Abstract
Background
The present study aimed to assess the impact of application of fluoridated- 10% carbamide peroxide (CP) with or without potassium iodide (KI) on silver diamine fluoride (SDF)-treated enamel surface in the primary teeth.
Methods
After stained-remineralized caries lesions (s-RCLs) creation, 96 teeth were randomly allocated to four experimental groups: Group 1:SDF-treated enamel followed by 8-h/day application of 10% CP for 2 weeks; Group 2: SDF-treated enamel followed by 15-min/day application of 10% CP for 3 weeks; Group 3: SDF + KI-treated enamel followed by 8-h/day application of 10% CP for 2 weeks; and Group 4: SDF + KI-treated enamel followed by 15-min/day application of 10% CP for 3 weeks. Enamel microhardness (EMH) test (n = 12) and spectrophotometric color assessment (n = 12) was performed at four stages: baseline (intact enamel), demineralized enamel, aged remineralized-stained enamel, and after final intervention. Sixteen samples were used for SEM evaluation. Data were analyzed with the paired t-test, one-way ANOVA, and Tukey’s post-hoc test (p < 0.05).
Results
EMH values in all groups showed significant decrease after demineralization (all, p < 0.00001). All samples showed complete recovery of EMH values (%REMH) after SDF application compared to demineralization (%REMHSDF) (p = 0.971). Bleaching caused a slight decrease in %REMH for all groups. However, the differences were not statistically significant (p = 0.979). SEM findings revealed no changes in enamel porosity after bleaching. Bleaching application ameliorated the discoloration in all groups (all, p < 0.00001). All samples in Groups 2 and 4 had significantly lighter color after 21 days as compared to 14-day exposure to the bleaching material (both, p < 0.00001).
Conclusions
SDF application on demineralized primary tooth enamel completely recovered enamel microhardness. 10% carbamide peroxide effectively bleached SDF stain without causing significant decrease in EMH values. Color improvement was more evident with the use of KI immediately after SDF application. Both 15-min and 8-h application of fluoridated CP resulted in statistically similar color enhancement in primary teeth.
Similar content being viewed by others
Introduction
Dental caries is a biofilm-mediated disease, characterized by dynamic episodes of demineralization and remineralization that deteriorates the tooth structure. The current caries control strategy consists of hindering the progression of lesions and promoting remineralization [1]. Fluoride compounds are the most commonly used materials for preventing and arresting dental caries [2]. The use of professionally applied fluoride materials such as acidulated phosphate fluoride (APF), sodium fluoride gel and varnish, and silver diamine fluoride (SDF) is a cost-effective and non-invasive modality [3].
Silver diamine fluoride (SDF) can remineralize tooth structure and arrest dental caries by formation of silver phosphate and calcium fluoride precipitates, and disruption of cariogenic microorganisms [4]. Various concentrations of SDF solution are commercially available, ranging from 10 to 38% [5]. However, its application causes permanent black staining on the porous tooth structure. The use of potassium iodide solution (KI) immediately after SDF application decreases the availability of silver ions by forming yellowish deposits of silver iodide, thus, reducing the degree of tooth staining [6].
Treatment options regarding esthetic enhancement of stained-remineralized caries lesions (s-RCLs) are few and mostly limited to dental microabrasions and restorations [7]. It is proposed that dental bleaching might help ameliorate these discolorations [7]. Many studies with controversial results have assessed the effect of bleaching products on mineral content and physical properties of the tooth surface [8,9,10,11,12]. Since the acidic nature of the whitening products raised concerns regarding the possibility of alteration in the enamel composition, fluoridated dental bleaching products were introduced to the market [13]. Studies have reported the remineralization potential of these fluoridated dental bleaching products [13, 14]. There are few studies, mostly case reports, to evaluate the use of bleaching agents in primary teeth [15,16,17,18,19,20]. Ten percent carbamide peroxide is the safest and the most effective bleaching material to be used in children, even in the existence of mild caries [15, 21].
Studies to evaluate the efficacy of bleaching agents on s-RCLs in the permanent dentition are limited [7, 22,23,24,25]. To the authors’ knowledge, this is the first study on this topic in the primary teeth. Therefore, this study aimed to design an in vitro model to create s-RCLs and to test the impact of duration of application of fluoridated- 10 percent carbamide peroxide with or without KI on surface microhardness, enamel morphology, and color change.
