Abstract
Background
Pain is one of the most common symptoms that has a severe impact on quality of life and is associated with numerous psychosocial issues in cancer patients. Palliative care, which is a recent development in China, mainly focuses on symptom control and provides psychosocial support in order to improve quality of life for terminally ill patients. This meta-analysis aimed to evaluate the effects of palliative care on cancer pain in China.
Methods
The four most comprehensive Chinese academic databases-CNKI, Wanfang, Vip and CBM-were searched from their inception until July 2019. Medline/PubMed, Web of Science, EBSCO and internet search (Google and Google Scholar) were also searched. Randomized controlled studies assessing the effects of palliative care on cancer pain were analyzed. The pooled random-effect estimates of standardized mean difference (SMD) and 95% confidence intervals (CI) were calculated. Subgroup analysis was conducted by moderating factors for heterogeneity.
Results
The present meta-analysis included 18 studies with a total of 1370 patients. The random-effect model showed a significant effect size of palliative care on cancer pain (SMD = 1.475, p < 0.001; 95% CI = 1.071–1.878). Age, pharmacological/non-pharmacological strategies and publication date could account for the heterogeneity through subgroup analysis to some extent.
Conclusions
Palliative care was largely effective for relieving pain among Chinese adults with cancer, indicating that an adequate system should be urgently established to provide palliative care for cancer patients in Chinese medical settings. However, given the extent of heterogeneity, our findings should be interpreted cautiously.
Similar content being viewed by others
Background
Pain is one of the most common and distressing symptoms in cancer patients, either because of the disease itself or the related treatments. The prevalence rate of pain was 50.7% in all cancer stages, and 66.4% in advanced stage; moderate to severe pain was reported by 38.0% of all patients, and by 51.9% in advanced stage [1]. Cancer pain could impair patients’ quality of life [2] and was associated with numerous psychosocial distresses [3]. Unfortunately, approximately 33% of untreated cancer pain have been amply documented [4], and thus pain control remains a core issue in cancer patient care.
Contrary to traditional oncologic care, advanced cancer patients received the treatment with palliative intent and the main emphasis placed on palliative care (PC) in some settings [5]. Considered as an interdisciplinary care, PC focuses on symptom control and provides psychosocial and other support (e.g., decision making) in order to improve quality of life for persons with serious illness [6]. Although pain control plays a central role in managing patients’ distress under the process of PC, not much is generally known regarding the effects of PC on cancer pain.
Due to multi-factorial etiology of cancer pain, the related treatment should be based upon the bio-psycho-social approach [7]. Compared to the traditional pain-control methods, PC considers not only biomedical factors but also patients’ psychosocial and spiritual distress [6]. However, relatively few studies have been carried out to explore the effects of PC on cancer pain. The randomized controlled trials (RCTs) of PC paid more attention to the evaluation of quality of life, symptom burden and psychological distress [8,9,10], and systematic reviews mainly focused on the prevalence rate and severity of cancer pain [1, 11]. To the best of our knowledge, few meta-analysis of RCTs have evaluated the effects of PC on cancer pain.
Among Chinese cancer patients, conducting such meta-analysis is vitally important for the following reasons. The first reason is attributed to the large number of Chinese cancer patients. The latest data revealed that the numbers of new cases and deaths were 4.292 million and 2.814 million, which accounted for 21.8 and 26.9% of global cancer population, respectively [12]; second, the development of PC originated in Western countries is in the initial stage in China, it is of importance to explore whether PC could effectively alleviate cancer pain; third, several RCTs of PC on cancer pain have been published in Chinese journals, but there has not been a comprehensive meta-analysis to review these literatures and assess the effects of PC; finally, Chinese researchers paid more attention to traditional Chinese medicine [13,14,15] rather than PC for reducing cancer pain, which could neglect the importance of PC to some extent.
The aim of this meta-analysis was to perform a systematic review and evaluate the effect of PC on reducing cancer pain reported by RCTs in an attempt to obtain accurate profile of cancer pain under PC in China.
