Abstract
Background
Medicinal plants are widely used for the treatment of different infectious diseases. Infectious diseases caused by bacteria have a large impact on public health. This study aimed to determine the in vitro antibacterial activity of the medicinal plants traditionally used in Vietnam against the bacterial strains associated with infectious diseases.
Methods
Methanol extracts of twelve Vietnamese medicinal plants were tested for their antibacterial activity against five bacterial species including Gram-positive bacteria (Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) using the broth microdilution method.
Results
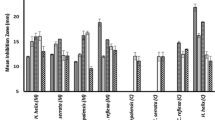
All the plant extracts showed antibacterial activity, especially against Gram-positive bacteria (Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus). Baeckea frutescens extract revealed a potent activity against the Gram-positive bacteria with the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of 62.5 μg/ml. High activity against all the three Gram-positive bacteria was also observed for the extracts of Cratoxylum formosum ssp. pruniflorum, Pogostemon cablin, and Pedilanthus tithymaloides with MICs of 125, 125 and 250 μg/ml and MBCs of 125–250, 125–250 and 250–500 μg/ml, respectively. The extracts of C. formosum ssp. pruniflorum and P. tithymaloides showed a broad-spectrum antibacterial activity against all the bacteria tested with the MICs of 125–2,000 μg/ml.
Conclusion
This study indicates clear evidence supporting the traditional use of the plants in treating infectious diseases related to bacteria. In particular, these plant species showed moderate to high antibacterial activity against the Gram-positive bacteria tested.
Similar content being viewed by others
Background
Since the mid-1970s, the emergence of a number of new pathogens and reemergence of older diseases has highlighted the fact that, contrary to expectations, epidemics of infectious disease remain a problem of public health concern [1]. Infectious diseases remain the largest global cause of death [1, 2]. They account for approximately one-half of all the deaths in tropical countries [2]. Many of these diseases are difficult to treat or have no specific effective therapy available. For most of these diseases, no vaccines are available [3]. Infectious diseases caused by bacteria have a large impact on public health [3, 4]. In recent years, the emergence of antibiotic resistance and the failure of chemotherapy are increasing [5]. Therefore, nowadays, the discovery of new natural antibacterial agents for treating infectious diseases is essential to prevent the spread of diseases and improve their treatment.
Similar to microorganisms, plants are a biologically and chemically diverse resource. It is estimated that there are 250,000 to 500,000 species of plants on the Earth [6]. Plants have been used as traditional medicines for the treatment of various diseases throughout most of human history. The use of plant extracts as medicinal treatments gained popularity in the late 1990s [7]. Plants are still an important source of medicines, especially in developing countries where the plant-based traditional medicines are still used to meet the health-care needs [8].
Despite the recent interest in drug discovery using molecular modeling, combinatorial chemistry, and other synthetic chemistry methods, natural product-derived compounds are still proving to be an invaluable source of medicines for humans [8]. Several recent studies have shown the increased interest in plant materials for their diverse pharmacological and biological properties including antibacterial activity [2, 9–12].
This study was aimed at validating the traditional use of selected Vietnamese medicinal plants against common bacteria, causing several human infections including Escherichia coli, Pseudomonas aeruginosa, Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus [2, 4, 13], by evaluating their in vitro antibacterial activity. The plants investigated in this study commonly used to treat the infectious diseases and the associated symptoms are listed in Table 1.
Methods
Plant materials and extraction
The plants were collected from different locations in Vietnam between March and October 2012. The collected species were authenticated by Associate Prof. Vu, Xuan Phuong, Dr. Tran, The Bach, and Dr. Nguyen, The Cuong from Institute of Ecology and Biological Resources, Vietnam. Voucher specimens of the plants were deposited in the Herbarium of the Department of Phytochemistry and Research and Development Center of Bioactive Compounds, Vietnam Institute of Industrial Chemistry (VIIC). The collected plant materials were air-dried and finely powdered using a blender. To prepare methanol extracts of the plant materials, 10 g of each powdered plant material was extracted twice with 100 ml of methanol for 48 h at room temperature. The extracted suspensions were filtered through Whatman No. 1 filter paper, and the filtrates were concentrated to dryness using a rotary evaporator, and then stored at −20 °C until further use. For the antibacterial activity assays, the extracts were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 100 mg/ml and stored at 4 °C as stock solutions.