Methods
Study design
Totally, 118 caries-free primary anterior teeth, extracted due to orthodontic treatment, were collected based on a protocol approved by the Ethics Review Committee of Shiraz University of Medical Sciences. Written informed consents for the use of the teeth were obtained. After removing the roots to the level of one mm under the cementoenamel junction, the specimens went through washing, disinfecting by immersion in 0.1% chloramine T solution for one month, and storing in a weekly renewed deionized water at 37 °C until use. Prior to the beginning of the experiment, the samples were assessed under a stereomicroscope (×10) to exclude teeth with cracks, anomalies, stains, or defects. 104 teeth fulfilled the selection criteria.
Sample preparation
To prepare enamel blocks, the crowns were embedded in acrylic resin with the labial surface parallel to the mold. Each tooth surface was serially polished with 600-, 800-, 1200-, 2400-, and 4000-grit waterproof silicon carbide paper followed by 1-μm aluminum oxide to obtain a horizontal and smooth surface. Next, the samples were washed for 20 s in distilled water, dried, and covered with two layers of nail polish except for a 2 × 4 mm window on the flattest portion of the enamel surface.
Stained-remineralized caries-like lesions creation
To create early caries lesions, each block was demineralized at 37 °C for 96 h in 15 mL of the demineralizing solution containing 0.1 mM lactic acid solution, 3 mM CaCl2, 3 mM KH2PO4, and 0.2% guar gum. The final pH was adjusted to 4.5 using 50% sodium hydroxide [26]. The solution was refreshed after 48 h. At the end of the fourth day, each sample was washed with deionized water for 20 s and allowed to air dry.
For s-RCLs creation, we applied 38% SDF solution (Caries arrest, Dengen dental, India) to all exposed enamel surfaces and agitated the solution with a micro-brush for 1 min. After 2 min, the excess and unreacted SDF was blotted with a cotton pellet.
Aging of the specimens
To better simulate the oral cavity environment, the samples underwent a thermocycling aging procedure for 1000 cycles at the temperatures between 5 and 55 °C with a dwell time of 30 s and a transfer time of 15 s. The SDF-treated samples were left in deionized water for 2 weeks.
Group allocation
Of 104 selected teeth, 8 samples were used for scanning electron microscopy (SEM) evaluation at baseline (n = 2), after demineralization (n = 2), after SDF application (n = 2) and after SDF + KI application (n = 2). The remaining 96 teeth were randomly divided into four experimental groups (n = 24) based on the bleaching protocol and time using random allocation software version 2.0. The samples in each group were arbitrarily divided into two sub-groups to assess enamel microhardness (EMH) (n = 12) and color change (n = 12).
Group 1: 10% CP 8 h/day for 14 days
Ten percent fluoridated-carbamide peroxide (CP) (pH 6.5; Opalescence®, Ultradent Products, Inc., USA) was applied on the enamel surface of each specimen (1 mm thick) and kept for 8 h at 37 °C. Then, the samples were rinsed with running deionized water for 20 s to remove the bleaching agent and left in the deionized water for the remainder of the day to prevent desiccation. This cycle was repeated for 14 days.
Group 2: 10% CP 15 min/day for 21 days
Ten percent CP (pH 6.5; Opalescence®, Ultradent Products, Inc., USA) was applied on the enamel surface of each specimen (1 mm thick) for 15 min at 37 °C as explained for Group 1. This cycle was repeated for 21 days.
Group 3: 10% CP 8 h/day for 14 days on SDF + KI-treated enamel
A generous amount of 10% potassium iodide (KI) solution was immediately applied after SDF application using a microbrush. This procedure was repeated until the creamy white color precipitate turned clear. Then the intervention was performed as explained for Group 1.
Group 4: 10% CP 15 min/day for 21 days on SDF + KI-treated enamel
After KI application, the intervention was performed as explained for Group 2.
