Abstract
Background
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death. Traditional Chinese medicine (TCM) has special advantages in relieving HCC, while Astragalus membranaceus is commonly used in TCM treatment. However, its underlying mechanisms for treatment of HCC are unclear.
Methods
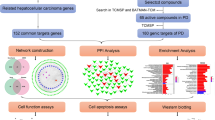
Differentially expressed genes (DEGs) of Astragalus membranaceus treatment in HepG2 cells were identified, and Astragalus membranaceus-gene network was constructed. The hub genes were then obtained via protein-protein interaction (PPI) analysis. Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Ontology (GO), and Gene Set Enrichment Analysis (GSEA) were subsequently performed. Furthermore, prognosis genes related to HCC from The Cancer Genome Atlas Program (TCGA) was identified to explore the correlation between Astragalus membranaceus treatment and prognosis of HCC. Finally, Astragalus membranaceus-component-target network was established through SymMap.
Results
Twenty five DEGs (15 up-regulated and 10 down-regulated) of Astragalus membranaceus treatment in HepG2 cells were identified. Among the 25 genes, MT1F, MT1G, MT1X and HMOX1 may play essential roles. Astragalus membranaceus mainly affects the Mineral absorption pathway in HCC. A total of 256 genes (p < 0.01) related to prognosis of HCC were identified, and MT1G is a common gene between prognosis genes and DEGs. Furthermore, Astragalus membranaceus may directly down-regulate MT1G through daidzein to promote ferroptosis of HCC cells and improve prognosis for HCC.
Conclusion
Our study provided new understandings of the pharmacological mechanisms by which Astragalus membranaceus improves the prognosis of HCC, and showed that the combination of transcriptomics and network pharmacology is helpful to explore mechanisms of TCM and traditional medicines from other nations.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC), the most common type of primary liver cancer, is the fourth main reason of cancer-related death around the world [1, 2]. As a type of aggressive and malignant tumor, the incidence and mortality rates of HCC have been on the rise since the 1990s [3, 4]. It is estimated that more than 1 million people will die because of HCC in 2030 worldwide [5, 6]. Radical surgery is still the major method for treating HCC at present, but most patients diagnosed with HCC are already in the advanced stage, and the efficacy of surgery is obviously declined [7, 8]. For advanced stage HCC patients, sorafenib (a multi-kinase inhibitor) is currently the first-line drug option [9].
Traditional Chinese medicine (TCM) has special advantages in relieving diseases, such as reducing recurrence, improving symptoms, enhancing the quality of life, reversing the multidrug resistance and prolonging survival [10]. Astragalus membranaceus is a commonly used TCM for patients with HCC [11]. A large number of pharmacological studies have shown that many components of Astragalus membranaceus have anti-HCC activity through different pathway. Astragaloside IV (AS-IV), a major active component of Astragalus membranaceus, could inhibit cell migration and viability of HCC via restraining long noncoding RNA ATB [12]. Astragalus polysaccharide (APS) could induce the apoptosis of HCC cells by suppressing the level of Notch1 [13]. Swainsonine, an extract from Astragalus membranaceus, may be a significant agent against HCC through inhibiting the growth of HCC cells [14]. Nevertheless, Astragalus membranaceus as a TCM, contains a variety of active ingredients, which could act on multiple targets. The mechanisms of Astragalus membranaceus on HCC is rarely reported, so its application is tremendously limited.
Perturbagens are reagents (chemical or genetic) to treat cells and measure biological responses caused by the reagents. Based on the concept, Broad Institute developed Connectivity Map to discover relationships between diseases, genes, and therapeutics [15]. This concept ignores the interaction of the reagent with cells and focuses on the downstream transcriptome changes. In view of the complexity of active components in TCM, thinking of TCM as perturbagens is an effective approach to reveal the mechanisms of TCM. Network pharmacology is a multidisciplinary approach that integrates systems biology, bioinformatics and pharmacology. It is able to systematically and holistically explore mechanisms of reagents on diseases, which is consistent with the holistic and systematic theory of TCM disease treatment [16].
In the present study, we combined transcriptomics and network pharmacology to comprehend the molecular mechanisms of Astragalus membranaceus in the treatment of HCC. The differentially expressed genes (DEGs) of Astragalus membranaceus were derived by GSE115506 [17]. The effective ingredients of Astragalus membranaceus and their targets were assayed by the SymMap database [18]. The mechanisms of Astragalus membranaceus against HCC were assessed by Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Set Enrichment Analysis (GSEA) analysis.