Bacterial strains and culture conditions
Five bacterial species including two Gram-negative (Escherichia coli American Type Culture Collection, ATCC 25922 and Pseudomonas aeruginosa ATCC 9027) and three Gram-positive strains (Bacillus cereus ATCC 21768, Bacillus subtilis ATCC 6633, and Staphylococcus aureus ATCC 6538) were obtained from the ATCC (Manassas, VA, USA). The bacterial strains were cultured aerobically on nutrient agar (NA) plates at 37 °C for 24 h. For the antibacterial activity test, the bacteria were aerobically cultured in nutrient broth (NB) at 37 °C for 24 h, and then suspended in sterile saline at a density equivalent to that of the 0.5 McFarland standard. Bacterial suspensions with a concentration of 105 cfu/ml were used for in vitro antibacterial activity test.
Determination of antibacterial activity
The minimum inhibitory concentration (MIC) of each plant extract was determined using the broth microdilution method as described in our previous report [14]. Briefly, two-fold serial dilutions of each plant extract were added to the wells of sterile 96-well plates containing inoculated NB medium (100 μl) with bacterial cells (105 cfu/ml). The final concentrations of the plant extracts ranged from 15.63 to 2,000 μg/ml. DMSO (2 %) was used as the negative control, which did not affect the bacterial growth. Streptomycin sulfate and chloramphenicol (Sigma-Aldrich, USA) were used as positive controls against all the bacteria. Following a 24–h incubation at 37 °C, the MIC was determined as the lowest concentration that completely inhibited the growth of the bacteria. The assay was repeated twice with two replicates for each extract against the individual bacterial species at all the test concentrations.
For the poorly water-soluble extracts, the MIC was determined according to the previously described method of Kurekci et al. with some modification [15]. Iodonitrotetrazolium chloride (20 μl, 0.2 mg/ml, INT, Sigma-Aldrich) was added to the test wells at the completion of the incubation period, and this was further incubated at 37 °C for 3 h. The presence of viable bacteria was determined based on the dye changing color from yellow to pink.
The minimum bactericidal concentration (MBC) of each extract was determined by withdrawing 20 μl of the bacterial broth suspension showing no color change, and then spreading it on NA plates, which were then incubated at 37 °C for 24–48 h. The lowest concentration of the extract at which no bacterial growth was observed, was considered as the MBC.
Results and discussion
In this study, 12 plants from 10 different families were selected according to their traditional usage for the treatment of infectious diseases and associated symptoms. The identification of the studied plants by their scientific and common names, traditional use, parts used, place of collection and voucher number is listed in Table 1. All the plants were extracted with methanol, because methanol is considered as the best solvent for the extraction of antimicrobial substances and may contain diverse chemical compounds with biological activity [2, 16].
The in vitro antibacterial activity of plants was evaluated against both Gram-positive and negative strains using the microdilution method to determine their MIC and MBC values. The MIC and MBC values of the extracts from various plant species against test bacteria are listed in Table 2. Most of the extracts exhibited a broad antibacterial spectrum against the strains tested. The extracts of Cratoxylum formosum ssp. pruniflorum and Pedilanthus tithymaloides displayed broad-spectrum antibacterial activities against all the five strains tested with the MIC values in the ranges 125–2,000 and 250–2,000 μg/ml, respectively. The extracts of Pogostemon cablin and Pluchea indica showed the MIC values in the ranges 125–2,000 and 500–2,000 μg/ml, respectively, against four bacterial strains except P. aeruginosa. On the other hand, streptomycin sulfate and chloramphenicol used as positive controls showed strong antibacterial activities against both Gram-positive and Gram-negative bacteria like as the results of previous studies [17–19].
Most of the plant extracts tested displayed impressive antibacterial efficacies against Gram-positive bacteria with the MIC values ≤ 1,000 μg/ml. In particular, the methanol extract of the leaves and branches of Baeckea frutescens showed a potent antibacterial activity against all the Gram-positive bacteria tested with the MIC and MBC values of 62.5 μg/ml each. High activity was also observed for the extracts of C. formosum ssp. pruniflorum and P. cablin (MIC, 125 μg/ml each and MBC, 125 and 250 μg/ml, respectively) followed by the extracts of Cinnamomum camphora and P. tithymaloides (MIC, 125–500 μg/ml and MBC values of 125–1,000 μg/ml). The extracts of Cannabis sativa and Cassytha filiformis were less active against the bacterial strains tested. In contrast, the antibacterial activity against the Gram-negative bacteria was shown only by some extracts with the MIC values of 1,000 and 2,000 μg/ml (Table 2). The results obtained in this study indicate that the Gram-negative bacteria were less susceptible to the plant extracts than the Gram-positive bacteria.