Methods
Literature search
A systematic search was conducted to identify published literatures about the effect of PC on cancer pain in China. The CNKI (China National Knowledge Infrastructure), Wanfang, Vip and CBM (Chinese Biomedical Literature Database), which are the four most comprehensive Chinese academic databases, were searched from their inception until July 2019. The search was constructed and performed by a professional medical librarian. In order to expand searches, Medline/PubMed, Web of Science (SCIE) and CINAHL (EBSCO) were also searched from their inception until July 2019 without language restrictions. Search strategies for domestic and international databases were shown in Additional file 1. The reference lists of relevant articles obtained were also screened. Additionally, the search keywords used for Google and Google Scholar search were: cancer, China and randomized controlled trials, in combination with palliative care, hospice care or terminal care. The screening of the abstracts/titles and full-text articles were performed twice by two authors (XXZ and MC) independently to reduce reviewer bias.
Inclusion and exclusion criteria
We included all studies in which: (1) subjects age ≥ 18 years; (2) RCTs; (3) subjects were diagnosed with cancer; (4) pain was evaluated by well-validated measures. We excluded studies in which: (1) PC was not described in details, which could not provide valuable leads for further research to conduct PC; (2) studies focused on the separate components of PC, such as psychosocial support, pain control or spiritual care; (3) studies did not provide the post-test score of cancer pain. Eligibility judgment and data extraction were recorded and carried out independently by two authors (YHG and XXZ) in a standardized manner. Any disagreements were resolved by discussion and the involvement of another author (YLY).
Quality assessment
The modified Jadad scale for assessing quality of RCTs was adopted in this study [16], which has been used in our previous meta-analysis [17]. The modified Jadad scale is an eight-item scale designed to assess randomization, blinding, withdrawals/dropouts, inclusion/exclusion criteria, adverse effects, and statistical analysis. In this meta-analysis, blinding (2 points) and adverse effects (1 point) were excluded due to the characteristics and effects of PC. Thus, the score for each study ranged from 0 (lowest quality) to 5 (highest quality). We defined three categories: the study was considered to have high quality (low risk of bias) if it scored 4 points or above, studies that scored 1 point or below were categorized as having low quality (high risk of bias), studies that scored 2 or 3 points were considered as having medium quality (moderate risk of bias). Any disagreements with authors (XXZ and MC) were resolved by discussion and the involvement of another author (YLY).
Data extraction
We used the Endnote to do the screening, and employed the Excel to do the data extraction, which is a standardized data abstraction form designed to capture and code all relevant study-level information required for analysis. For the included studies, two authors independently extracted data (YHG and MC). Disagreements were resolved by discussion and the involvement of another author (YLY). Extracted data included author name, year of publication, age range and mean age, simple size, assessment instruments, cancer type, pharmacological/non-pharmacological strategies, care time, settings and the values of mean and standard deviation (SD) in experimental-control group.
Pain control in PC mainly included both pharmacological and non-pharmacological strategies. Analgesic and adjuvant drugs are the most commonly used pharmacological strategies, which were endorsed and promoted by World Health Organization (WHO) in the now-famous ‘analgesic ladder’ for managing cancer pain properly. Non-pharmacological strategies included several bio-psycho-social approaches, such as music therapy, psychological interventions (e.g., relaxation), traditional Chinese medicine (e.g., herbal, acupuncture, moxibustion, massage) and others. Data could not be extracted when psychological interventions mainly focused on psychological distress rather than cancer pain.
Meta-analysis
Assessment of overall effect size
We computed the effect size of standardized mean difference (SMD) for each study by subtracting the average post-test score of the control group from that of the experimental group and dividing the result by the pooled SDs of the experimental-control group. Means and SDs of cancer pain were used for computation of SMD (Cohen’s d). A SMD of 1 indicates a relatively stronger improvement in experimental group by one standard deviation larger than the mean of control group. According to the expected heterogeneity across studies, the pooled random-effect estimates of SMD and 95% confidence intervals (CI) were used as the summary measure of effect size. Cohen’s criteria were used to interpret the magnitude of SMD [18]: a value of 0.20–0.50 corresponds to small effect sizes, 0.50–0.80 to medium and a value over 0.80 to large effect sizes, which is also employed and supported by other studies [19,20,21]. A two-tailed P value of < 0.05 was considered to be significant. Overall effects and other statistical analyses were analyzed using the statistical software Stata 11.0.