Methods
DEGs acquisition and screening
The DEGs of Astragalus membranaceus were acquired from GEO database (https://www.ncbi.nlm.nih.gov/geo/) (Series: GSE115506, Samples: GSM3179695, GSM3179696, GSM3179697, GSM3179701, GSM3179702, GSM3179703). In GSE115506, total RNA was obtained from HepG2 cells treated with 3 mg/mL Astragalus membranaceus aqueous extracts for 24 h in vitro. We used limma R packages to perform differential analysis [19], and the genes with |log2 fold change| > 1 and adjusted p-value <0.05 were considered to be DEGs.
Collection of components and targets
The components and targets of Astragalus membranaceus were collected from SymMap (https://www.symmap.org/), an integrative database of traditional Chinese medicine [18].
Network building and hub genes identifying
Protein-protein interaction (PPI) network was established using STRING (https://string-db.org/) [20]. The Astragalus membranaceus-gene network, PPI network and Astragalus membranaceus-component-target network were visualized by Cytoscape. MCODE plug-in of Cytoscape was used to identify hub genes.
Prognostic analysis
Based on The Cancer Genome Atlas Liver Hepatocellular Carcinoma (TCGA-LIHC) data, we identified 256 genes (p < 0.01) related to prognosis of HCC via the Kaplan-Meier (K-M) survival curves. The survival curve of MT1G was visualized by survminer R package.
Functional enrichment analysis
We conducted KEGG pathway analysis, biological process of GO analysis, and GSEA using clusterProfiler (a kind of R package) [21].
The ferroptosis potential index (FPI) calculating
We establish the FPI by referring to Zekun Liu’s article [22]. The calculation of FPI was based on the expression of core positive and negative genes associated with ferroptosis. The enrichment score (ES) of gene set was calculated by single sample gene set enrichment analysis (ssGSEA), and the normalized differences between the ES of the positive genes minus negative genes was defined as FPI to represent the ferroptosis levels.
Sequence alignment and functional identity analysis
We got the sequences of MT1G (mRNA accession: NM_005950.3, protein accession: NP_005941.1) and MT2A (mRNA accession: NM_005953.5, protein accession: NP_005944.1) from GenBank. Then we compared the identity of the sequences by nucleotide and protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The correlation between GO semantic similarity and gene expression profile has been validated and GO semantic similarity has been applied in protein–protein interaction analysis, pathway analysis and gene function analysis [23]. Here, we measured the functional identity between MT1G and MT2A through the geometric mean of semantic similarities in biological process (BP), molecular function (MF) and cellular component (CC).
Results
Astragalus membranaceus-gene network and PPI analysis
We identified 25 DEGs (15 up-regulated and 10 down-regulated) from GEO database (GSE115506). A volcano plot and a heatmap were established to display the distribution of DEGs in HepG2 cells (a kind of human hepatocellular carcinoma cell line) after Astragalus membranaceus treatment (Fig. 1A, B). These DEGs with |log2 fold change| > 1 and adjusted p-value <0.05 were considered to be DEGs (Table S1). Hence, we built a Astragalus membranaceus-gene network (Fig. 1C). To further explore the possible connections among these DEGs, we conducted the PPI network analysis for the 25 DEGs by STRING [20]. The final PPI network includes 8 nodes and 9 edges (Fig. 1D). Furthermore, we identified 1 up-regulated gene (HMOX1) and 3 down-regulated genes (MT1G, MT1X, MT1F), which were hub genes in PPI network by Molecular Complex Detection (MCODE) (a plug-in of Cytoscape).
Astragalus membranaceus-gene network and PPI analysis. A Volcano plot and (B) heatmap of DEGs showed that significant changes in genes caused by Astragalus membranaceus treatment in HepG2 cells. C Astragalus membranaceus-gene network. D The PPI analysis of DEGs showed that MT1F, MT1G, MT1X and HMOX1 were hub genes
GO and KEGG analysis
Only 2 pathways that were notably affected (p.adjust <0.05) by Astragalus membranaceus in the process of treating HepG2 cells by the KEGG pathway analysis (Fig. 2A). HMGCS1 and IDI1 were enriched in Terpenoid backbone biosynthesis, and 4 hub genes (MT1F, MT1G, MT1X and HMOX1) were enriched in Mineral absorption. In total, 20 biological processes (GO terms) were significantly enriched (p.adjust <0.05) (Table S2). Top 5 biological processes were chosen and displayed in Fig. 2B. The highly enriched biological processes contained detoxification of inorganic compound, cellular response to cadmium ion, detoxification of copper ion, stress response to copper ion and response to cadmium ion. The results suggest that Astragalus membranaceus mainly affects the Mineral absorption pathway in HCC cells.