Microhardness test
Twelve samples in each group underwent the Vickers microhardness test at four stages: baseline (intact enamel), demineralized enamel, aged remineralized-stained enamel (SDF-/ SDF + KI-treated surfaces), and after bleaching. For Groups 2 and 4, the microhardness values were also recorded at day 14. For EMH evaluation, a Vickers diamond indenter (MHV-1000Z, SCTMC, China) was used at 200 g force for 15 s, at five points of 100 µm distance per sample. The percentage recovery of enamel microhardness (%REMH) after s-RCLs creation and the final exposure to bleaching material was determined as follows:
Surface morphology assessment
At the end of the intervention, two samples out of the 12 samples of the colorimetric assessment in each group, were randomly selected for SEM evaluation (n = 8). As explained before, we also prepared extra 8 enamel blocks with 2 × 4 mm window for microscopic assessment at baseline (n = 2), after demineralization (n = 2), after SDF application (n = 2) and after SDF + KI application (n = 2). The selected 16 samples were dehydrated with series of ethanol solutions and sputter-coated with gold in a vacuum evaporator. The surface morphology was examined with SEM (VEGA, Tescan, Brno, Czech Republic) at 20 kV in high-vacuum mode and 1500× magnification.
Color assessment
L*a*b* values (Commision Internationale de l’Eclairage) of each sample was acquired for each specimen at baseline (intact enamel), demineralized enamel, aged remineralized-stained enamel (SDF-/ SDF + KI-treated surfaces), and after final exposure to bleaching material. For groups 2 and 4, the L*a*b* values were also recorded at day 14. L* value expresses brightness as numbers from 0 (dark) to 100 (bright), a* value describes redness (+ a*) to greenness (− a*), and the b* value represents yellowness (+ b*) to blueness (− b*). A silicone putty jig with a 2 × 4 mm window (equal to the area of the exposed enamel) was fabricated on each block to allow for repeated measurements. One examiner performed all the measurements using a spectrophotometer (Minolta Chromameter CR- 241, Minolta Camera Co., Osaka, Japan) three times for each specimen at each time period over a gray background (L* = 49.2, a* = −0.4, b* = 0.0) and recorded the mean values. The color difference (ΔE) between 2 stages was calculated using the following equation: ΔE = \(\surd\)(ΔL*)2 + (Δa*)2 + (Δb*)2, where ΔL*, Δa*, and Δb* represent changes in lightness, red-green coordinate, and yellow-blue coordinate, respectively [5]. ΔE and ΔL were calculated after demineralization (ΔE and ΔLdemineralization-baseline), staining (ΔE and ΔLSDF-demineralization), after 2-week intervention (ΔE and ΔL2-week intervention-staining), and after 3-week intervention (ΔE and ΔL3-week intervention-staining).
Statistical analysis
All measurements are represented as mean value ± the standard deviation (± SD) of the mean using SPSS version 22.0 (IBM Corp, Armonk, NY, USA) software. The EMH, ΔE, and ΔL values, except for the 3-week intervention data, were analyzed with one-way ANOVA and the Tukey’s post-hoc test. The paired t-test was used to compare the EMH, %REMH, ΔE, and ΔL values of Groups 2 and 4 at the end of the exposure to bleaching material. Besides, the paired t-test was used to compare %REMH between two time points. The significance level was set at p < 0.05.
Results
Table 1 shows the mean ± SD for EMH in each group at different times. At the baseline, EMH ranged from 304.46 to 358.7 VHN (mean: 333.25 ± 14.06 VHN) with no significant differences among the groups (p = 0.998). The demineralizing solution significantly reduced EMH values in all groups (all, p < 0.00001). EMH values were not statistically different among groups after demineralization (p > 0.05). Two weeks after SDF or SDF + KI application, EMH values dramatically increased in all groups as compared to the demineralized status (all, p < 0.00001). EMH values were not significantly different among groups after SDF or SDF + KI application (p > 0.05). Notably, EMH values at this stage did not show significant difference from the baseline values (p = 0.327). Neither 2-week nor 3-week exposure to bleaching material did not cause significant decrease in EMH values compared to both the s-RCLs and the baseline status (all, p > 0.05).
To better compare the EMH values at different stages, the percentage recovery of enamel microhardness (%REMH) was calculated using the formula explained previously. Interestingly, all intervention groups showed complete recovery of EMH values after SDF application compared to demineralization (%REMHSDF) (p > 0.05). Bleaching caused a slight decrease in %REMH for all groups. However, the differences were not statistically significant (p > 0.05). Table 2 demonstrates the mean ± SD for %REMH in each group at different periods.