Assessment of heterogeneity
Heterogeneity was evaluated with the Q statistic and I2 statistic. The Q statistic is used to assess whether differences in results are compatible with chance alone, but it is sensitive to the number of studies [22]. The I2 statistic which denotes the variance among studies as a proportion of the total variance was also calculated and reported, because I2 statistic is not sensitive to the number of studies [22]. Larger values of I2 show increasing heterogeneity. An I2 of 0% shows no observed heterogeneity, while 25% shows low, 50% moderate, and 75% high level of heterogeneity [23].
Subgroup analysis
When the hypothesis of homogeneity was rejected by the Q statistic and I2 statistic, subgroup analysis was conducted to explore the potential moderating factors for heterogeneity. For subgroup analyses, the heterogeneity within groups was tested. In this meta-analysis, subgroup analysis was conducted for moderating factors, including age, cancer type, pharmacological strategies (experimental-control group), non-pharmacological strategies in the experimental group and publication date. If subgroup analysis restricted to studies of these factors could reduce the variance (i.e., Q statistic and I2 statistic), and these moderating factors were indeed behind the heterogeneity observed.
Results
Study selection
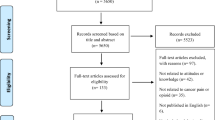
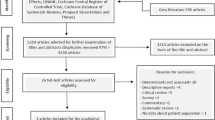
The total number of included studies was 18 in the present study. As shown in Fig. 1, we identified the eligible articles through the database of CNKI (n = 420), Wangfang (n = 320), Vip (n = 232) and CBM (n = 145). The titles and abstracts of these articles were respectively reviewed by the three authors (MC, YHG, YLY), and the full-text articles without duplicates (n = 72) were selected for further examination. Based on these 72 studies, 54 did not meet the inclusion criteria. In total, 18 RCTs about the effect of PC on cancer pain were included [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41].
In order to expand searches (see Additional file 2), we also searched the international databases of Medline/PubMed (n = 32), SCIE (n = 22) and EBSCO (n = 3), but there were no studies that met our inclusion criteria.
Characteristics of included studies
Study characteristics were listed in Table 1. The included studies published from 2006 to 2019 comprised 1370 subjects (experimental group = 705, control group = 665) with mean age of 62.07 years (median: 61.31; range: 43–76.26). Cancer pain was assessed by single-item unidimensional tools, such as Visual Analogue Scales (VAS) and Numerical Rating Scales (NRS). Approximately 38.9% of studies included heterogeneous samples of cancer patients, and 66.7% adopted non-pharmacological strategies in experimental group. The most frequently used non-pharmacological strategy was musicotherapy (55.6%). For pharmacological strategies, 61.1% of experimental groups used WHO three-step analgesic ladder and/or analgesic pumps, and 16.7% of control groups employed WHO three-step ladder. More than half of the included studies (66.7%) adopted a combination of pharmacological and non-pharmacological strategies. Dosage and type of analgesic were reported in detail in only one study [25]. Finally, less than half of studies (44.4%) provided care time (mean: 4.63 weeks, median: 4; range: 2–12) and most studies (83.3%) were conducted in general hospital.
Study quality assessment
Study quality ratings for each criteria of the modified Jadad were indicated in Table 2. Higher scores reflected the better study quality, and the average scores of all studies were above 3 (mean: 3, median: 3; range: 2–4). Six studies were judged to have high quality, and other studies were rated as medium quality.
Effects of PC on cancer pain
A pooled random-effect meta-analysis was conducted using data from 18 studies, which estimated the post-test effects of PC on cancer pain compared with care-as-usual control group. As shown in Fig. 2, the random-effect model indicated an overall effect size of 1.475 (95% CI = 1.071–1.878, p < 0.001). The heterogeneity analysis (Q = 184.81, p < 0.001; I2 = 90.8%) revealed that there was a relatively high amount of heterogeneity in our meta-analysis.