Astragalus membranaceus improved prognosis of HCC by down-regulating MT1G
A total of 256 genes (p < 0.01) related to prognosis of HCC were identified from The Cancer Genome Atlas Program (TCGA) (Table S3). According to the intersection of 256 genes related to prognosis of HCC and 25 DEGs, MT1G was a common gene (Fig. 3A). Figure 3B showed that high expression of MT1G was related to the poor prognosis of HCC. On the contrary, the expression of MT1G was decreased by Astragalus membranaceus in HCC cells (Fig. 1D). These results imply that Astragalus membranaceus may improve prognosis of HCC by down-regulating MT1G.
Astragalus membranaceus promoted ferroptosis of HCC cells
To further explore the potential effect of Astragalus membranaceus, we conducted the GSEA analysis for ferroptosis and calculated the ferroptosis potential index to assess ferroptosis level after Astragalus membranaceus treatment for HCC. The GSEA analysis (Fig. 4A) showed that ferroptosis increased in HepG2 cells treated with Astragalus membranaceus. In Fig. 4B, we observed that the FPI of Astragalus membranaceus treatment group was significantly increased. The above results suggests that Astragalus membranaceus promoted ferroptosis of HCC cells.
Astragalus membranaceus may targeted MT1G by daidzein in HepG2 cells
We established a Astragalus membranaceus-component-target network (Fig. 5A). The network revealed that Astragalus membranaceus targeted MT2A by daidzein. MT2A and MT1G are both belong to the metallothioneins (MTs) superfamily. Through identity analysis of sequence (mRNA and protein) and function (GO), MT1G and MT2A are highly homologous and similar (Fig. 5B). However, our result showed that MT1G is the hub gene and MT2A is not (Fig. 1). Could daidzein really target MT2A or MT1G? To address this question, we found that daidzein tended to up-regulate MT2A and down-regulate MT1G in human fibroblasts (Fig. S1). The result implies that daidzein can affect MT1G and MT2A expression, and the regulation degree and trend of daidzein on MT1G and MT2A are different in variant cell types. Consequently, Astragalus membranaceus may directly target MT1G via daidzein in HepG2 cells.
Astragalus membranaceus targeted MT1G by daidzein. A Astragalus membranaceus-component−target network showed that Astragalus membranaceus targeted MT2A by daidzein. Red dots represent components, and blue spots represent targets. B The identity analysis of sequence (mRNA and protein) and function (GO) showed that MT1G and MT2A are highly similar and homologous
Discussion
In China, TCM prescriptions are extensively used in the treatment of HCC [24]. Astragalus membranaceus is prescribed frequently in patients with HCC [11]. TCM therapy is a unique and useful theoretical system formed after thousands of years of clinical practice and exploration in China. Different from small molecule drugs, a single TCM usually contains multiple ingredients and targets. There have been some effective pretreatment methods to retain the active components, of which hydrogen peroxide presoaking prior to ammonia fiber expansion (HAFEX) pretreatment could destroy cell wall to release the active ingredients, suggesting its potential in pretreatment method of Astragalus membranaceus [25]. In addition, a series of studies reported that miRNAs in herbal medicines could be absorbed by body to regulate the disease process [26,27,28]. Consequently, although a variety of active components of Astragalus membranaceus have been confirmed to have anti-HCC effect [12,13,14], the mechanisms of Astragalus membranaceus as a whole is still unclear.
In our study, we constructed a Astragalus membranaceus-gene network using 25 significant DEGs in HepG2 cells treated with Astragalus membranaceus (Fig. 1C). Among the 25 DEGs, we identified that MT1F, MT1G, MT1X and HMOX1 were hub genes (Fig. 1D). The 4 hub genes were not only enriched in the Mineral absorption pathway, but also the biological process of their enrichment is mainly in response to metal ions (Fig. 2A, B). A study revealed that Mineral absorption is significantly associated with the occurrence and development of HCC [29]. In addition, the metabolism of metal ions plays important roles in HCC progression and therapy [30, 31]. Therefore, Astragalus membranaceus is likely to treat HCC by affecting the Mineral absorption pathway. It is worth noting that the biological process of negative regulation of growth was significantly enriched (Table S2). Therefore, GSEA analysis was performed to explore the changes in the overall negative regulation of growth biological process (Fig. S3). We observed that negative regulation of growth was increased after Astragalus membranaceus treatment in HepG2 cells, suggesting that the growth of HepG2 cell line is likely to be inhibited by Astragalus membranaceus (Fig. S3).
