Abstract
Background
To compare the mean mineral density (MMD) and examine the remineralization of carious dentin after cavity disinfection with chlorhexidine gluconate (CHX) and restoration with high viscosity glass ionomer cement (H-GIC) in vitro.
Methods
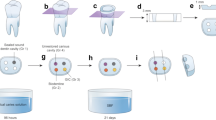
Selective caries removal to leathery dentin was performed in 40 extracted primary molars. The samples were scanned using micro-computed tomography (micro-CT) to determine the MMD baseline and randomly divided into 4 groups (n = 10): Equia™ group, applied dentin conditioner and restored with H-GIC (Equia Forte™), CHX-Equia™ group, disinfected the cavity with 2% CHX before applying dentin conditioner and restored with H-GIC (Equia Forte™), Ketac™ group, restored with H-GIC (Ketac Universal™) and CHX-Ketac™ group, disinfected the cavity with 2% CHX before restored with H-GIC (Ketac Universal™). The samples underwent micro-CT scanning post-restoration and post-pH-cycling to determine their respective MMDs. One sample from each group was randomly selected to analyze by scanning electron microscopy (SEM).
Results
The MMD gain in the 4 groups post-restoration was significantly different between the Equia™ and CHX-Ketac™ groups (oneway ANOVA with Post hoc (Tukey) test, P = 0.045). There was a significant difference in MMD gain post-restoration between the Equia™ and CHX-Equia™ groups (Independent t-test, P = 0.046). However, the Ketac™ and CHX-Ketac™ group’s MMD were similar. The SEM images revealed that the CHX-Ketac™ group had the smallest dentinal tubule orifices and the thickest intertubular dentin among the groups. However, the CHX-Equia™ group had thicker intertubular dentin than the Equia™ group.
Conclusion
Applying 2% CHX on demineralized dentin enhances the remineralization of the dentin beneath the restoration.
Similar content being viewed by others
Background
Dental caries management has evolved into a preventive and restorative strategy known as minimal intervention, which promotes preserving tooth structure and emphasizes maximum tooth function [1,2,3,4,5]. One evidence-based management technique is selective caries removal that is used in deep caries without any signs or symptoms of pulpal degeneration [6]. The concepts of selective caries removal are removing the surrounding axial-wall caries and leaving the pulpal wall caries in the cavity.
Selective caries removal is often used in atraumatic restorative treatment (ART), which is a method to manage deep caries lesions using only hand instruments to reduce trauma to the pulp [7]. ART was developed mainly for treating caries in children living in under-served areas where resources are limited [8]. In addition to reducing pulpal damage, ART results in a reduced pain experience, increased patient cooperation, and is more cost-effective than the conventional treatment [8,9,10]. Therefore, ART is suitable for pediatric patients who have multiple severe caries, prevention programs, and arresting caries progression. Although the selective caries removal method has a high survival rate, defective restorations, pulpal inflammation, and secondary caries can cause ART failure [11].
There are several ways to improve the success rate of ART. Selecting the appropriate restorative material is an important concern for ART. Glass ionomer cement (GIC) is commonly used in pediatric dentistry, with desirable properties, such as being biocompatible to the tooth or soft tissue, fluoride release, antimicrobial activity, coefficient of material expansion that is similar to tooth expansion, and physio-chemical bonding with tooth structure. The other advantages of glass ionomer are its white color and being more tolerant to moisture compared with resin composite [12]. High viscosity glass ionomer cement (H-GIC) is a material that has been used in ART [13]. ART using H-GIC makes dental treatment easier, faster, and more comfortable than the conventional restorative treatment [14]. A low evidence-based study found that ART using H-GIC demonstrated a higher restoration failure rate in both primary and permanent teeth compared with the conventional treatment [8]. The survival rate in a 2-year follow-up of single surface ART using GIC was high in both primary and permanent posterior teeth, while the multiple surface restorations had a medium survival rate [15]. Although H-GIC was not recommended for multiple surface restoration in primary molars in the past [16], currently a new generation of H-GIC, such as Equia Forte™ (GC Corporation, Tokyo, Japan) and Ketac™ Universal Aplicap™ (3M ESPE Dental Products, St. Paul, USA), are claimed by the manufacturers to be appropriate for restoring cavity class II cavities. The hybrid technology in Equia Forte™ increases flexural strength [17], which prevents material deformation against chewing forces [18].
