Abstract
Background
To evaluate the efficacy of fluoride-containing toothpastes with different technologies to remineralize artificial caries lesions in enamel.
Methods
Bovine enamel blocks were divided into three thirds: intact (untreated), demineralized (artificial caries lesion), and treated (caries lesion, pH cycling with dentifrices). Enamel blocks were randomly distributed into five groups (n = 12): Fluoride-free toothpaste, Colgate Oral Care (NC); Arginine-containing toothpaste, Colgate Total Daily Repair (PC); Silicate-based fluoride toothpaste: REFIX technology, regenerador + sensitive (RDC), NR-5 technology, Regenerate Enamel Science (RES), and NOVAMIN technology, Sensodyne Repair and Protect (SRP). The specimens were submitted to a pH cycling model for 6 days. The efficacy of the toothpastes was estimated by calculating the surface microhardness recovery (%SMHR) and the fluorescence recovery (ΔFRE) with quantitative light-induced fluorescence. The cross-sectional micromorphology of the enamel surface was also assessed using scanning electron microscopy. Elemental analyses (weight%) were determined with an energy-dispersive X-ray spectrometer (EDS). The results were compared to that of the control (NC). Data were statistically analyzed (5%).
Results
%SMHR could be ranked as follows: RDC = PC = RES = SRP > NC. Significantly higher %SMHR and ΔFRE means were observed after enamel treatment with RDC (22.7 and 46.9, respectively). PC (%SMHR = 18.8) was as efficacious as RDC to recover the surface microhardness with a significantly lower mean of ΔFRE (19.5). Only RDC was able to promote the formation of a mineralized layer on the surface of enamel enriched with silicon on the surface.
Conclusions
The silicate-based fluoride toothpaste containing REFIX technology demonstrated greater efficacy in the remineralizing artificial caries than the other products.
Similar content being viewed by others
Background
Tooth brushing with fluoride toothpaste is claimed to significantly reduce the risk of caries associated with sugar intake [1]. Many fluoridated products are available on the market, with different compositions and applications, and are widely used due to their easy access and low cost [2, 3]. Brushing with fluoridated toothpastes is the most effective non-professional intervention to prevent tooth decay, especially in places where the water is not fluoridated [4, 5].
Currently, manufacturers are seeking for innovative compounds to improve the remineralization of dental tissues, with or without fluoride [6,7,8]. The innovative aspects aim not only to boost the remineralization process but also to increase the regeneration potential of these formulations [7]. Along these lines, these biomimetic agents function as fluoride and calcium carriers for tooth enamel [8]. Biological apatite has been found to be composed of small crystals and characterized by poor crystallinity and relatively high solubility [9]. These alternative mechanisms seem to mimic the natural remineralization, promoting the formation of less soluble and porous hydroxyapatite [10, 11].
The remineralization process can be enhanced by the substituting of ionic species in the sites of the hydroxyapatite molecule [12]. The presence of substitute ions, either incorporated within the apatite lattice or only adsorbed on the surface, including both anionic(e.g., F−, Cl−, SiO44−, and CO32−) and cationic substitutions (e.g., Na+, Mg2+, K+, Sr2+, Zn2+, Ba2+, Al3+), changes the hydroxyapatite solubility, depending on the substitution at the different sites of the hydroxyapatite molecule (calcium, phosphate, and hydroxyl sites) [9, 13].
Silica, a component of bioactive glass, has been incorporated in some toothpaste formulations in order to enhance their bioactivity and apatite-forming ability of hydroxyapatite [14, 15]. In this manner, silica acts as a site for the precipitation of calcium and phosphate ions, forming calcium silicate, which leads to the nucleation of hydroxyapatite and mineral formation and intensifies the remineralization process [16, 17]. Calcium silicate is responsible for a protective effect on the surface, stimulating the deposition of other minerals and reducing the effects of demineralization [17]. Although it is claimed that these changes seem to occur mainly at the hydroxyapatite surface [18], it seems to be the reason for retaining fluoride in the composition, even at lower concentrations [19].
Therefore, in view of the current efforts in search of effective components against caries, the purpose of this in vitro study was to analyze the efficacy of fluoride-containing toothpastes containing REFIX technology, NR-5 and NOVAMIN technologies to remineralize enamel after pH cycling. The research hypothesis was that the remineralization of the enamel would be positively affected by the use of fluoride toothpastes after pH cycling, irrespective of the technology contained in the products tested.