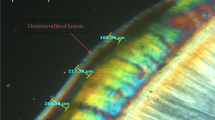
The effects of SDF and bleaching material application on the primary tooth enamel are presented in SEM images (Fig. 1a–h). Demineralization increased the porosities and spaces in the enamel surface due to dissolved minerals and organic materials (Fig. 1b). SEM images after SDF application (with and without KI) revealed agglomeration of mineral deposits with the irregular surfaces (Fig. 1c, d). Mineral precipitates, silver and fluoride-containing compounds, could nicely fill the porous areas and voids of the demineralized enamel. No microsurface alteration was evident after the bleaching process with 10% carbamide peroxide (Fig. 1e–h). However, the dissolution of the precipitated minerals in the enamel porosities was more evident in Groups 1 and 3 (Fig. 1e, g).
Scanning electron microscopy (SEM) images of the primary tooth enamel. a at the baseline; b after demineralization. An increase in the porosities and spaces in the enamel surface is evident; c aged remineralized-stained enamel (SDF-treated surface); d aged remineralized-stained enamel (SDF + KI-treated surface). The globular arrangement of mineral deposits has filled the porous areas and voids of the demineralized enamel; e–h after final intervention. Note that CP did not cause significant changes to the enamel surface. e SDF-treated enamel followed by 8-h/day application of 10% CP for 2 weeks; f SDF-treated enamel followed by 15-min/day application of 10% CP for 3 weeks; g SDF + KI-treated enamel followed by 8-h/day application of 10% CP for 2 weeks; h SDF + KI-treated enamel followed by 15-min/day application of 10% CP for 3 weeks
All samples were lighter in color after demineralization compared to their baseline color (Table 3). However, ΔE and ΔL were not significantly different among groups (p = 0.565 and p = 0.634, respectively). SDF application caused significantly darker colors in all samples (p < 0.00001). Groups 1 and 2 showed more severe changes in color than Groups 3 and 4 (all, p < 0.00001). No significant difference was observed between ΔESDF-Demin and ΔLSDF-Demin of Groups 1 and 2 (p = 0.998 and p = 0.989, respectively) and Groups 3 and 4 (both, p = 0.998). Two-week exposure to bleaching material ameliorated the discoloration in all groups (all, p < 0.00001). Group 3 and 4 had significantly lighter colors than group 1 and 2 after a 2-week intervention (all, p < 0.00001). No significant change was observed between ΔE2-week int-SDF of Groups 1 and 2 (p = 0.249) and Groups 3 and 4 (p = 0.979). ΔL2-week int-SDF values of Groups 1 and 2 (p = 0.175) and Groups 3 and 4 (p = 0.967) showed no statistically significant differences. Groups 2 and 4 were exposed to the bleaching material for 3 weeks. All samples in Groups 2 and 4 had significantly lighter color after 21 days as compared to their 14-day exposure to the bleaching material (both, p < 0.00001). The changes in ΔE and ΔL values after the 3-week exposure to bleaching material showed significant differences among Groups 2 and 4 (both, p < 0.00001). The exemplary photos of the samples at different stages are illustrated in Additional file 1: Figure S1.
Discussion
In this study, we evaluated the impact of time of exposure of artificially created metallic s-RCLs of enamel surface to fluoridated bleaching material on EMH, surface topography, and color. We created initial caries-like lesions on the enamel surface as the porous structure of carious lesions are more prone to chromogen penetration than the sound enamel.