The antibacterial activities of most of the plants evaluated in this study were previously tested against a few bacterial strains as listed in Table 1. However, not all of them were tested against the strains used in this study. Interestingly, no previous study has reported the antibacterial activity of Clematis vitalba, which showed a good activity against the Gram-positive bacteria with the MIC values in the range 250–500 μg/ml. To the best of our knowledge, this study is the first report on the antibacterial activity of C. vitalba.
The methanol extract of B. frutescens exhibited the most potent antibacterial activity with the MIC values of 62.5 μg/ml against the Gram-positive bacteria (Table 2). Traditionally, decoction of leaves and flowers of this plant has been used to treat menstrual irregularities and essential oil of the plant to treat symptoms such as influenza, sores, and indigestion [20]. The plant was also used as a medicinal plant in China, Malaysia, and Indonesia [21]. Its antibacterial activity was reported in some previous studies against only Streptococcus mutans and S. aureus [21, 22]. However, detailed information on the antibacterial properties of B. frutescens is lacking.
C. formosum ssp. pruniflorum belongs to the family Clusiaceae. The Cratoxylum genus distributed in several Southeast Asian countries. Some species of this genus have been used for the treatment of diuretic, stomachic and tonic effects, as well as diarrhea and food poisoning [23]. This plant has been used traditionally to aid digestion and treat ailments in Vietnam [20]. Plants of this genus produce various types of secondary metabolites, including xanthones, triterpenoids, and flavonoids. Some compounds such as prenylated xanthones and anthraquinones isolated from the roots and barks of this plant showed strong antibacterial activities against several bacteria including B. subtilis, S. aureus, and P. aeruginosa [23]. In this study, the methanol extract of the leaves of C. formosum ssp. pruniflorum also displayed a broad-spectrum and potent antibacterial activity against all the bacterial strains tested. The antibacterial activity of this extract is likely to be associated with the presence of these compounds.
This study also indicates that methanol extract of P. tithymaloides leaves possesses a broad-spectrum antibacterial activity against all the test bacterial strains. The previous studies of Vidotti et al. [24] and Ghosh et al. [25] showed the same behavior of its methanol and ethanol extracts against B. subtilis, S. aureus, E. coli, and P. aeruginosa, but the methanol extract in our study is less effective against the Gram-negative strains.
In this study, the methanol extract of P. cablin was found to be active against all the bacterial strains tested except P. aeruginosa. However, polar solvent extracts (ethanol, methanol, and aqueous) of this plant did not form growth inhibition zones against S. aureus, E. coli, and P. aeruginosa by the disc diffusion method, whereas its hexane extract did [26]. In addition, essential oil and its major compounds from this plant showed a broad-spectrum antibacterial against various bacterial strains including B. subtilis, S. aureus, E. coli, and P. aeruginosa [27, 28]. The results of this study are in agreement with the literature reports.
Most of the previous studies reported the use of different methods or bacterial species, therefore, a direct comparison of literature data with our present study was difficult. The contrasting results might be largely attributed to the different locations where the plants were collected and the solvent used in the extraction.
Conclusions
All the plant species evaluated in this study are currently used traditionally for the treatment of various infectious diseases (Table 1), and showed promising antibacterial activity against the Gram-positive bacteria including B. cereus, B. subtilis, and S. aureus. The antibacterial activity of B. frutescens was highly significant with the MIC values of 62.5 μg/ml. Furthermore, this is the first study to report the antibacterial activities of C. vitalba. Further phytochemical studies are necessary to provide relevant information for the development of these plants as potential effective treatments against bacterial infections and diseases. Finally, the results of this study clearly elucidate the antibacterial activities of these plants and provide an evidence to support their use in folk medicine.
References
Avery G. Infectious diseases, a resurgent problem: developing effective public health responses. In: Charney W, editor. Emerging infectious diseases and the threat to occupational health in the U.S. and Canada. Boca Raton: Taylor & Francis; 2006. p. 223.
Tekwu EM, Pieme AC, Beng VP. Investigations of antimicrobial activity of some Cameroonian medicinal plant extracts against bacteria and yeast with gastrointestinal relevance. J Ethnopharmacol. 2012;142:265–73.
Hamer D, Griffiths JK, Maguire JH, Heggenhougen HK, Quah SR. Public health and infectious diseases. 1st ed. San Diego: Academic Press of Elsevier; 2010.
Khan UA, Rahman H, Niaz Z, Qasim M, Khan J, Tayyaba, et al. Antibacterial activity of some medicinal plants against selected human pathogenic bacteria. Eur J Microbiol Immunol. 2013;3:272–4.