Methods
Sample preparation
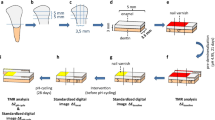
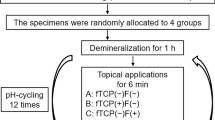
The recently extracted bovine incisor teeth were obtained from the commercial establishment: Honorato e Araújo LTDA Fridge (CNPJ: 01.179.091/0001-37) and stored in a 0.08% thymol solution until use. Enamel blocks (4 × 4 × 2 mm) were made from these teeth and then were embedded in self-curing acrylic resin using circular molds of 16 mm in diameter and 3 mm in depth. The outer enamel surface was ground flat with grit papers (600–1500 grades) under water refrigeration and polished with 1 µm diamond paste (Extec Corporation, Enfield, CT) in a rotating polishing machine PSK-2 V (Skill-tec Comércio e ManutençãoLtda, São Paulo, SP, Brazil). Baseline enamel Vickers surface microhardness (SH0) analysis was performed with a microhardness tester (Shimadzu HMV—AD Easy Test Version 3.0). Five indentations spaced 100 µm from each other were made at the center of the enamel surface (50 g, 10 s). Enamel blocks between 360 and 400 VHN surface microhardness were selected for the study. Sixty enamel blocks were randomly distributed into 5 groups (n = 12) according to the products used [5, 20, 21].
Tested groups and interventions
Toothpastes were selected among commercial products containing fluoride associated with different technologies, as indicated by the manufacturers. The characteristics of the products are listed in Table 1. A fluoride-free product was chosen as the control toothpaste (NC) and an arginine-containing toothpaste, Colgate® Total Daily Repair, as the positive control (PC).
Lesion formation
Subsurface enamel demineralization was carried out using a modified model [22]. Following 5 min sonication in water using an ultrasonic device, one-third of the exposed enamel surface was covered with two layers of nail varnish (Risqué, Niasi, Taboão da Serra, São Paulo, Brazil) as a reference sound area. The enamel blocks were immersed individually in 32 mL of a demineralizing solution containing 1.3 mM/L Ca(NO3)2·4H2O, 0.78 mM/L NaH2PO4·H2O in 0.05 M/L acetate buffer, 0.03 μgF/mL (NaF), pH 5.0, 32 mL/specimen, during 16 h at 37 °C. After that, the blocks were submitted to a post-demineralization surface hardness (SH1) with the same parameters described previously.
Remineralizing pH-cycling
Before the remineralization pH cycling model [23], the enamel specimens had another one third of its surface covered with two layers of nail varnish (Risqué, Niasi, Taboão da Serra, São Paulo, Brazil) as a reference for caries lesion area. The blocks were submitted to a pH cycling model at 37 °C for 6 days. The blocks were immersed individually in a remineralization solution (1.5 mM L−1 calcium, 0.9 mM L−1 phosphate, 150 mM L−1 potassium chloride in 0.02 mM L−1cacodylic buffer, pH 7.0; 0.02 µgF/mL, 1 mL/mm2), for 22 h. The cariogenic challenge was performed by a demineralization solution (2.0 mM L−1 calcium and phosphate in 75 mM L−1 acetate buffer, pH 4.7; 0.03 µgF/mL, 3 mL/mm2) during 2 h per day (12–2 pm). Twice a day, at 10 am and 2 pm, enamel samples were exposed to toothpaste slurries (toothpaste: deionized water, 1:3 w/w; 2 mL/enamel specimen) for 1 min, under agitation. Deionized water rinses were performed between each step. In between treatments, the enamel blocks were individually immersed in a remineralization solution at 37 °C. De- and remineralizing solutions were changed daily.