Determination of EMH is a non-destructive easy technique that has been frequently used to assess the impact of bleaching procedures on the enamel surface. All samples of our study had baseline EMH ranging from 304.46 to 358.7 VHN. The sound enamel specimens have surface hardness between 250 and 360 VHN [27]. We artificially created initial caries lesions. As a result, EMH values of all samples dramatically reduced compared to baseline, due to mineral loss from the enamel. Based on our results, the application of SDF (with or without KI) completely recovered the EMH values to the baseline level. According to the literature, SDF-arrested caries lesions have the same hardness values as the sound enamel [28] and are twice as hard as the sound dentin [29]. The main mechanism of SDF-induced increased hardness can be explained by the formation of silver phosphate (Ag3PO4), calcium fluoride (CaF2), and fluorapatite [Ca10(PO4)6F2] on treated lesions [30]. We kept the SDF-treated samples in deionized water for 2 weeks to create s-RCLs. It is suggested that caries lesion starts to arrest and reharden by 2 weeks [29, 31]. Existing literature reports controversial bleaching effects on surface microhardness, surface porosity, and susceptibility to further demineralization [7,8,9,10, 32, 33]. Based on our results, neither 2-week nor 3-week intervention did not cause significant decrease in EMH values compared to the baseline status. This finding is in line with previous studies [9, 10, 32]. The differences in study variables (composition, concentration, and duration of application of the bleaching material) and evaluation methods can explain the controversies in results [7]. Besides, we used fluoridated CP, which according to the manufacturer, contains 0.11% fluoride ion [13]. Fluoridated bleaching materials were previously reported to maintain EMH [13, 14].
We noted the globular arrangement of mineral deposits and an increase in the mineral density of the demineralized enamel in SEM images. As a water-soluble solution with a specific gravity of 1.35, SDF can be dissolved in the trapped water of enamel and dentin. The molecular and osmotic differences between water and SDF can pull SDF through the surface porosity [30]. The depth of silver and fluoride ions penetration is deeper in primary teeth (with an average depth of 744 µm) than the permanent ones (25–200 microns) [30]. This penetration potential might explain the increase in the microhardness after SDF treatment. Although the acidic nature of CP (pH 6.5) caused some dissolution of the precipitated minerals from the enamel surface, SEM analysis revealed no changes in enamel porosity after bleaching, which is in line with other studies [9,10,11]. Possible explanations for the conflicting results [8, 12] are the differences in the bleaching material with less pH levels than carbamide peroxide, and the differences in the methodology. We applied carbamide peroxide on SDF-treated carious lesions with evidently less porous areas than the demineralized enamel surface. Besides, fluoridated carbamide peroxide can cause further remineralization [13]. The longer period of CP application in Groups 1 and 3 can explain the minor differences in the surface mineral precipitates.
We used 38% SDF, which is composed of 24–27% silver, 8.5–10% ammonia, and 5–6% fluoride with a highly alkaline pH (pH 12.5) [34]. Despite its benefits, SDF application causes dark stains, which limits its clinical use [5]. In our study, all samples became significantly darker after SDF application, as indicated by negative ΔLSDF-Demin values (Table 3). These results implied the successful incorporation of the metallic stains in the created lesions. In line with our findings, the immediate use of potassium iodide (KI) after the application of SDF has been suggested to minimize this adverse effect [6].
ΔE* values greater than 3.3 correlates with clinically visible changes in tooth color [23]. In this regard, all samples demonstrated a significant whitening effect after the 2-week exposure to bleaching material. This effect was more remarkable in KI-treated samples (Groups 3 and 4). The 3-week exposure to bleaching material resulted in visible changes in tooth color in both Groups 2 and 4. This effect was more remarkable in Group 4. This result corroborates previous studies suggesting that longer enamel bleaching time caused better color improvement regardless of the concentration of bleaching agents [7, 11].
There are few studies, mostly case reports, to evaluate the use of bleaching agents in primary dentition [15,16,17,18,19,20]. Determination of the appropriate duration of bleaching procedure more suitable for children was suggested by Lee et al. [17]. We found that both 8-h and 15-min application of the bleaching material could effectively lighten the metallic staining. This finding might be explained by the porous structure of the caries-induced enamel and the acidic pH of the bleaching material, which might help better penetration to tooth structure. Besides, primary tooth enamel has a higher interprismatic fraction (interprismatic area related to intraprismatic area) than its permanent counterpart [35]. It implies that primary tooth enamel is more porous and permeable than the permanent enamel [35].