Fankam AG, Kuiate JR, Kuete V. Antibacterial activities of Beilschmiedia obscura and six other Cameroonian medicinal plants against multi-drug resistant Gram-negative phenotypes. BMC Complement Altern Med. 2014;14:241.
Borris RP. Natural products research: perspectives from a major pharmaceutical company. J Ethnopharmacol. 1996;51:29–38.
Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;2:564–82.
Salim AA, Chin YW, Kinghorn AD. Drug discovery from plants. In: Ramawat KG, Merillon JM, editors. Bioactive Molecules and Medicinal Plants. Berlin Heidelberg: Springer; 2008. p. 1–24.
Bussmann RW, Malca-Garcia G, Glenn A, Sharon D, Chait G, Diaz D, et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J Ethnopharmacol. 2010;32:1–8.
Moura-Costa GF, Nocchi SR, Ceole LF, de Mello JCP, Nakamura CV, Filho BPD, et al. Antimicrobial activity of plants used as medicinals on an indigenous reserve in Rio das Cobras, Parana. Brazil J Ethnopharmacol. 2012;143:631–8.
Fomogne-Fodjo MCY, Vuuren SV, Ndinteh DT, Krause RWM, Olivier DK. Antibacterial activity of plant from Central Africa used traditionally by the Bakola pygmies for treating respiratory and tuberculosis-related symptoms. J Ethnopharmacol. 2014;115:123–31.
Ocheng F, Bwanga F, Joloba M, Borg-Karlson AK, Gustafsson A, Obua C. Antibacterial activity of extracts from Ugandan medicinal plants used for oral care. J Ethnopharmacol. 2014;155:852–5.
Pushparaj A, Raubbin RS, Balasankar T. Antibacterial activity of Kappaphycus alvarezii and Ulva lactuca extracts against human pathogenic bacteria. Int J Curr Microbiol App Sci. 2014;3:432–6.
Vu TT, Kim JC, Choi YH, Choi GJ, Jang KS, Choi TH, et al. Effect of gallotannins derived from Sedum takesimense on tomato bacterial wilt. Plant Dis. 2013;97:1593–8.
Kurekci C, Bishop-Hurley SL, Vercoe PE, Durmic Z, Al Jassim RAM, McSweeney CS. Screening of Autralian plants for antimicrobial activity against Campylobacter jejuni. Phytother Res. 2012;26:186–90.
Robles-Zepeda RE, Coronado-Aceves EW, Velazquez-Contreras CA, Ruiz-Bustos E, Navarro-Navarro M, Garibay-Escobar A. In vitro anti-mycobacterial activity of nine medicinal plants used by ethnic groups in Sonora, Mexico. BMC Complement Altern Med. 2013;13:329.
Khan N, Abbasi AM, Dastagir G, Nazir A, Shah GM, Shah MM, et al. Ethnobotanical and antimicrobial study of some selected medicinal plants used in Khyber Pakhtunkhwa (KPK) as potential source to cure infectious diseases. BMC Complement Altern Med. 2014;14:122.
Rejiniemon TS, Arasu MV, Duraipandiyan V, Ponmurugan K, Al-Dhabi NA, Arokiyarai S, et al. In-vitro antimicrobial, antibiofilm, cytotoxic, antifeedant and larvicidal properties of novel quinone isolated from Aegle marmelos (Linn.) Correa. Ann Clin Microbiol Antimicrob. 2014;13:48.
Abu-Al-Basal MA. In vitro and in vivo anti-microbial effects of Nigella sativa Linn. seed extracts against clinical isolates from skin wound infections. Am J Applied Sci. 2009;6:1440–7.
Do TL. Medicinal plants and drugs from Vietnam. Hanoi: Medical Publisher House; 2004.
Habsah M, Muhammad Amin M, Rosmeira MA, Zulhelmi, Hamdan S, Ku Halim KB, et al. Comparison of hydrodistillation methods for extraction of essential oils from Beackea frutescens and evaluation for the antibacterial activity. J Sustain Sci Manag. 2008;3:66–75.
Hwang JK, Shim JS, Chung JH. Anticariogenic activity of some tropical medicinal plants against Streptococcus mutans. Fitoterapia. 2004;75:596–8.
Boonnak N, Karalai C, Chantrapromma S, Ponglimanont C, Fun HK, Kanjana-Opas A, et al. Bioactive prenylated xanthones and anthraquinones from Cratoxylum formosum ssp. pruniflorum. Tetrahedron. 2006;62:8850–9.
Vidotti GJ, Zimmermann A, Sarragiotto MH, Nakamura CV, Filho BPD. Antimicrobial and phytochemical studies on Pedilanthus tithymaloides. Fitoterapia. 2006;77:43–6.