Microhardness test analysis
The baseline surface microhardness (SH0) was evaluated using a microhardness tester with the previously described settings. The enamel surface microhardness after enamel demineralization (SH1) and after treatment with the toothpastes associated with pH cycling (SH2) was also determined using the same parameters. For this determination, the acid-resistant nail varnish was removed, and the hardness of each third of each specimen was tested. Each indentation in the center of the thirds was separated from the others by a distance of 100 μm. The percentage of surface hardness recovery (%SMHR) was then calculated [5, 24, 25], as follows (Eq. 1):
Quantitative light-induced fluorescence (QLF) analysis
The bovine enamel blocks were evaluated for fluorescence loss in caries lesions and treated areas, using the Qraycam Pro device (Inspektor Research System BV, Amsterdam, The Netherlands). The nail varnish in each window was carefully removed with a surgical blade and cotton swabs soaked in diluted acetone. Then, the specimens were water rinsed with deionized water and dried with a cotton roll. A camera was attached to a stand in the same position for all the images to standardize the QLF measurements. The images were taken in a dark room, with an exposure of 0, a contrast of 0, and a distance between the device and a sample of 8 cm [26, 27]. A software (Q-ray version 1.38, (Inspektor Research System BV, Amsterdam, The Netherlands) analyzed the changes in the amount of mineral in the enamel based on the ΔF value. The ΔF value represents the percentage decrease in the autofluorescence intensity in a carious lesion and treated areas compared with that of sound enamel, reflecting the changes in the mineral contents of enamel [26]. The measurements were made in two stages for the calculation of the percentage fluorescence recovery (ΔFRE): ΔF0, which represents a loss of initial fluorescence, passing through the difference between the sound and demineralized enamel, and ΔF1, which represents a difference in final fluorescence, using the difference between the sound enamel and the area treated with the toothpastes[28, 29]. Then, the percentage of fluorescence recovery was calculated as follows[29] (Eq. 2):
Scanning electronic microscopy (SEM) plus energy-dispersive X-ray spectroscopy (EDS)
The morphological analysis of the specimens was performed in a scanning electron microscope (EGA 3, TESCAN, LMU, Kohoutovice, Czech Republic), operating at 15 kV. For the morphological analysis of the specimens, the blocks were previously sputter-coated with gold in a vacuum evaporator (MED 010; Balzers, Balzer, Liechtenstein), and then microscopically analyzed to obtain photomicrographs of the surface morphology of the treated specimens (1000× magnification). Representative images of selected regions of the sputter-coated specimens were taken in order to characterize the morphological aspect of the surface [5, 20, 21]. The EDS point analysis (80 mm2, SDD Detector, Oxford Instruments, Concord, MA, USA) was performed to determine a qualitative elemental analysis of specimens, operating in high vacuum mode and an accelerating voltage of 15 kV. For each sample, five points were randomly selected for each sample (300 µm2 for each point), and the mean values were calculated [20, 21].
For the subsurface analysis, cross-sections of the bovine blocks were obtained by longitudinally sectioning the specimens under water-cooling. Both half-blocks were used for the SEM and the elemental analyses. The halves were dehydrated in silica gel for 3 h. The specimens were then gold-sputtered and evaluated using an SEM coupled with an EDS [20, 21].
Statistical analysis
Data were analyzed statistically using the SPSS package for Windows, version 21.0 (SPSS, Inc., Chicago, IL, USA). The Shapiro–Wilk test and Levene’s test were used to determine the normality and homogeneity of variances, respectively. As the data demonstrated equal variances and Gaussian distribution, no data transformation was needed. The following tests were performed: (1) ANOVA followed Tukey for the analysis of differences between groups regarding SH0, SH1, SH2, %SMHR, and ΔFRE; (2) ANOVA repeated measures, followed by Bonferroni, for the analysis of the variables SH0, SH1, SH2 into the same group at the different analysis times; (3) Pearson's correlation between variables. The level of significance considered was 5% [5, 21].
Results
The average means and standard deviation of surface microhardness for the variables SH0, SH1, and SH2 are displayed in Table 2. In the mineralized surface, the SH0 means (baseline enamel surface microhardness) varied from 373.5 (NC) to 384.4 VHN (RDC), with no significant differences between the groups (p > 0.05). No significance was also observed when the SH1 means (post-demineralization surface hardness) were compared, varying from 31.4 (RES) to 32.8 VHN (RDC) (p > 0.05). For the variable SH2 (surface hardness after pH cycling), the highest mean found was 112.5 (RDC) and the lowest was 38.1 (NC). Significantly higher means of SH2 were observed when the enamel was treated with fluoride-containing toothpastes, regardless of the different technology, compared to the control fluoride-free toothpaste (p < 0.05).
Table 3 displays the results of QLF analysis for tested areas, ΔF0 (sound vs. demineralized area) and ΔF1 (sound × treated area) in the experimental groups. No significance was observed for ΔF0 when the means were compared (p > 0.05). Conversely, RDC exhibited a significantly higher mean of ΔF1 (− 13.5) than all experimental groups (p < 0.05). PC, RES, and SRP presented intermediary means (− 24.1, − 21.3, and − 29.5, respectively). The control group (NC) exhibited a significantly lower mean (− 34.8), than the experimental groups.
In general, the surface microhardness recovery (%SMHR) could be ranked as follows: RDC = PC = RES = SRP > NC. When the %SMHR results were compared, RDC and PC exhibited significantly higher means, followed by RES and SRP (p < 0.05)(Fig. 1). After treatment with the fluoride-free toothpaste, a significantly lower mean of %SMHR was observed (p < 0.05). RDC exhibited the higher mean ΔFRE, being statistically different from all groups, followed by PC and RES. SRP exhibited the lowest ΔFRE (0.27) of the fluoride-containing toothpastes (Fig. 1). The fluoride-free toothpaste (NC) exhibited a negative mean of ΔFRE (− 6.63). The statistical analysis demonstrated that significantly higher means of %SMHR and ΔFRE were observed after enamel treatment with RDC (22.7 and 46.9, respectively). The anti-erosive, arginine-containing toothpaste (PC) was as effective as RDC to recover the surface microhardness (%SMHR = 18.8), but comparatively exhibited a significantly lower mean of ΔFRE (19.5). A strong positive correlation was found when variables ΔFRE and %SMHR were plotted (r2 = 0.9371, p < 0.001).