Among 4 hub genes, 3 genes (MT1F, MT1G and MT1X) belong to the metallothioneins (MTs) superfamily. MTs are small cysteine-rich intracellular proteins, including at least ten known functional subtypes (MT1A, MT1B, MT1E, MT1F, MT1G, MT1H, MT1X, MT2A, MT3, and MT4) [32]. A bulk of studies have shown that the changes of MTs expression level could be associated with the process of carcinogenesis, such as cell proliferation, migration, and angiogenesis [33]. Interestingly, high expression of MT1G was related to the poor prognosis of HCC (Fig. 3B), and Astragalus membranaceus could significantly down-regulate MT1G in HCC cells (Fig. 1C), suggesting that Astragalus membranaceus may improve prognosis of HCC by down-regulating MT1G. In addition, researchers observed that protein level of MT1 was increased in some patients with HCC taking sorafenib, and found that the phenomenon associated with lower overall survival [34], as well as MT1G could enhance sorafenib resistance via inhibiting ferroptosis in HCC [35]. These results indicate that Astragalus membranaceus has the potential to assist sorafenib in the treatment of HCC.
Ferroptosis, a recently identified cell death type of non-apoptotic regulation, is a kind of programmed cell necrosis primarily caused by extra-mitochondrial lipid peroxidation due to an iron-dependent reactive oxygen species (ROS) accumulation [36, 37]. Ferroptosis is closely related to the progression of cancer, so a massive effort has been devoted to the design and development of anticancer drugs based on ferroptosis [38]. High expression of MT1G could inhibit ferroptosis in HepG2 cells [35], while Astragalus membranaceus could significantly reduce the level of MT1G and promote ferroptosis in HepG2 cells (Fig. 4). These results imply that Astragalus membranaceus have the potential to relieve sorafenib resistance via down-regulating MT1G and enhancing ferroptosis.
Through the network pharmacology strategies, we built a Astragalus membranaceus-component-target network (Fig. 5A). We could draw a preliminary conclusion from the network that Astragalus membranaceus may directly target MT2A via daidzein. Furthermore, we observed that daidzein tends to reduce the expression of MT1G and up-regulates MT2A in human fibroblasts (Fig. S1A, C). In some types of cancer, up-regulation of MT2A seems to play an adverse role in treatment [39]. For example, over-expression of MT2A is related with chemoresistance in ductal breast cancer [40], and predicts poor prognosis in non-small cell lung cancer [41]. On the contrary, higher levels of MT2A are associated with favorable outcome in patients with gastric cancer, and MT2A exerts anti-gastric cancer effects by complexing with MZF1 to target NFKBIA [42]. These studies demonstrated the diversity of MT2A functions in different cancers. In fact, the up/down-regulation of MT2A depends on the type of tumors, as well as other environmental stimuli or gene mutations [39]. In human hepatocellular carcinomas, the expression of MT2A is drastically reduced [43, 44], suggesting down-regulation of MT2A is an imbalance in liver cancer, while MT2A is able to preserve homeostasis of biologically essential metals and to scavenge the toxic metals [43], therefore the up-regulation of MT2A by daidzein may contribute to restoration of homeostasis.
MT2A and MT1G are both belong to the MTs superfamily, and they have a high degree of identity (Fig. 5B), implying that Astragalus membranaceus also may directly target MT1G via daidzein. Daidzein, a kind of phytoestrogen, could affect the cell cycle, inhibit cell proliferation and angiogenesis in different types of cancer [45, 46]. However, there are few studies on transcriptomic changes induced by daidzein in HCC, and the causal mechanisms were still unclear. Here, we found that daidzein may enhance ferroptosis of HCC cells via directly targeting MT1G. Furthermore, we found that daidzein could down-regulate MT1G and significantly increase the ferroptosis level (Fig. S1, S2) through GSE43692, a dataset of daidzein affecting gene expression in human fibroblasts. This result implies that daidzein is able to decrease the expression of MT1G and stimulate ferroptosis in vitro, which to some extent supported our conclusion that daidzein may directly target MT1G and promote ferroptosis in HCC.