Subgroup analysis
As shown in Table 3, age, pharmacological/non-pharmacological strategies and publication date were the significant sources of heterogeneity to some extent, and their moderating effects were significant for the effect of PC on cancer pain (p < 0.01). Effect size was the largest in patients less than 60 years old (SMD = 1.859, 95% CI = 1.348–2.369), but it was the smallest (SMD = 0.348, 95% CI = 0.081–0.616) among patients aged above 70. Compared with pharmacological strategies used in experimental-control group (SMD = 1.054, 95% CI = 0.423–1.686), the effect size was larger than the pharmacological strategies only used in experimental group (SMD = 1.75, 95% CI = 1.255–2.245). Effect size was larger (SMD = 1.954, 95% CI = 1.473–2.435) in the experimental group using non-pharmacological strategies than studies without non-pharmacological strategies (SMD = 0.564, 95% CI = 0.233–0.895). Additionally, effect size was the smallest (SMD = 0.3, 95% CI = − 0.104 to 0.705) for studies published before 2015.
Discussion
We performed strict inclusion criteria and subgroup analysis to reduce the heterogeneity. However, heterogeneity was still relatively high within some subgroups, and the conclusion should be considered with some caution. On the other hand, according to the modified Jadad scale assessing study quality, studies in our meta-analysis with the lack of description about withdrawals/dropouts might weaken the internal validity to some extent.
The present meta-analysis, to our knowledge, was the first to explore the effect and associated moderator variables of PC on pain among Chinese cancer patients, and PC was proven largely effective to relieve pain (SMD = 1.475, 95% CI = 1.071–1.878). The possible explanation to this finding could be attributed to the interface between cancer pain and PC. Contrary to pain traditionally considered as a physiological experience, cancer pain is a complex subjective human experience affected by physical, psychological, social and spiritual components [42], which is of great importance in the care of advanced cancer patients. Correspondently, the aim of PC is to control pain and other symptoms, manage mental, social and spiritual problems, and improve the lives of patients with terminal illness [6], which could capture the complexity of cancer pain.
Age, pharmacological/non-pharmacological strategies and publication date contributed to the significantly substantial heterogeneity to some extent. Compared with other age groups, the effect size was the smallest (SMD = 0.348, 95% CI = 0.081–0.616) among patients aged above 70. Among older cancer patients, there was an attitude of ‘getting on with life’ regardless of pain in comparison with younger counterparts, indicating that older patients revealed acceptance and tolerance of pain to some extent [43]. Several studies also found a relation between more advanced age and less cancer pain [44, 45]. Furthermore, older patients are considered to be more susceptible to opioid-related side effects, and chronic disease treatment might render the pain control more difficult [46]. The opinions mentioned above might lead to that cancer pain were found to be more likely to be unrecognized and undertreated among older patients [46, 47]. As a result, the effect of PC on older patients’ pain were significantly smaller than that on younger patients’ pain in our meta-analysis.
For pain relief, the effects of pharmacological strategies used alone in experimental group were larger than that both used in experimental-control group. The effectiveness of pharmacological approach has been confirmed in the vast majority of patients with cancer pain according to WHO three-step ladder [46, 48, 49]. Additionally, the effect of PC on cancer pain was still significant even though both experimental group and control group adopted pharmacological strategies, which could be explained by the different pharmacological approaches. Several studies suggested better overall pain control and fewer complications with intravenous analgesic pumps than conditional provider-administered analgesia [50, 51]. Another possible explanation might be the non-pharmacological strategies also used in these included studies [25, 31, 38, 39]. RCT found that both non-pharmacological strategies and intravenous analgesic pumps adopted could better improve pain control compared with analgesia pumps adopted alone [52].
Besides of physiological components, other components of human functioning (e.g., personality, mood, behavior, social relations) also play an important role in cancer pain management. By comparison with non-pharmacological strategies not used in the experimental groups, non-pharmacological strategies used in the experimental groups had more beneficial effects on reducing cancer pain. The findings were in line with the literature indicating that in addition to pharmacological strategies, various methods of non-pharmacological treatments, such as psychological, cognitive and behavior therapies, could also relieve cancer pain [13, 53, 54].
With regard to publication date, studies published before 2015 had the smallest effect size of PC on cancer pain [28, 30, 35], mainly because pharmacological strategies were both used in experimental and control group, and non-pharmacological strategies were not used among these studies. It should be noted, however, that because the statistical power of subgroup analysis in this subgroup was limited due to the small number of available studies (n = 3), the present result might be overestimated, and clearly there is need for further studies.