Means and standard deviation of remineralization in terms of fluorescence recovery (ΔFRE) and microhardness measurements (%SMHR) in the experimental groups. Distinct letters, lower case for %SMHR, upper case for ΔFRE: significant, p < 0.05. Vertical bars = ± 1 standard deviation. Abbreviations: NC: Fluoride-free toothpaste Colgate; PC: Colgate Total 12; RDC: Regenerador + Sensitive; RES: regenerate enamel science; SRP: sensodyne repair and protect
Figure 2 shows representative scanning electron micrographs of the enamel cross-sections and demonstrates the differences among the experimental groups. RDC induced the formation of a mineralized layer onto the enamel surface when associated with pH cycling. This mineralized surface layer was not observed when the enamel blocks were treated with other toothpastes. Table 4 shows the elemental mapping analysis was shown demonstrating the differences among the groups. RDC exhibited the highest silicon content (7.14%). Variability in the Ca/P ratio was observed in the analyzed specimens can also be seen in Table 4, with the lowest ratio for PC (1.58) and the highest for RES (1.96). EDS also showed that the element fluoride was more frequent in surfaces treated with SRP and PC and less present in RDC and RES.
Discussion
The results of the present study demonstrated different values for enamel caries recovery among the analyzed toothpastes, as related in other studies [30,31,32,33,34,35,36]. Although no significance was observed when comparing the results of the microhardness after pH cycling (SH2), irrespective of the treatment technology, RDC and PC exhibited significantly higher means of microhardness, followed by RES and SRP (Fig. 1.). The calculus of %SMHR, which considers the microhardness in the different testing areas, demonstrated a significantly higher mean when the enamel was treated with RDC compared to other experimental groups.
QLF is a visible light system used to quantitatively and nondestructively monitor the progression or regression of enamel demineralization [37]. As related in Gomez et al. [38] both microhardness and QLF methods were able to distinguish in vitro remineralization models using different fluoridated toothpastes. The differences between the initial and post treatment QLF measurements were observed in all experimental groups with the significant recovery of fluorescence and reduction of the lesion area. Despite the statistical equivalence when the means were compared, a higher microhardness means after pH cycling was observed when the specimens were treated with RDC (112.5) (Table 2). The high fluorescence recovery mean presented by the RDC demonstrated a greater mineral gain (ΔFRE) in the specimens treated with the REFIX-containing toothpaste (Fig. 1).
Reasons that explain the better results for the REFIX-containing toothpaste rely on its effects on the surface morphology of the specimens after treatment. The SEM micrographs show a mineralized layer formed on the enamel surface after pH cycling interspersed with the REFIX toothpaste treatment (Fig. 2c). A previous study demonstrated that the formation of a silicon-enriched mineral layer on the enamel surface induced by the REFIX-based toothpaste was favored by the formation of complexes of the bioactive particles of calcium, phosphorus, and sodium [39]. The elemental mapping analysis of RDC also corroborated the presence of silicon (7.14%) in the formulation (Table 3). Substituting phosphate groups with silicon affects the mechanical properties of the silicon-enriched hydroxyapatite [40]. When associated with fluorine and phosphate groups, the silicon content enhanced the bioactivity and apatite-forming ability of hydroxyapatite, substituting with silicon in to the remineralizing hydroxyapatite [14, 15]. These findings were corroborated in previous studies [21, 39], that also demonstrated the formation of a mineral layer rich in calcium and silicon in the specimens treated with this same toothpaste.
Like RDC, the anti-erosive toothpaste PC, which contains tetrasodium pyrophosphate associated with sodium fluoride, was found to be as effective as RDC at remineralizing the enamel according to the %SMHR analysis. PC contains the highest content of calcium associated with phosphates, in the form of calcium carbonate, dicalcium phosphate dihydrate, and calcium pyrophosphate [41]. PC also contains a proprietary Pro-Argin technology with 8% arginine, which is indicated against tooth hypersensitivity [42]. This arginine-containing toothpaste has been regarded as offering a potential caries prevention [43]. Unlike RDC, no mineralized layer on the enamel surface was observed in the microscopy analysis (Fig. 2b).