The latest revision of American Academy of Pediatric Dentistry policy has considered adult/dentist-supervised bleaching procedure as a safe and beneficial modality for children and adolescents [21]. The General Dental Council (GDC) in its 2016 position statement on tooth whitening stated that products containing or releasing between 0.1 and 6% hydrogen peroxide cannot be used in any person under 18 years of age, “except where such use is intended wholly for the purpose of treating or preventing disease” [36]. Noteworthy, ten percent carbamide peroxide falls within the permitted range as it equivalents to 3 percent hydrogen peroxide [21]. Despite the GDC announcement, the use of bleaching products containing more than 0.1% hydrogen peroxide in patients under 18 years of age in countries belonging to the European Union is illegal (EU Cosmetics Regulation directive 2011/84/EU) [37].
Despite the limitation of in vitro studies, we tried to imitate the oral cavity condition by thermocycling aging and accurately following the manufacturer's instructions for materials application. As we only wanted to focus on the effect of SDF and bleaching materials on EMH, we did not preserve the samples in saliva. The absence of enough studies in primary teeth that have assessed the efficacy of bleaching materials precluded appropriate comparisons. For this reason, we compared our results with studies performed on permanent teeth. Besides, we did not extend our microscopic evaluation to measure the depth of penetration and changes in mineral content, which is another limitation. Initial grinding of enamel surface removes its aprismatic enamel and changes optical properties, and the use of unground specimens is desirable for correlating the results to daily practice. However, the unground enamel has surface irregularity and curvature, which causes a wide range of variability in the measurements. Therefore, we initially polished the enamel surface to provide a standardized surface, which helps reduce the biological sample variations. Further experimental and clinical studies in primary dentition are highly suggested to find effective and safe methods to eliminate metallic stains in primary teeth.
Conclusion
Within the limitations of this in vitro study, we concluded that SDF could highly remineralize the demineralized enamel surface, and the SDF-treated surfaces were highly resistant to further acidic challenges due to bleaching material application. The use of fluoridated CP could effectively improve the color change caused by SDF application (with and without KI). Color improvement was more evident with the use of KI immediately after the SDF application. As both 15-min and 8-h application of fluoridated CP resulted in statistically similar color enhancement in primary teeth, a shorter duration of bleaching material application is highly acknowledged in pediatric patients.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CP:
-
Carbamide peroxide
- KI:
-
Potassium iodide
- SDF:
-
Silver diamine fluoride
- s-RCLs:
-
Stained-remineralized caries lesions
- EMH:
-
Enamel microhardness
- SEM:
-
Scanning electron microscopy
- %REMH:
-
Percentage recovery of enamel microhardness
- APF:
-
Acidulated phosphate fluoride
- VHN:
-
Vickers hardness number
References
Schwendicke F, Splieth C, Breschi L, Banerjee A, Fontana M, Paris S, Burrow MF, Crombie F, Page LF, Gatón-Hernández P, et al. When to intervene in the caries process? An expert Delphi consensus statement. Clin Oral Investig. 2019;23(10):3691–703.
Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51–9.
Munteanu A, Holban AM, Păuna MR, Imre M, Farcașiu AT, Farcașiu C. Review of professionally applied fluorides for preventing dental caries in children and adolescents. Appl Sci. 2022;12(3):1054.
Chu CH, Mei L, Seneviratne CJ, Lo ECM. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent. 2012;22(1):2–10.
Sayed M, Matsui N, Hiraishi N, Inoue G, Nikaido T, Burrow MF, Tagami J. Evaluation of discoloration of sound/demineralized root dentin with silver diamine fluoride: In-vitro study. Dent Mater J. 2019;38(1):143–9.
Uchil SR, Suprabha BS, Suman E, Shenoy R, Natarajan S, Rao A. Effect of three silver diamine fluoride application protocols on the microtensile bond strength of resin-modified glass ionomer cement to carious dentin in primary teeth. J Indian Soc Pedod Prev Dent. 2020;38(2):138–44.
Al-Angari SS, Lippert F, Platt JA, Eckert GJ, González-Cabezas C, Li Y, Hara AT. Dental bleaching efficacy and impact on demineralization susceptibility of simulated stained-remineralized caries lesions. J Dent. 2019;81:59–63.
de Arruda AM, dos Santos PH, Sundfeld RH, Berger SB, Briso AL. Effect of hydrogen peroxide at 35% on the morphology of enamel and interference in the de-remineralization process: an in situ study. Oper Dent. 2012;37(5):518–25.
