moulin F, Raymond J, Lebon P: [Coincidental outbreaks of rotavirus and respiratory syncytial virus in Paris: a survey from 1993 to 1998]. Arch Pediatr. 1999, 6: 735-739. 10.1016/S0929-693X(99)80355-8.
Gleizes O, Desselberger U, Tatochenko V, Rodrigo C, Salman N, Mezner Z, Giaquinto C, Grimprel E: Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J. 2006, 25: S12-S21. 10.1097/01.inf.0000197563.03895.91.
Meakins SM, Adak GK, Lopman BA, O'Brien SJ: General outbreaks of infectious intestinal disease (IID) in hospitals, England and Wales, 1992–2000. J Hosp Infect. 2003, 53: 1-5. 10.1053/jhin.2002.1326.
Castilla J, Beristain X, Martinez-Artola V, Navascues A, Garcia Cenoz M, Alvarez N, Polo I, Mazon A, Gil-Setas A, Barricarte A: Effectiveness of rotavirus vaccines in preventing cases and hospitalizations due to rotavirus gastroenteritis in Navarre, Spain. Vaccine. 2011, 30: 539-543. 10.1016/j.vaccine.2011.11.071.
Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperriere N, Abalea L, Poulhazan E, Jossens A, Auzanneau L, Tran A, Payan C, Jay N, de Parscau L, Oger E: Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine. 2011, 29: 3753-3759. 10.1016/j.vaccine.2011.03.035.
Martinon-Torres F, Bouzon Alejandro M, Redondo Collazo L, Sanchez Lastres JM, Pertega Diaz S, Seoane Pillado MT, Martinon Sanchez JM: Effectiveness of rotavirus vaccination in Spain. Hum Vaccin. 2011, 7: 757-761. 10.4161/hv.7.7.15576.
Vesikari T, Uhari M, Renko M, Hemming M, Salminen M, Torcel-Pagnon L, Bricout H, Simondon F: Impact and Effectiveness of Rotateq(R) Vaccine Based on Three Years of Surveillance Following Introduction of a Rotavirus Immunization Program in Finland. Pediatr Infect Dis J 2013..
Giaquinto C, Dominiak-Felden G, Van Damme P, Myint TT, Maldonado YA, Spoulou V, Mast TC, Staat MA: Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: a systematic review of the experience in industrialized countries. Hum Vaccin. 2011, 7: 734-748. 10.4161/hv.7.7.15511.
Fourquet F, Desenclos JC, Maurage C, Baron S: [Acute gastro-enteritis in children in France: estimates of disease burden through national hospital discharge data]. Arch Pediatr. 2003, 10: 861-868. 10.1016/S0929-693X(03)00459-7.
Harrington M, Butler K, Cafferkey M: Rotavirus infection in hospitalised children: incidence and impact on healthcare resources. Ir J Med Sci. 2003, 172: 33-36. 10.1007/BF02914784.
Haas H, Suau B, Allaert FA, Sana C, Caulin E: [Assessment of workload in pediatric emergency units due to acute gastroenteritis during the epidemic season in France]. Med Mal Infect. 2008, 38: 642-647. 10.1016/j.medmal.2008.10.013.
Hwang U, Concato J: Care in the emergency department: how crowded is overcrowded?. Acad Emerg Med. 2004, 11: 1097-1101. 10.1111/j.1553-2712.2004.tb00686.x.
Solberg LI, Asplin BR, Weinick RM, Magid DJ: Emergency department crowding: consensus development of potential measures. Ann Emerg Med. 2003, 42: 824-834. 10.1016/S0196-0644(03)00816-3.
Weiss SJ, Derlet R, Arndahl J, Ernst AA, Richards J, Fernandez-Frackelton M, Schwab R, Stair TO, Vicellio P, Levy D, Brautigan M, Johnson A, Nick TG: Estimating the degree of emergency department overcrowding in academic medical centers: results of the National ED Overcrowding Study (NEDOCS). Acad Emerg Med. 2004, 11: 38-50. 10.1197/j.aem.2003.07.017.