Conclusions
The present study revealed that the effect of Astragalus membranaceus against HCC may be mainly related to Mineral absorption pathway. Furthermore, Astragalus membranaceus may directly down-regulate MT1G through daidzein to promote ferroptosis of HCC cells and improve prognosis. Our study showed that the combination of transcriptomics and network pharmacology is helpful to explore mechanisms of TCM and traditional medicines from other nations.
Availability of data and materials
The datasets analysed during the current study are available in the GEO repository, GSE115506 and GSE43692.
Abbreviations
- APS:
-
Astragalus polysaccharide
- AS-IV:
-
Astragaloside IV
- BP:
-
biological process
- CC:
-
cellular component
- DEG:
-
Differentially expressed gene
- ES:
-
enrichment score
- HAFEX:
-
hydrogen peroxide presoaking prior to ammonia fiber expansion
- FPI:
-
ferroptosis potential index
- GO:
-
Gene Ontology
- GSEA:
-
Gene Set Enrichment Analysis
- HCC:
-
Hepatocellular carcinoma
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- K-M:
-
Kaplan-Meier
- MCODE:
-
Molecular Complex Detection
- MF:
-
molecular function
- MT:
-
metallothioneins
- PPI:
-
protein-protein interaction
- ROS:
-
reactive oxygen species
- TCGA:
-
The Cancer Genome Atlas Program
- TCM:
-
Traditional Chinese medicine
References
Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17(3):139–52. https://doi.org/10.1038/s41575-019-0229-4.
Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745–61. https://doi.org/10.1053/j.gastro.2016.11.048.
Liu Z, Jiang Y, Yuan H, Fang Q, Cai N, Suo C, et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the global burden of disease study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–83. https://doi.org/10.1016/j.jhep.2018.12.001.
Liu J, Tang W, Budhu A, Forgues M, Hernandez MO, Candia J, et al. A viral exposure signature defines early onset of hepatocellular carcinoma. Cell. 2020;182(2):317–28.e10. https://doi.org/10.1016/j.cell.2020.05.038.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–62. https://doi.org/10.1056/NEJMra1713263.
Nault J-C, Villanueva A. Biomarkers for Hepatobiliary cancers. Hepatology. 2021;73(S1):115–27. https://doi.org/10.1002/hep.31175.
Jun L, Yang G, Zhisu L. The utility of serum exosomal microRNAs in hepatocellular carcinoma. Biomed Pharmacother= Biomedecine & pharmacotherapie. 2019;111:1221–7. https://doi.org/10.1016/j.biopha.2018.12.131.
Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019;18(1):114. https://doi.org/10.1186/s12943-019-1043-x.
Lee SK, Jang JW, Nam H, Sung PS, Kim HY, Kwon JH, et al. Sorafenib for advanced hepatocellular carcinoma provides better prognosis after liver transplantation than without liver transplantation. Hepatol Int. 2021;15(1):137–45. https://doi.org/10.1007/s12072-020-10131-0.
Jiang Z, Hua H. Progress on prevention and treatment of Chinese medicine to molecular mechanism of liver cancer. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = Chi J Chin Mat Med. 2009;34(10):1310–3.
Ting CT, Kuo CJ, Hu HY, Lee YL, Tsai TH. Prescription frequency and patterns of Chinese herbal medicine for liver cancer patients in Taiwan: a cross-sectional analysis of the National Health Insurance Research Database. BMC Complement Altern Med. 2017;17(1):118. https://doi.org/10.1186/s12906-017-1628-0.
Li Y, Ye Y, Chen H. Astragaloside IV inhibits cell migration and viability of hepatocellular carcinoma cells via suppressing long noncoding RNA ATB. Biomed Pharmacother= Biomedecine & pharmacotherapie. 2018;99:134–41. https://doi.org/10.1016/j.biopha.2017.12.108.
Huang WH, Liao WR, Sun RX. Astragalus polysaccharide induces the apoptosis of human hepatocellular carcinoma cells by decreasing the expression of Notch1. Int J Mol Med. 2016;38(2):551–7. https://doi.org/10.3892/ijmm.2016.2632.