RES contains NR-5 technology, which combines calcium silicate, sodium phosphate salts, and fluoride, seems to improve the remineralization of hydroxyapatite by the nucleation of minerals in tooth enamel in the presence of saliva [44]. SRP contains NOVAMIN technology which comprises an amorphous inorganic of sodium and calcium phosphosilicate [45]. According to the technical profile, serial chemical reactions occurs when bioactive glass is in contact with an aqueous solution, leading to the formation of an insoluble mineralized carbonated hydroxyapatite layer on the surface of the dentin tissue. Conversely, in enamel, this technology favors another mechanism of action, which seems to reinforce the structure of the enamel hydroxyapatite rather than forming a superficial mineralized layer on the tissue. Despite the claim of the formation of a less-soluble surface hydroxyapatite that is resistant to acid challenges [46], the presence of a mineralized layer on the enamel surface was not confirmed in microscopic analysis (Fig. 2).
The difference in the outcomes of the present study support speculation that changes in the hydroxyapatite structure may occur due to treatment of enamel with the fluoride toothpastes associated with different technologies. Depending on the substitution at the different sites of the hydroxyapatite structure, changes in calcium phosphates nucleation, hydroxyapatite growth and crystallization thermodynamics and kinetics, and ultimately its stability may occur in an oral environment rich in ions released by the toothpastes [47]. In this manner, changes in the mineral phase of teeth may to occur in terms of stoichiometric hydroxyapatite. Unfortunately, the mineral phase of teeth has been erroneously attributed to the stoichiometry of hydroxyapatite of 1.67 [9]. In fact, biological apatite usually comprises a non-stoichiometric hydroxyapatite, being calcium deficient (Ca/P < 1.67), with small crystals and poor crystallinity, leading to a relatively high solubility [9, 48]. Except for PC, which exhibited the lowest Ca/P ratio (1.58), the silicon-rich fluoride toothpastes RDC, RES, and SRP presented the highest ratios, higher than 1.86 (Table 4). Variability in the Ca/P ratio may explain the different results regarding hardness and mineral quantification (QLF). Thus, the research hypothesis, which anticipated that the remineralization of the enamel would be positively affected by the use of fluoride toothpastes after pH cycling, irrespective of their technology, was accepted.
Despite the limitations of this in vitro methodology, every effort was made to simulate the variables in the oral environment, specifically the pH cycle in which the demineralization and remineralization processes that occur in situ or in vivo analyses are intercalated with toothpaste exposure [49]. These analyses allowed a complex control of conditions at a reduced cost to test the efficacy of products designated to remineralize the enamel tissue. It is important to mention that the positive control (PC) used in this study did not only contain sodium fluoride but also had arginine, tetrasodium pyrophosphate, and triclosan as bioactive ingredients. This may have influenced the good performance of this toothpaste, highlighting the suggestion for further studies using only a fluoride toothpaste as a control, without the addition of other compounds.
Although this study did not use cross-sectional microhardness, different analyses were performed on the specimens, such as surface microhardness. This provides data on surface mineral deposition and surface hardening, suggesting a pattern of remineralization and recovery. In addition, the QLF is a validated method for verifying the remineralization of caries lesions in vivo, and can be extrapolated to in vitro studies. The analyses with SEM and EDS also allowed us to identify the formation of a mineral layer containing silicon, suggesting a remineralizing potential of the RDC, which was the initial objective of this study [26, 28, 38, 39, 50, 51].
Conclusion
The present study demonstrated that all fluoride-containing toothpastes showed remineralizing potential for demineralized enamel associated with pH cycling, compared to toothpaste without fluoride. The toothpaste containing the REFIX technology had the highest mean surface hardness and light-induced fluorescence recovery from carious lesions. The anti-erosive toothpaste containing arginine and tetrasodium pyrophosphate also proportioned surface mineral deposition according to surface microhardness recovery analysis. On the other hand, only the REFIX technology was able to promote the formation of a silicon-rich mineralized layer on the enamel surface.
Availability of data and materials
All the data generated or analyzed during this study are included in this published article.
Abbreviations
- SH0 :
-
Baseline enamel Vickers surface microhardness
- VHN:
-
Vickers hardness
- NC:
-
Negative control (Fluoride-free toothpaste Colgate)
- PC:
-
Positive control (Colgate total 12)
- SH1 :
-
Post-demineralization surface hardness
- SH2 :
-
After treatment surface hardness
- %SMHR :
-
Surface microhardness recovery
- QLF:
-
Quantitative light-induced fluorescence
- ΔF value:
-
Percentage decrease in the auto fluorescence intensity and changes in the mineral contents of enamel
- ΔF0 :
-
A loss of initial fluorescence, passing through the difference between the sound and demineralized enamel
- ΔF1 :
-
Difference in final fluorescence, using the difference between the sound enamel and the area treated with the toothpastes
- ΔFRE :
-
Percentage of fluorescence recovery
- SEM:
-
Scanning electronic microscopy
- EDS:
-
Energy-dispersive X-ray spectroscopy
- kV:
-
Kilovolts
- ANOVA:
-
One-way analysis of variance
- RDC:
-
Regenerador + sensitive dentalclean
- RES:
-
Regenerate enamel science
- SRP:
-
Sensodyne repair and protect
References
van Loveren C. Sugar restriction for caries prevention: amount and frequency: which is more important? Caries Res. 2019;53(2):168–75.