Llena C, Esteve I, Forner L. Effect of hydrogen and carbamide peroxide in bleaching, enamel morphology, and mineral composition: In vitro study. J Contemp Dent Pract. 2017;18(7):576–82.
Borges AB, Zanatta RF, Barros ACSM, Silva LC, Pucci CR, Torres CRG. Effect of hydrogen peroxide concentration on enamel color and microhardness. Oper Dent. 2015;40(1):96–101.
Farawati FAL, Hsu SM, O’Neill E, Neal D, Clark A, Esquivel-Upshaw J. Effect of carbamide peroxide bleaching on enamel characteristics and susceptibility to further discoloration. J Prosthet Dent. 2019;121(2):340–6.
Wijetunga CL, Otsuki M, Abdou A, Luong MN, Qi F, Tagami J. The effect of in-office bleaching materials with different ph on the surface topography of bovine enamel. Dent Mater J. 2021;40(6):1345–51.
Pessanha S, Silva S, Silveira JM, Otel I, Luis H, Manteigas V, Jesus AP, Mata A, Fonseca M. Evaluation of the effect of fluorinated tooth bleaching products using polarized Raman microscopy and particle induced gamma-ray emission. Spectrochim Acta A Mol Biomol Spectrosc. 2020;236:118378.
Bollineni S, Janga RK, Venugopal L, Reddy IR, Babu PR, Kumar SS. Role of fluoridated carbamide peroxide whitening gel in the remineralization of demineralized enamel: an in vitro study. J Int Soc Prev Community Dent. 2014;4:117–21.
Brantley DH, Barnes KP, Haywood VB. Bleaching primary teeth with 10% carbamide peroxide. Pediatr Dent. 2001;23(6):514–6.
Campos SFF, César ICR, Munin E, Liporoni PCS, Do Rego MA. Analysis of photoreflectance and microhardness of the enamel in primary teeth submitted to different bleaching agents. J Clin Pediatr Dent. 2007;32(1):9–12.
Lee SS, Zhang W, Lee DH, Li Y. Tooth whitening in children and adolescents: a literature review. Pediatr Dent. 2005;27(5):362–8.
Haywood VBJDT. Bleaching a retained primary tooth. Dent Today. 2018;37(8).
Donly KJ, Donly AS, Baharloo L, Rojas-Candelas E, Garcia-Godoy F, Zhou X, Gerlach RW. Tooth whitening in children. Compend Contin Educ Dent. 2002;23(1):22–8.
Bussadori SK, Roth F, Guedes CC, Fernandes KP, Domingues MM, Wanderley MT. Bleaching non vital primary teeth: case report. J Clin Pediatr Dent. 2006;30(3):179–82.
AAPD: Policy on dental bleaching for child and adolescent patients. Pediatr Dent. 2019:116–119.
Al-Angari SS, Lippert F, Platt JA, Eckert GJ, González-Cabezas C, Li Y, Hara AT. Bleaching of simulated stained-remineralized caries lesions in vitro. Clin Oral Investig. 2019;23(4):1785–92.
Al-Shahrani AA, Levon JA, Hara AT, Tang Q, Lippert F. The ability of dual whitening anti-caries mouthrinses to remove extrinsic staining and enhance caries lesion remineralization—an in vitro study. J Dent. 2020;4:100022.
Al-Angari SS, Alhadlaq M, Abahussain N, Alazzam N. Bleaching stained arrested caries Lesions: in vivo clinical study. Eur J Dent. 2021;15(1):127–32.
Al-Angari SS, Eisa SI. Bleaching efficacy and re-staining susceptibility of stained arrested caries lesions in-vitro. J Int Dent Med Res. 2020;13(3):979–84.
Patil N, Choudhari S, Kulkarni S, Joshi SR. Comparative evaluation of remineralizing potential of three agents on artificially demineralized human enamel: an in vitro study. J Conserv Dent. 2013;16(2):116–20.
Gutierrez-Salazar MP, Reyes-Gasga J. Microhardness and chemical composition of human tooth. Mater Res. 2003;6(3):367–73.
Farhadian N, Farhadian M, Borjali M, Ghaderi E. The effect of silver diamine fluoride versus sodium fluoride varnish on the microhardness of demineralized enamel: an in vitro study. Avicenna J Dent Res. 2020;12(1):13–8.