You N, Liu W, Wang T, Ji R, Wang X, Gong Z, et al. Swainsonine inhibits growth and potentiates the cytotoxic effect of paclitaxel in hepatocellular carcinoma in vitro and in vivo. Oncol Rep. 2012;28(6):2091–100. https://doi.org/10.3892/or.2012.2035.
Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell. 2017;171(6):1437–52.e17. https://doi.org/10.1016/j.cell.2017.10.049.
Chen S, Jiang H, Cao Y, Wang Y, Hu Z, Zhu Z, et al. Drug target identification using network analysis: taking active components in Sini decoction as an example. Sci Rep. 2016;6:24245. https://doi.org/10.1038/srep24245.
Ko PH, Huang CW, Chang HH, Chuang EY, Tsai MH, Lai LC. Identifying the functions and biomarkers of Codonopsis pilosula and Astragalus membranaceus aqueous extracts in hepatic cells. Chin Med. 2019;14:10. https://doi.org/10.1186/s13020-019-0233-1.
Wu Y, Zhang F, Yang K, Fang S, Bu D, Li H, et al. SymMap: an integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019;47(D1):D1110–d7. https://doi.org/10.1093/nar/gky1021.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. https://doi.org/10.1093/nar/gkv007.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D13. https://doi.org/10.1093/nar/gky1131.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics-a J Integr Biol. 2012;16(5):284–7. https://doi.org/10.1089/omi.2011.0118.
Liu Z, Zhao Q, Zuo Z-X, Yuan S-Q, Yu K, Zhang Q, et al. Systematic analysis of the aberrances and functional implications of Ferroptosis in cancer. iScience. 2020;23(7):101302. https://doi.org/10.1016/j.isci.2020.101302.
Han Y, Yu G, Sarioglu H, Caballero-Martinez A, Schlott F, Ueffing M, et al. Proteomic investigation of the interactome of FMNL1 in hematopoietic cells unveils a role in calcium-dependent membrane plasticity. J Proteome. 2013;78:72–82. https://doi.org/10.1016/j.jprot.2012.11.015.
Liao X, Bu Y, Jia Q. Traditional Chinese medicine as supportive care for the management of liver cancer: past, present, and future. Genes Dis. 2020;7(3):370–9. https://doi.org/10.1016/j.gendis.2019.10.016.
Zhao C, Qiao X, Shao Q, Hassan M, Ma Z. Evolution of the lignin chemical structure during the bioethanol production process and its inhibition to enzymatic hydrolysis. Energy Fuel. 2020;34(5):5938–47. https://doi.org/10.1021/acs.energyfuels.0c00293.
Zhou LK, Zhou Z, Jiang XM, Zheng Y, Chen X, Fu Z, et al. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discov. 2020;6:54. https://doi.org/10.1038/s41421-020-00197-3.
Xia C, Zhou H, Xu X, Jiang T, Li S, Wang D, et al. Identification and investigation of miRNAs from Gastrodia elata Blume and their potential function. Front Pharmacol. 2020;11(1477). https://doi.org/10.3389/fphar.2020.542405.
Zhou Z, Li X, Liu J, Dong L, Chen Q, Liu J, et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza a viruses. Cell Res. 2015;25(1):39–49. https://doi.org/10.1038/cr.2014.130.
Li Y, Chen R, Yang J, Mo S, Quek K, Kok CH, et al. Integrated bioinformatics analysis reveals key candidate genes and pathways associated with clinical outcome in hepatocellular carcinoma. Front Genet. 2020;11:814. https://doi.org/10.3389/fgene.2020.00814.
Wachsmann J, Peng F. Molecular imaging and therapy targeting copper metabolism in hepatocellular carcinoma. World J Gastroenterol. 2016;22(1):221–31. https://doi.org/10.3748/wjg.v22.i1.221.
Wang W, Xie Q, Zhou X, Yao J, Zhu X, Huang P, et al. Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Lett. 2015;358(1):47–58. https://doi.org/10.1016/j.canlet.2014.12.025.
Ji XF, Fan YC, Gao S, Yang Y, Zhang JJ, Wang K. MT1M and MT1G promoter methylation as biomarkers for hepatocellular carcinoma. World J Gastroenterol. 2014;20(16):4723–9. https://doi.org/10.3748/wjg.v20.i16.4723.
Merlos Rodrigo MA, Jimenez Jimemez AM, Haddad Y, Bodoor K, Adam P, Krizkova S, et al. Metallothionein isoforms as double agents – their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist Updat. 2020;52:100691. https://doi.org/10.1016/j.drup.2020.100691.