Ghosh S, Samanta A, Mandal NB, Bannerjee S, Chattopadhyay D. Evaluation of the wound healing activity of methanol extract of Pedilanthus tithymaloides (L.) Poit leaf and its isolated active constituents in topical formulation. J Ethnopharmacol. 2012;142:714–22.
Pullagummi C, Rao NB, Singh BCS, Bheemagani AJ, Kumar P, Venkatesh K, et al. Comparitive studies on antibacterial activity of Patchouli [Pogostemon cablin (Blanco) Benth] and Geranium (Pelargonium graveolens) aromatic medicinal plants. Afr J Biotechnol. 2014;13:2379–84.
Karimi A. Characterization and antimicrobial activity of Patchouli essential oil extracted from Pogostemon cablin [Blanco] Benth. [lamiaceae]. Adv Environ Biol. 2014;8:2301–9.
Yang X, Zhang X, Yang SP, Liu WQ. Evaluation of the antibacterial activity of Patchouli oil. Iran J Pharm Res. 2013;12:307–16.
Joycharat N, Thammavong S, Voravuthikunchai SP, Plodpai P, Mitsuwan W, Limsuwan S, et al. Chemical constituents and antimicrobial properties of the essential oil and ethanol extract from the stem of Aglaia odorata Lour. Nat Prod Res. 2014;28:2169–72.
Appendino D, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, et al. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J Nat Prod. 2008;71:1427–30.
Verma RS, Padalia RC, Verma SK, Chauhan A, Darokar MP. The essential oil of ‘bhang’ (Cannabis sativa L.) for non-narcotic application. Curr Sci. 2014;107:645–50.
Nasrullah, Suliman, Rahman K, Ikram M, Nisar M, Khan I. Screening of antibacterial activity of medicinal plants. Int J Pharm Sci Rev Res. 2012;14:25–9.
Adonu CC, Esimone CO, Attama AA, Ugwueze MC. In vitro evaluation of antibacterial activity of extracts from Cassytha filiformis Linn against urogenital clinical Gram-negative bacteria. Int J Pharm Bio Sci. 2013;3:99–107.
Chen W, Vermaak I, Viljoen A. Camphor-a fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon-a review. Molecules. 2013;18:5434–54.
Mokbel MS, Hashinaga F. Evaluation of the antimicrobial activity of extract from buntan (Citrus grandis Osbeck) fruit peel. Pak J Biol Sci. 2005;8:1090–5.
Mokbel MS, Suganuma T. Antioxidant and antimicrobial activities of the methanol extracts from pummelo (Citrus grandis Osbeck) fruit albedo tissues. Eur Food Res Technol. 2006;224:39–47.
Tao N, Gao Y, Liu Y, Ge F. Carotenoids from the peel of Shatian pummelo (Citrus grandis Osbeck) and its microbial activity. American-Eurasian J Agri Environ Sci. 2010;7:110–5.
Perumal S, Mahmud R, Pillai S, Lee WC, Ramanathan S. Antimicrobial activity and cytotoxicity evaluation of Euphorbia hirta (L.) extracts from Malaysia. APCBEE Procedia. 2012;2:80–5.
Singh G, Kumar P. Phytochemical study and screening for antimicrobial activity of flavonoids of Euphorbia hirta. Int J App Basic Med Res. 2013;3:111–6.
Sittiwet C. In vitro antimicrobial activity of Pluchea indica aqueous extract: the potential for urinary tract infection treatment. J Pharmacol Toxicol. 2009;4:87–90.
Kundu A, Chatterjee TK. In vitro antimicrobial activity of thiophene derivative PITC-2 of Pluchea indica and its mechanism of action. Asian J Pharm Clin Res. 2013;6:115–7.
Acknowledgements
This study was financially supported by the Chonnam National University in 2014. We thank Associate Prof. Vu, Xuan Phuong, Dr. Tran, The Bach and Dr. Nguyen, The Cuong of the Institute of Ecology and Biological Resources, Vietnam, for assisting in the identification of the plant materials.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
TTV carried out the main experimental work and wrote the manuscript. QL, HTN, HK and ISK participated in sampling and generation of extraction. GJC and JCK designed and supervised the study. HK and VKT provided guidance on data analysis and drafting the manuscript. JCK critically evaluated and revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vu, T.T., Kim, H., Tran, V.K. et al. In vitro antibacterial activity of selected medicinal plants traditionally used in Vietnam against human pathogenic bacteria. BMC Complement Altern Med 16, 32 (2015). https://doi.org/10.1186/s12906-016-1007-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-016-1007-2