The hypermineralized zone of the dentin adjacent to a GIC restoration decreases the progression of secondary caries [19, 20]. The hypermineralized zone occurs from the exchange of charged ions between the restorative material and tooth structure [19]. H-GIC contains several minerals that promote a hypermineralized zone in the adjacent dentin [21]. Fluoride and strontium ions from H-GIC can penetrate deep into carious demineralized dentin and induce remineralization [21].
Importantly, antimicrobial agents reduce long-term treatment failure by inhibiting the growth of residual bacteria in deep caries [22]. Antimicrobial agents, such as triclosan, cetylpyridinium chloride, povidone iodine, hydrogen peroxide, sodium hypochlorite, and chlorhexidine gluconate (CHX) are used in different dental products [23,24,25,26]. Several studies evaluated using antimicrobial agents as a cavity disinfectant before placing the restoration [27, 28]. CHX is a well-known antimicrobial agent used as a cavity disinfectant in ART [27]. CHX is a broad-spectrum synthetic disinfectant agent that is active against Gram-positive, Gram-negative bacteria and against fungi and viruses [29]. CHX increases the permeability of the bacterial cell membrane, resulting in macromolecules leaking into the cytoplasm and causing cell lysis [29]. CHX is positively charged and eliminates microorganisms by interacting with their negatively charged membrane. CHX reduces Enterococcus faecalis, which is difficult to eliminate and known to induce pulpal and periapical inflammation over the long-term, in deep caries lesions [22, 30].
In addition to its antimicrobial effect, several studies found that using CHX with polyacrylic acid increased the GIC bond strength [31,32,33,34]. CHX neutralized the dentin surface that was applied with an acid conditioner [33] and also increased the surface energy of the dentin [33]. However, the effect of CHX on the remineralization of the affected dentin after GIC restoration is not clear [35].
Therefore, the aim of this study was to investigate the remineralization effect of CHX used as a cavity disinfectant on dentin carious lesions restored with H-GIC.
Methods
Sample size calculation and teeth selection
The study protocols were approved by the Human Research Ethics Committee of the Faculty of Dentistry, Chulalongkorn University (HREC-DCU2020-043), and were approved by the Institutional Biosafety Committee of the Faculty of Dentistry, Chulalongkorn University (DENT CU-IBC 032/2020). The sample size calculation using the G*Power 3.1 program indicated that 6.25 samples were required per group (α = 0.05, β = 0.20). To increase the power of the study, the sample size per group in this study was 10 (40 samples total). The primary molars were collected from the Pediatric Dentistry Department Clinic, Faculty of Dentistry, Chulalongkorn University. The inclusion criteria were extracted primary molars with an occlusal or proximal dentin carious lesion with or without pulpal exposure. If the carious lesion exposed the dental pulp, the exposure size must less than 1 × 1 mm2 after selective caries removal. If the carious lesion did not expose the dental pulp, the lesion must invade the dentin based on visual examination. The remaining tooth structure must be more than 1/3 of the crown, and the roots of the teeth must be at least 1 mm long.
Specimen preparation
The extracted primary molars with carious lesions were stored in 0.9% sodium chloride solution and 10% formalin solution at room temperature at least 2 weeks as previously described [36]. The teeth were cleaned with pumice, rinsed in deionized water, and dried with tissue paper. To prepare a horizontal surface, the cusps of the teeth were cut to a flat occlusal surface with a slow speed cutting machine. The teeth were placed in a prefabricated 18 × 22 × 20 mm3 resin mold and attached to the resin mold with dental pink wax. The tooth caries was removed with the ART method using only a spoon excavator, rinsed with water, and dried with sterile cotton pellets. The soft carious tissue in the lesion was removed followed by selective caries removal to leathery dentin. The samples’ dentin mean mineral density (MMD) was measured using micro-CT as baseline.