Houessinon A, François C, Sauzay C, Louandre C, Mongelard G, Godin C, et al. Metallothionein-1 as a biomarker of altered redox metabolism in hepatocellular carcinoma cells exposed to sorafenib. Mol Cancer. 2016;15(1):38. https://doi.org/10.1186/s12943-016-0526-2.
Sun X, Niu X, Chen R, He W, Chen D, Kang R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64(2):488–500. https://doi.org/10.1002/hep.28574.
Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26(3):165–76. https://doi.org/10.1016/j.tcb.2015.10.014.
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–79. https://doi.org/10.1038/cdd.2015.158.
Liang C, Zhang X, Yang M, Dong X. Recent Progress in Ferroptosis inducers for cancer therapy. Adv Mat (Deerfield Beach, Fla). 2019;31(51):e1904197. https://doi.org/10.1002/adma.201904197.
Si M, Lang J. The roles of metallothioneins in carcinogenesis. J Hematol Oncol. 2018;11(1):107. https://doi.org/10.1186/s13045-018-0645-x.
Yap X, Tan HY, Huang J, Lai Y, Yip GW, Tan PH, et al. Over-expression of metallothionein predicts chemoresistance in breast cancer. J Pathol. 2009;217(4):563–70. https://doi.org/10.1002/path.2489.
Werynska B, Pula B, Muszczynska-Bernhard B, Gomulkiewicz A, Piotrowska A, Prus R, et al. Metallothionein 1F and 2A overexpression predicts poor outcome of non-small cell lung cancer patients. Exp Mol Pathol. 2013;94(1):301–8. https://doi.org/10.1016/j.yexmp.2012.10.006.
Lin S, Wang X, Pan Y, Tian R, Lin B, Jiang G, et al. Transcription factor myeloid zinc-finger 1 suppresses human gastric carcinogenesis by interacting with Metallothionein 2A. Clin Cancer Res. 2019;25(3):1050–62. https://doi.org/10.1158/1078-0432.Ccr-18-1281.
Datta J, Majumder S, Kutay H, Motiwala T, Frankel W, Costa R, et al. Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein alpha by phosphatidylinositol 3-kinase signaling cascade. Cancer Res. 2007;67(6):2736–46. https://doi.org/10.1158/0008-5472.Can-06-4433.
Li H, Lu YF, Chen H, Liu J. Dysregulation of metallothionein and circadian genes in human hepatocellular carcinoma. Chronobiol Int. 2017;34(2):192–202. https://doi.org/10.1080/07420528.2016.1256300.
Chan KKL, Siu MKY, Jiang Y-X, Wang J-J, Leung THY, Ngan HYS. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018;18:65. https://doi.org/10.1186/s12935-018-0559-2.
Salama AAA, Allam RM. Promising targets of chrysin and daidzein in colorectal cancer: Amphiregulin, CXCL1, and MMP-9. Eur J Pharmacol. 2021;892:173763. https://doi.org/10.1016/j.ejphar.2020.173763.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ZL1 and HM contributed equally to this work; ZL2 conceived and designed this study; ZL1 and HM performed the acquisition and analysis of data, and all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
25 DEGs in HepG2 cells after Astragalus membranaceus treatment. Table S2 All significantly enriched biological processes (GO terms). Table S3 256 genes significantly associated with HCC prognosis. Figure S1. (A) MT1G expression level tended to decrease in human fibroblasts in daidzein treatment group. (B) After excluding outliers, MT1G expression level was significantly decreased in human fibroblasts in daidzein treatment group. (C) MT2A expression level tended to increase in human fibroblasts in daidzein treatment group. Figure S2. (A) GSEA analysis of ferroptosis showed that ferroptosis was increased after daidzein treatment in human fibroblasts. (B) The ferroptosis potential index (FPI) was significantly increased after daidzein treatment in human fibroblasts. Figure S3. GSEA analysis of negative regulation of growth showed that Astragalus membranaceus may inhibit the growth of HepG2 cells.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Z., Ma, H. & Lai, Z. Revealing the potential mechanism of Astragalus membranaceus improving prognosis of hepatocellular carcinoma by combining transcriptomics and network pharmacology. BMC Complement Med Ther 21, 263 (2021). https://doi.org/10.1186/s12906-021-03425-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-021-03425-9