Vilhena FV, Polassi MR, Paloco EAC, Alonso RC, Guiraldo RD, D’Alpino PH. Effectiveness of toothpaste containing REFIX technology against dentin hypersensitivity: a randomized clinical study. J Contemp Dent Pract. 2020;21(6):609–14.
Cruz M, Narvai PC. Caries and fluoridated water in two Brazilian municipalities with low prevalence of the disease. Rev Saude Publica. 2018;52:28.
Ganavadiya R, Shekar BR, Goel P, Hongal SG, Jain M, Gupta R. Comparison of anti-plaque efficacy between a low and high cost dentifrice: a short term randomized double-blind trial. Eur J Dent. 2014;8(3):381–8.
Tomaz PLS, Sousa LA, Aguiar KF, Oliveira TS, Matochek MHM, Polassi MR, D’Alpino PHP. Effects of 1450-ppm fluoride-containing toothpastes associated with boosters on the enamel remineralization and surface roughness after cariogenic challenge. Eur J Dent. 2020;14(1):161–70.
Cardoso Cde A, Lacerda B, Mangueira DF, Charone S, Olympio KP, Magalhaes AC, Pessan JP, Vilhena FV, Sampaio FC, Buzalaf MA. Mechanisms of action of fluoridated acidic liquid dentifrices against dental caries. Arch Oral Biol. 2015;60(1):23–8.
Farooq I, Ali S, Siddiqui IA, Al-Khalifa KS, Al-Hariri M. Influence of thymoquinone exposure on the micro-hardness of dental enamel: an in vitro study. Eur J Dent. 2019;13(3):318–22.
Philip N. State of the art enamel remineralization systems: the next frontier in caries management. Caries Res. 2019;53(3):284–95.
Cacciotti I. Cationic and anionic substitutions in hydroxyapatite. In: Antoniac IV, editor. Handbook of bioceramics and biocomposites. Cham: Springer; 2016. p. 145–211.
Volponi AA, Zaugg LK, Neves V, Liu Y, Sharpe PT. Tooth repair and regeneration. Curr Oral Health Rep. 2018;5(4):295–303.
Xiao Z, Que K, Wang H, An R, Chen Z, Qiu Z, Lin M, Song J, Yang J, Lu D, et al. Rapid biomimetic remineralization of the demineralized enamel surface using nano-particles of amorphous calcium phosphate guided by chimaeric peptides. Dent Mater. 2017;33(11):1217–28.
Vallet-Regí M, Arcos D. Silicon substituted hydroxyapatites: a method to upgrade calcium phosphate based implants. J Mater Chem. 2005;15(15):1509–16.
Palard M, Champion E, Foucaud S. Synthesis of silicated hydroxyapatite Ca10(PO4), 6–x(SiO4)x(OH)2–x. J Solid State Chem. 2008;181(8):1950–60.
Carrouel F, Viennot S, Ottolenghi L, Gaillard C, Bourgeois D. Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: a review of the current situation. Nanomaterials. 2020;10(1):1–32.
Gibson IR, Huang J, Best SM, Bonfield W, Enhanced in vitro cell activity and surface apatite layer formation on novel silicon-substituted hydroxyapatites. In: 12th international symposium on ceramics in medicine: 1999; Nam, Japan: World Scientific Publishing Co. Pte. Ltd.; 1999.
Carvalho SM, Moreira CDF, Oliveira ACX, Oliveira AAR, Lemos EMF, Pereira MM. Bioactive glass nanoparticles for periodontal regeneration and applications in dentistry. In: Subramani K, Ahmed W, editors. Nanobiomaterials in clinical dentistry. Amsterdam: Elsevier; 2019. p. 351–83.
Parker AS, Patel AN, Al Botros R, Snowden ME, McKelvey K, Unwin PR, Ashcroft AT, Carvell M, Joiner A, Peruffo M. Measurement of the efficacy of calcium silicate for the protection and repair of dental enamel. J Dent. 2014;42(Suppl 1):S21-29.
Turner IG. Ceramics and glasses. In: Narayan R, editor. Biomedical materials. New York: Springer; 2009. p. 3–39.