Weiss SJ, Ernst AA, Derlet R, King R, Bair A, Nick TG: Relationship between the National ED Overcrowding Scale and the number of patients who leave without being seen in an academic ED. Am J Emerg Med. 2005, 23: 288-294. 10.1016/j.ajem.2005.02.034.
Rasanen S, Lappalainen S, Halkosalo A, Salminen M, Vesikari T: Rotavirus gastroenteritis in Finnish children in 2006–2008, at the introduction of rotavirus vaccination. Scand J Infect Dis. 2010, 43: 58-63. 10.3109/00365548.2010.508462.
Feikin DR, Scott JA, Gessner BD: Use of vaccines as probes to define disease burden. Lancet. 2014, 383: 1762-1770. 10.1016/S0140-6736(13)61682-7.
Martinon-Torres F, Martinon-Torres N, Bouzon Alejandro M, Redondo Collazo L, Pertega-Diaz S, Seoane-Pillado MT, Aboal Vinas J, San-Martin M: Acute gastroenteritis hospitalizations among children aged < 5 years before and after introduction of rotavirus vaccines: a hospital-based surveillance study in Galicia, Spain. Hum Vaccin Immunother. 2012, 8: 946-952. 10.4161/hv.20178.
Atchison C, Lopman B, Edmunds WJ: Modelling the seasonality of rotavirus disease and the impact of vaccination in England and Wales. Vaccine. 2010, 28: 3118-3126. 10.1016/j.vaccine.2010.02.060.
Tate JE, Parashar UD: Monitoring impact and effectiveness of rotavirus vaccination. Expert Rev Vaccines. 2011, 10: 1123-1125. 10.1586/erv.11.94.
Salo H, Ollgren J, Linna M, Sintonen H, Kilpi T: Economic Evaluation of rotavirus vaccination in Finland. Eur J Public Health. 2007, 17: 210-211.
Ruuska T, Vesikari T: Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990, 22: 259-267. 10.3109/00365549009027046.
Ruuska T, Vesikari T: A prospective study of acute diarrhoea in Finnish children from birth to 2 1/2 years of age. Acta Paediatr Scand. 1991, 80: 500-507. 10.1111/j.1651-2227.1991.tb11893.x.
Vesikari T, Rautanen T, Von Bonsdorff CH: Rotavirus gastroenteritis in Finland: burden of disease and epidemiological features. Acta Paediatr Suppl. 1999, 88: 24-30. 10.1111/j.1651-2227.1999.tb14322.x.
Joensuu J, Koskenniemi E, Pang XL, Vesikari T: Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997, 350: 1205-1209. 10.1016/S0140-6736(97)05118-0.
Pang XL, Koskenniemi E, Joensuu J, Vesikari T: Effect of rhesus rotavirus vaccine on enteric adenovirus–associated diarrhea in children. J Pediatr Gastroenterol Nutr. 1999, 29: 366-369. 10.1097/00005176-199909000-00026.
Takala AK, Koskenniemi E, Joensuu J, Makela M, Vesikari T: Economic evaluation of rotavirus vaccinations in Finland: randomized, double-blind, placebo-controlled trial of tetravalent rhesus rotavirus vaccine. Clin Infect Dis. 1998, 27: 272-282. 10.1086/514650.
Vesikari T, Itzler R, Matson DO, Santosham M, Christie CD, Coia M, Cook JR, Koch G, Heaton P: Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries). Int J Infect Dis. 2007, 11 (Suppl 2): S29-S35. 10.1016/S1201-9712(07)60019-8.
Dey A, Wang H, Menzies R, Macartney K: Changes in hospitalisations for acute gastroenteritis in Australia after the national rotavirus vaccination program. Med J Aust. 2012, 197: 453-457. 10.5694/mja12.10062.
Jayasinghe S, Macartney K: Estimating rotavirus gastroenteritis hospitalisations by using hospital episode statistics before and after the introduction of rotavirus vaccine in Australia. Vaccine. 2012, 31: 967-972. 10.1016/j.vaccine.2012.11.099.