Clinical implications
The present meta-analysis provided several clinical implications. First, PC has long been recognized to provide better pain management and symptom control for patients with advanced illness in developed countries, but aggressive treatment of advanced cancer patients is prevalent in China [55], which poses problems for the development of PC. Based on our findings, PC has been proven to be effective for relieving pain in Chinese cancer patients; second, our findings also provided pharmacological and non-pharmacological guidance in developing optimal approach and appropriate standards of PC in clinical practice. For instance, intravenous analgesic pumps should be adopted for patients with cancer pain at PC units, especially for patients who are ineffectual to conventional pain control. Moreover, non-pharmacological strategies are undoubtedly essential issues in cancer pain management, which should be recommended as pain control methods used concurrently with pharmacological interventions.
Limitation
Our meta-analysis had several drawbacks that should be taken into consideration in interpreting the findings. First, subgroup analysis was used to explore potential sources of heterogeneity, but substantial heterogeneity of SMD was still observed in both the overall analysis and subgroups analysis (I2 > 75%). Thus, we assumed that there were other factors, which are not provided by the included studies, likely influencing heterogeneity; second, as a psychometrically satisfactory instrument for assessment of pain intensity, VAS was used in the included studies, but it cannot provide information about the duration, frequency or interference of pain [56]; third, due to the lack of follow-up results, it is not confirmed whether there were long-term PC effects; fourth, most of the included studies were conducted in general hospitals, which might be difficult to reflect the whole picture of Chinese PC for cancer pain; fifth, further studies need to be conducted to examine whether the findings of our meta-analysis are suitable to other countries; finally, the evaluation of publication bias generally is not useful when less than 20 studies are included in a meta-analysis. Therefore, we did not evaluate the publication bias neither graphically nor using a statistical test in this context due to the small number of included studies (N = 18).
Conclusion
Although there are several limitations (small number of included studies and high heterogeneity) in this meta-analysis, a tentative and preliminary conclusion could be reached that PC was effective for relieving pain in Chinese cancer patients. Besides, medical personnel should pay more attention to the moderating effects of age and pharmacological/non-pharmacological strategies on cancer pain in the process of PC service. However, the findings should be interpreted cautiously, with consideration of high heterogeneity.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CBM:
-
Chinese Biomedical Literature Database
- CI:
-
Confidence interval
- CNKI:
-
China National Knowledge Infrastructure
- NRS:
-
Numerical Rating Scales
- PC:
-
Palliative care
- RCTs:
-
Randomized controlled trials
- SD:
-
Standard deviation
- SMD:
-
Standardized mean difference
- VAS:
-
Visual Analogue Scales
- WHO:
-
World Health Organization
References
van den Beuken-van MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag. 2016;51:1070–90.
Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. 2018;167:157–69.
Lee YP, Wu CH, Chiu TY, Chen CY, Morita T, Hung SH, et al. The relationship between pain management and psychospiritual distress in patients with advanced cancer following admission to a palliative care unit. BMC Palliat Care. 2015;14:69.
Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149–54.
Are M, McIntyre A, Reddy S. Global disparities in cancer pain management and palliative care. J Surg Oncol. 2017;115:637–41.
Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747–55.
Kumar SP. Reporting characteristics of cancer pain: a systematic review and quantitative analysis of research publications in palliative care journals. Indian J Palliat Care. 2011;17:57–66.
Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza PA, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–30.
Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35:834–41.
El-Jawahri A, LeBlanc T, VanDusen H, Traeger L, Greer JA, Pirl WF, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316:2094–103.
Roberto A, Deandrea S, Greco MT, Corli O, Negri E, Pizzuto M, et al. Prevalence of neuropathic pain in cancer patients: pooled estimates from a systematic review of published literature and results from a survey conducted in 50 Italian palliative care centers. J Pain Symptom Manag. 2016;51:1091–102.
Stewart BW, Wild CP. World Cancer report 2014. Switzerland: WHO Press; 2014.
Chen TH, Tung TH, Chen PS, Wang SH, Chao CM, Hsiung NH, et al. The clinical effects of aromatherapy massage on reducing pain for the cancer patients: meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2016;2016:9147974.