Walsh T, Worthington HV, Glenny AM, Appelbe P, Marinho VC, Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2010;20(1):CD007868.
Fernandes NLS. Eficácia de dentifrício com tecnologia inovadora para remineralização de lesões iniciais de cárie e erosão: abordagem in vitro e in vivo. João Pessoa: Universidade Federal da Paraíba; 2021.
Fernandes NLS, Juliellen LDC, Andressa FBO, D’Alpino HPP, Sampaio CF. Resistance against erosive challenge of dental enamel treated with 1450-PPM fluoride toothpastes containing different biomimetic compounds. Eur J Dent. 2021;15(3):433–9.
Queiroz CS, Hara AT, Paes Leme AF, Cury JA. pH-cycling models to evaluate the effect of low fluoride dentrifice on enamel de- and remineralization. Braz Dent J. 2008;19(1):21–7.
Vieira AE, Delbem AC, Sassaki KT, Rodrigues E, Cury JA, Cunha RF. Fluoride dose response in pH-cycling models using bovine enamel. Caries Res. 2005;39(6):514–20.
Forcin LV, Oliveira TS, Tomaz PLS, Matochek MHM, Polassi MR, Vilhena FV, Svizero NR, D’Alpino PHP. Enamel remineralization and surface roughness after treatment with herbal-containing toothpastes. J Clin Exp Dent. 2021;13(9):e849–58.
Joao-Souza SH, Lussi A, Baumann T, Scaramucci T, Aranha ACC, Carvalho TS. Chemical and physical factors of desensitizing and/or anti-erosive toothpastes associated with lower erosive tooth wear. Sci Rep. 2017;7(1):17909.
Park SW, Kim SK, Lee HS, Lee ES. de Josselin de Jong E, Kim BI: Comparison of fluorescence parameters between three generations of QLF devices for detecting enamel caries in vitro and on smooth surfaces. Photodiagnosis Photodyn Ther. 2019;25:142–7.
Diniz MB, Campos PH, Wilde S, Cordeiro RCL, Zandona AGF. Performance of light-emitting diode device in detecting occlusal caries in the primary molars. Lasers Med Sci. 2019;34(6):1235–41.
Gokce G, Savas S, Kucukyilmaz E, Veli I. Effects of toothpastes on white spot lesions around orthodontic brackets using quantitative light-induced fluorescence (QLF): an in vitro study. J Orofac Orthop. 2017;78(6):480–6.
Kim H-E, Cho Y-K, Kim B-R, Jung E-H, Kim B-I. Cutoff fluorescence loss for the recovery of incipient carious lesions after fluoride application in primary teeth: a clinical study. Photodiagnosis Photodyn Ther. 2018;23:367–72.
Akyildiz M, Sonmez IS. Comparison of remineralising potential of nano silver fluoride, silver diamine fluoride and sodium fluoride varnish on artificial caries: an in vitro study. Oral Health Prev Dent. 2019;17(5):469–77.
Alhussain AM, Alhaddad AA, Ghazwi MM, Farooq I. Remineralization of artificial carious lesions using a novel fluoride incorporated bioactive glass dentifrice. Dent Med Probl. 2018;55(4):379–82.
Almohefer SA, Levon JA, Gregory RL, Eckert GJ, Lippert F. Caries lesion remineralization with fluoride toothpastes and chlorhexidine: effects of application timing and toothpaste surfactant. J Appl Oral Sci. 2018;26:e20170499.
Creeth JE, Karwal R, Hara AT, Zero DT. A randomized in situ clinical study of fluoride dentifrices on enamel remineralization and resistance to demineralization: effects of zinc. Caries Res. 2018;52(1–2):129–38.
Grewal N, Sharma N, Kaur N. Surface remineralization potential of nano-hydroxyapatite, sodium monofluorophosphate, and amine fluoride containing dentifrices on primary and permanent enamel surfaces: an in vitro study. J Indian Soc Pedod Prev Dent. 2018;36(2):158–66.
Lippert F, Gill KK. Carious lesion remineralizing potential of fluoride- and calcium-containing toothpastes: a laboratory study. J Am Dent Assoc. 2019;150(5):345–51.
Oliveira PHC, Oliveira MRC, Oliveira LHC, Sfalcin RA, Pinto MM, Rosa EP, Melo Deana A, Horliana A, Cesar PF, Bussadori SK. Evaluation of different dentifrice compositions for increasing the hardness of demineralized enamel: an in vitro study. Dent J. 2019;7(1):14.
Lee YE, Baek HJ, Choi YH, Jeong SH, Park YD, Song KB. Comparison of remineralization effect of three topical fluoride regimens on enamel initial carious lesions. J Dent. 2010;38(2):166–71.