Leino T, Ollgren J, Salo H, Tiihonen P, Kilpi T: First year experience of rotavirus immunisation programme in Finland. Vaccine. 2012, 31: 176-182. 10.1016/j.vaccine.2012.10.068.
Msimang VM, Page N, Groome MJ, Moyes J, Cortese M, Seheri M, Kahn K, Chagan M, Madhi SA, Cohen C: Impact of Rotavirus Vaccine on Childhood Diarrheal Hospitalization Following Introduction into the South African Public Immunization Program. Pediatr Infect Dis J 2013..
Borg MA: Bed occupancy and overcrowding as determinant factors in the incidence of MRSA infections within general ward settings. J Hosp Infect. 2003, 54: 316-318. 10.1016/S0195-6701(03)00153-1.
Kaier K, Meyer E, Dettenkofer M, Frank U: Epidemiology meets econometrics: using time-series analysis to observe the impact of bed occupancy rates on the spread of multidrug-resistant bacteria. J Hosp Infect. 2010, 76: 108-113. 10.1016/j.jhin.2010.04.010.
Virtanen M, Kurvinen T, Terho K, Oksanen T, Peltonen R, Vahtera J, Routamaa M, Elovainio M, Kivimaki M: Work hours, work stress, and collaboration among ward staff in relation to risk of hospital-associated infection among patients. Med Care. 2009, 47: 310-318. 10.1097/MLR.0b013e3181893c64.
Virtanen M, Pentti J, Vahtera J, Ferrie JE, Stansfeld SA, Helenius H, Elovainio M, Honkonen T, Terho K, Oksanen T, Kivimaki M: Overcrowding in hospital wards as a predictor of antidepressant treatment among hospital staff. Am J Psychiatry. 2008, 165: 1482-1486. 10.1176/appi.ajp.2008.07121929.
Braun B: Ethical issues of hospital crowding solutions. J Emerg Nurs. 2011, 37: 381-385. 10.1016/j.jen.2011.04.015.
Higginson I: Emergency department crowding. Emerg Med J. 2012, 29: 437-443. 10.1136/emermed-2011-200532.
Hostetler MA, Mace S, Brown K, Finkler J, Hernandez D, Krug SE, Schamban N: Emergency department overcrowding and children. Pediatr Emerg Care. 2007, 23: 507-515. 10.1097/01.pec.0000280518.36408.74.
Kinnula SE, Renko M, Tapiainen T, Knuutinen M, Uhari M: Hospital-associated infections during and after care in a paediatric infectious disease ward. J Hosp Infect. 2008, 68: 334-340. 10.1016/j.jhin.2008.02.004.
Acknowledgements
The authors take full responsibility for the content of this contribution and thank Nicolas Voirin in behalf of alpha005 (supported by Sanofi Pasteur MSD) for assistance in preparing the manuscript. The authors would like to thank François Simondon (scientific advice) and Laurence Pagnon (project coordination).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
This work was supported by Sanofi Pasteur MSD. Susanne Hartwig is an employee of Sanofi Pasteur MSD. Matti Uhari has received grants and fees for review activities from Sanofi Pasteur MSD. Marjo Renko has received grants and travel compensation from Sanofi Pasteur MSD. Perrine Bertet was an employee of Sanofi Pasteur MSD at the time of study initiation. Maria Hemming declared no competing interests. Timo Vesikari is an advisory board member for Sanofi Pasteur MSD.
Authors’ contributions
SH contributed to the literature research, data analysis, interpretation of findings and drafting of the manuscript. MU contributed to the data collection and interpretation of findings. MR contributed to the data collection. PB contributed to the data collection and analysis. MH contributed to the trial design. TV contributed to the trial design, data collection and interpretation of findings. All authors critically reviewed the manuscript and approved the final version.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hartwig, S., Uhari, M., Renko, M. et al. Hospital bed occupancy for rotavirus and all cause acute gastroenteritis in two Finnish hospitals before and after the implementation of the national rotavirus vaccination program with RotaTeq®. BMC Health Serv Res 14, 632 (2014). https://doi.org/10.1186/s12913-014-0632-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-014-0632-z