Yanju B, Yang L, Hua B, Hou W, Shi Z, Li W, et al. A systematic review and meta-analysis on the use of traditional Chinese medicine compound kushen injection for bone cancer pain. Support Care Cancer. 2014;22:825–36.
Chiu HY, Hsieh YJ, Tsai PS. Systematic review and meta-analysis of acupuncture to reduce cancer-related pain. Eur J Cancer Care. 2017;26:e12457.
Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord. 2001;12:232–6.
Yang YL, Sui GY, Liu GC, Huang DS, Wang SM, Wang L. The effects of psychological interventions on depression and anxiety among Chinese adults with cancer: a meta-analysis of randomized controlled studies. BMC Cancer. 2014;14:956.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Earlbaum: Hillsdale; 1988.
Berben L, Sereika SM, Engberg S. Effect size estimation: methods and examples. Int J Nurs Stud. 2012;49:1039–47.
Sosner P, Guiraud T, Gremeaux V, Arvisais D, Herpin D, Bosquet L. The ambulatory hypotensive effect of aerobic training: a reappraisal through a meta-analysis of selected moderators. Scand J Med Sci Sports. 2017;27:327–41.
Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res. 2014;158:156–62.
Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to meta-analysis. Oxford: Wiley; 2009.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Fei HL, Wang XH, Hu X. Hospice care analysis of patients with advanced liver cancer. Chin Health Stand Manag. 2016;7:189–90 (in China).
Su HX, Yang L. Efficacy of evidence-based nursing in patients with advanced primary liver cancer. Chin J Clin Oncol Rehabil. 2016;23:1261–4 (in China).
Gao DD, Zhao X. Nursing of palliative care for the terminal cancer patients. Psychol Doct. 2017;23:214–5 (in China).
He X, Liu LJ, Ding Y, Yuan WR. Application effect of palliative care in nursing for cancer patients with terminal stage. Nurs Pract Res. 2017;14:113–4 (in China).
Huang MZ, Chen Y, Zhu Q. An intervention study of hospice care about the elderly patients at the community hospital. Chin Med J Metall Indus. 2010;27:641–2 (in China).
Kang HQ, Qi KN, Wu H, Wang SL. The application value of hospice care in patients with advanced liver cancer. Chin Contin Med Educ. 2015;7:249–50.
Li YQ, Zhang MX, Fu GX, Zhao LH, Li WY, Li H, et al. Effect of hospice care on the quality of life and psychological state of aged mortal inpatients. J Cent South Univ. 2006;31:538–42 (in China).
Liu YL, Guo LZ. Effect of palliative comprehensive nursing intervention on pain and anxiety of patients with advanced liver cancer. J Bethune Med Sci. 2016;14:389–91 (in China).
Luo YL, Feng SZ, Zhou XP, Yang QY. Pain management and hospice nursing intervention among primary late liver cancer patients. Guangdong Med J. 2017;38:2421–3 (in China).
Wu L. Application of high quality nursing service on hospice care of patients with terminal stages of cancer. Med Inf. 2015;28:161 (in China).
Wu L. Effects of pain care and hospice care on quality of life in patients with advanced gastric cancer. Med Inf. 2017;30:139–40 (in China).
Xu YF. The effect of comprehensive nursing intervention in negative emotions and pain of patients with advanced cancer. Chin Contin Med Educ. 2014;6:71–2 (in China).
Yao LP. Hospice care analysis of patients with advanced liver cancer. Oriental Diet Therapy and Health Care. 2017;10:314 (in China).
Zhang M, Kang LY, Li QY. Application of hospice care in the management of pain in patients with advanced primary lung cancer. Chin J Clin Oncol Rehabil. 2018;25:198–201 (in China).
You Y. Effect of palliative care on pain control of patients with cancer at terminal stage. Gen Nurs. 2018;5:109 (in China).
Rao JF, Zhu T, Zhang L. Study on the effect of hospice care in the nursing of advanced liver cancer. Psychologies. 2018;3:62 (in China).
Cao Y. Application effect of palliative care service in nursing for patients with advanced liver cancer. Psychol Doct. 2018;24:246–7 (in China).
Yang QY, Zhang P. Effect of family palliative care on quality of life and mental states in patients with end stage lung cancer. Shanxi Med J. 2019;48:1505–7 (in China).