Young DA, Quock RL, Horst J, Kaur R, MacLean JK, Frachella JC, Duffin S, Semprum-Clavier A, Ferreira Zandona AG. Clinical instructions for using silver diamine fluoride (SDF) in dental caries management. Compend Contin Educ Dent. 2021;42(6):e5–9.
Li Y, Liu Y, Psoter WJ, Nguyen OM, Bromage TG, Walters MA, Hu B, Rabieh S, Kumararaja FC. Assessment of the silver penetration and distribution in carious Lesions of deciduous teeth treated with silver diamine fluoride. Caries Res. 2019;53(4):431–40.
Crystal YO, Marghalani AA, Ureles SD, Wright JT, Sulyanto R, Divaris K, Fontana M, Graham L. Use of silver diamine fluoride for dental caries management in children and adolescents, including those with special health care needs. Pediatr Dent. 2017;39(5):135–45.
Lopes GC, Bonissoni L, Baratieri LN, Vieira LCC, Monteiro S Jr. Effect of bleaching agents on the hardness and morphology of enamel. J Esthet Restor Dent. 2002;14(1):24–30.
Azer SS, Machado C, Sanchez E, Rashid R. Effect of home bleaching systems on enamel nanohardness and elastic modulus. J Dent. 2009;37(3):185–90.
Rossi G, Squassi A, Mandalunis P, Kaplan A. Effect of silver diamine fluoride (SDF) on the dentin-pulp complex: ex vivo histological analysis on human primary teeth and rat molars. Acta Odontol Latinoam. 2017;30(1):5–12.
Sabel N, Robertson A, Nietzsche S, Norén JG. Demineralization of enamel in primary second molars related to properties of the enamel. Sci World J. 2012;2012:587254.
Greenwall-Cohen J, Greenwall L, Haywood V, Harley K. Tooth whitening for the under-18-year-old patient. Br Dent J. 2018;225(1):19–26.
Monteiro J, Ashley PF, Parekh S. Vital bleaching for children with dental anomalies: EAPD members’ survey. Eur Arch Paediatr Dent. 2020;21(5):565–71.
Acknowledgements
The authors thank the Vice-Chancellery of Research of Shiraz University of Medical Sciences, Shiraz, Iran, for supporting this research (Grant No. 22906). The authors also thank Dr. Kamran Mirzaie of the Center for Improvement, Shiraz Dental School for statistical analysis. This article is based on thesis by Dr. Hadi Benam.
Funding
Funding for this study was provided by the Vice-chancellery of Shiraz University of Medical Sciences for the study design (Grant No. 22906).
Author information
Authors and Affiliations
Contributions
AR: conceptualized and designed the study, supervised data collection, interpreted the data, drafted the manuscript, and approved the final manuscript as submitted. MM: Conceptualized and designed the study, supervised data collection and interpretation, reviewed the manuscript, and approved the final manuscript as submitted. HB: Designed the study, assisted in data collection, carried out the initial analyses and interpretation of data, reviewed the manuscript, and approved the final manuscript as submitted. All authors agreed to be accountable for all aspects of this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Review Committee of the School of Dentistry, Shiraz University of Medical Sciences (IR.SUMS.DENTAL.REC.1400.046). All methods were performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki). Written informed consents for the use of the teeth were obtained from the participants.
Consent for publication
Not applicable.
Competing interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Figure S1:
Tooth samples: (a) SDF-treated enamel, (b) SDF+KI-treated enamel, (c) SDF-treated enamel followed by 8-hour/day application of 10% CP for two weeks; (d) SDF-treated enamel followed by 15-min/day application of 10% CP for three weeks; (e) SDF+KI-treated enamel followed by 8-hour/day application of 10% CP for two weeks; (f) SDF+KI-treated enamel followed by 15-min/day application of 10% CP for three weeks
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rafiee, A., Memarpour, M. & Benam, H. Evaluation of bleaching agent effects on color and microhardness change of silver diamine fluoride-treated demineralized primary tooth enamel: An in vitro study. BMC Oral Health 22, 347 (2022). https://doi.org/10.1186/s12903-022-02371-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-022-02371-3