Gomez J, Pretty IA, Santarpia RP 3rd, Cantore B, Rege A, Petrou I, Ellwood RP. Quantitative light-induced fluorescence to measure enamel remineralization in vitro. Caries Res. 2014;48(3):223–7.
Vilhena FV, Oliveira SML, Matochek MHM, Tomaz PLS, Oliveira TS, D’Alpino PHP. Biomimetic mechanism of action of fluoridated toothpaste containing proprietary REFIX technology on the remineralization and repair of demineralized dental tissues: an in vitro study. Eur J Dent. 2021;15(2):236–41.
Surmeneva MA, Mukhametkaliyev TM, Tyurin AI, Teresov AD, Koval NN, Pirozhkova TS, Shuvarin IA, Shuklinov AV, Zhigachev AO, Oehr C, et al. Effect of silicate doping on the structure and mechanical properties of thin nanostructured RF magnetron sputter-deposited hydroxyapatite films. Surf Coat. 2015;275:176–84.
Fu D, Pei D, Huang C, Liu Y, Du X, Sun H. Effect of desensitising paste containing 8% arginine and calcium carbonate on biofilm formation of Streptococcus mutans in vitro. J Dent. 2013;41(7):619–27.
Cummins D. Clinical evidence for the superior efficacy of a dentifrice containing 8.0% arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity. J Clin Dent. 2011;22(4):97–9.
Zheng X, He J, Wang L, Zhou S, Peng X, Huang S, Zheng L, Cheng L, Hao Y, Li J, et al. Ecological effect of arginine on oral microbiota. Sci Rep. 2017;7(1):7206.
Gorrepati EA, Wongthahan P, Raha S, Fogler HS. Silica precipitation in acidic solutions: mechanism, pH effect, and salt effect. Langmuir. 2010;26(13):10467–74.
Joshi S, Gowda AS, Joshi C. Comparative evaluation of NovaMin desensitizer and Gluma desensitizer on dentinal tubule occlusion: a scanning electron microscopic study. J Periodontal Implant Sci. 2013;43(6):269–75.
Burwell AK, Litkowski LJ, Greenspan DC. Calcium sodium phosphosilicate (NovaMin): remineralization potential. Adv Dent Res. 2009;21(1):35–9.
Wang L, Nancollas GH. Calcium orthophosphates: crystallization and dissolution. Chem Rev. 2008;108(11):4628–69.
Weiner S, Wagner HD. The material bone: structure-mechanical function relations. Annu Rev Mater Sci. 1998;28(1):271–98.
Amaechi BT. Protocols to study dental caries in vitro: pH cycling models. Methods Mol Biol. 2019;1922:379–92.
Krishnan G, George S, Anandaraj S, John SA, Mathew V, Shanavas NM. Efficacy of four remineralizing agents on primary teeth: in vitro evaluation using microhardness testing and quantitative light-induced fluorescence. Pediatr Dent. 2017;39(3):233–7.
Al-Khateeb S, ten Cate JM, Angmar-Månsson B, de Jong EJ, Sundström G, Exterkate RA, Oliveby A. Quantification of formation and remineralization of artificial enamel lesions with a new portable fluorescence device. Adv Dent Res. 1997;11(4):502–6.
Acknowledgements
The authors are grateful to the assistance provided by the Postgraduate Program in Dentistry at the Federal University of Paraiba, by the Department of Clinical and Community Dentistry, and by the Department of Morphology at the Federal University of Paraiba and National Council for Scientific and Technological Development (CNPq-BR) who granted a Master's Scholarship to Nayanna Lana Soares Fernandes, from 04/01/2019 to 11/30/2019, with process no 133080/2019-6.
Funding
The present study was supported by the Federal University of Paraiba and National Council for Scientific and Technological Development (CNPq- BR) who granted a Master's Scholarship to Nayanna Lana Soares Fernandes, from 04/01/2019 to 11/30/2019, with process no 133080/2019-6. The funding contributed to the purchase of study materials and instrumental testing.
Author information
Authors and Affiliations
Contributions
NLFS, JGVCS, EBGS and PHPD contributed to the conceptualization and curation of data, as well as research and methodology. FCS, AFBO, EJJ designated the research methodology, project management, resources, supervision and validation. All authors were responsible for writing the original draft, writing the final article and reviewing it.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fernandes, N.L.S., Silva, J.G.V.C., de Sousa, E.B.G. et al. Effectiveness of fluoride-containing toothpastes associated with different technologies to remineralize enamel after pH cycling: an in vitro study. BMC Oral Health 22, 489 (2022). https://doi.org/10.1186/s12903-022-02429-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-022-02429-2