Dalal S, Tanco KC, Bruera E. State of art of managing pain in patients with cancer. Cancer J. 2013;19:379–89.
McPherson CJ, Hadjistavropoulos T, Lobchuk MM, Kilgour KN. Cancer-related pain in older adults receiving palliative care: patient and family caregiver perspectives on the experience of pain. Pain Res Manag. 2013;18:293–300.
Gagliese L, Jovellanos M, Zimmermann C, Shobbrook C, Warr D, Rodin G. Age-related patterns in adaptation to cancer pain: a mixed-method study. Pain Med. 2009;10:1050–61.
Strömgren AS, Groenvold M, Petersen MA, Goldschmidt D, Pedersen L, Spile M, et al. Pain characteristics and treatment outcome for advanced cancer patients during the first week of specialized palliative care. J Pain Symptom Manag. 2004;27:104–13.
Barbera L, Seow H, Husain A, Howell D, Atzema C, Sutradhar R, et al. Opioid prescription after pain assessment: a population-based cohort of elderly patients with cancer. J Clin Oncol. 2012;30:1095–9.
Lindskog M, Tavelin B, Lundström S. Old age as risk indicator for poor end-of-life care quality-a population-based study of cancer deaths from the Swedish register of palliative care. Eur J Cancer. 2015;51:1331–9.
Prommer EE. Pharmacological management of cancer-related pain. Cancer Control. 2015;22:412–25.
Wood H, Dickman A, Star A, Boland JW. Updates in palliative care–overview and recent advancements in the pharmacological management of cancer pain. Clin Med. 2018;18:17–22.
Chao PW, Lin SP, Tsou MY, Kuo IT, Chang KY. Assessing the impact of renal function on trajectory of intravenous patient-controlled analgesic demands over time after open and laparoscopic colorectal surgery using latent curve analysis. Clin J Pain. 2016;32:695–701.
Zhou Y, Huang JX, Lu XH, Zhang YF, Zhang W. Patient-controlled intravenous analgesia for non-small cell lung cancer patient after thoracotomy. Cancer Res Ther. 2015;11:128–30.
Wang Y, Tang H, Guo Q, Liu JS, Liu XH, Luo JM, et al. Effects of intravenous patient-controlled sufentanil analgesia and music therapy on pain and hemodynamics after surgery for lung cancer: a randomized parallel study. J Altern Complement Med. 2015;21:667–72.
Tse MMY, Wong ACF, Ng HN, Lee HY, Chong MH, Leung WY, et al. The effect of a pain management program on patients with cancer pain. Cancer Nurs. 2012;35:438–46.
Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ, et al. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32:1703–11.
Wu Y, Li L, Su H, Yao X, Wen M. Hospice and palliative care: development and challenges in China. Clin J Oncol Nurs. 2016;20:E16–9.
Hølen JC, Hjermstad MJ, Loge JH, Fayers PM, Caraceni A, et al. Pain assessment tools: is the content appropriate for use in palliative care? J Pain Symptom Manag. 2006;32:567–80.
Acknowledgements
The authors wish to acknowledge the bio-statistical assistance of Bin Ma.
Funding
This work was supported by the Basic Research Projects of the Higher Education Department of Liaoning Province [grant numbers: LQNK201732]. The funding source had no role in study design, data collection, analysis, interpretation, writing of the manuscript, or decision to submit for publication.
Author information
Authors and Affiliations
Contributions
XXZ and MC was responsible for conception and design of the review, carried out the literature search, performed inclusion criteria and data analysis, and wrote the manuscript. YHG carried out the literature search, performed data extraction and quality assessment, and participated in conception and design of the review. XXZ and MC performed data extraction and quality assessment. YLY supervised the data collection and statistical analysis, and was a major contributor in writing and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Search strategies for all databases. (PDF 118 kb)
Additional file 2:
Selection process of studies for the meta-analysis (international databases). (PDF 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, XX., Cui, M., Geng, YH. et al. A systematic review and meta-analysis of randomized controlled trials of palliative care for pain among Chinese adults with cancer. BMC Palliat Care 18, 69 (2019). https://doi.org/10.1186/s12904-019-0456-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12904-019-0456-z