The teeth were assigned to 4 groups (n = 10) using Permuted block randomization. Equia™ group: the samples were treated with a dentin conditioner (GC Corporation, Tokyo, Japan) for 20 s, rinsed with water, restored with H-GIC (Equia Forte™) and coated with petroleum jelly. CHX-Equia™ group: the sample cavities were applied with 2% CHX liquid for 1 min with a micro-brush according to previous studies [27, 37, 38]. The cavity was treated with a dentin conditioner for 20 s, rinsed with water, restored with H-GIC (Equia Forte™) and coated with petroleum jelly. Ketac™ group: the samples were restored with H-GIC (Ketac Universal Aplicap™). CHX-Ketac™ group: the samples’ cavities were applied with 2% chlorhexidine gluconate liquid for 1 min with a micro-brush. The cavity was restored with H-GIC (Ketac Universal Aplicap™). The samples were stored in sterilized artificial saliva (0.75 gr Potassium chloride, 0.07 gr Magnesium chloride, 0.199 gr Calcium chloride, 0.965 gr, di-Potassium hydrogen phosphate, 0.439 gr Potassium dihydrogen phosphate, 6 gr Sodium carboxymethyl cellulose, 36 gr Sorbital and 2.4 gr Sodium benzoate in a final volume of 1000 ml. The artificial saliva were sterilized in an autoclave at 121 °C, 15 psi for 15 min). The sample were stored at 37 °C for 24 h before micro-CT scanning as the MMD post-restoration.
pH cycling
The demineralization-remineralization cycling was performed based on Dias et al. [39], as derived from Ten Cate [40]. The samples were immersed in a demineralization solution for 8 h and a remineralization solution for 16 h per day. The samples were stored in individual tubes. The cycle was performed for 14 days at room temperature without stirring. After pH cycling, the samples were soaked in water for 5 min before undergoing micro-CT scanning to determine the post-pH cycling MMD. The solutions used in the pH cycling were prepared by the Biochemistry Department, Faculty of Dentistry, Chulalongkorn University. The demineralization solution (pH 4.8) was composed of 2.2 mM calcium chloride, 2.2 mM sodium phosphate, and 50 mM acetic acid. The remineralization solution (pH 7.0) was composed of 1.5 mM calcium chloride, 0.9 mM sodium phosphate, and 0.15 mM potassium chloride.
Micro-CT MMD assessment and data analysis
The samples were scanned using micro-CT at baseline, post-restoration, and post-pH cycling. The MMD of each sample was calculated by Micro-CT programs (Micro-CT Ray Version 4.2 and Micro-CT Evaluation Program Version 6.6). The Micro-CT scanning programs were set at a resolution of 1024 × 1024 megapixels compared with hydroxyapatite mineral density 1200 mg/cm3, 70 kVp, and 57 μA.
Before the micro-CT scanning, the image was shown in the program in the 2D sagittal plane. The scanned area was demarcated with green lines. The upper green straight line was the upper scanned limit; the lower green dashed line was the lower scanned limit (Fig. 1A). The scanned area of interest comprised the area from the first slice of the occlusal surface to the first slice of the roof of pulp chamber. The scanning results are presented as slices in the horizontal plane (Fig. 1B, C). The multiple slices of the area of interest were drawn anti-clockwise around the outer surface of the tooth for all tooth surface selection.
Representative micro-CT scanned images. A The micro-CT scanned area. Upper green straight line showed the first occlusal limit which was included the first occlusal slide, lower green dash line showed the lower limit including the roof of pulp chamber. B The first slide of the occlusal surface from the baseline sample. C The first slide of the roof of pulp chamber from the baseline sample. D The after-restoration slide showed the difference contrast between H-GIC and dentin. E The preview selecting area of slide with restoration in contrast management, black area showed the excluding area such as restoration and enamel which had high resolution like the restoration and white area showed the including dentin area for mineral density calculation
The 3D bone morphology analysis program was used to analyze the MMD. The contrast setting for the analysis was determined using the after-restoration samples because the contrast resolution between the H-GIC and dentin in the after-restoration samples was differentiated easier than the dentin alone in the baseline samples (Fig. 1D, E). The contrast resolution was derived from the lowest mean values between 2 examiners who identified the difference in contrast excluding the restoration in all samples. Therefore, the micro-CT contrast value setting was performed at − 1000 for the lower threshold and + 550 for the upper threshold on the micro-CT 3D program. After setting the contrast, the 3D image was constructed from the area of interest slices at baseline, post-restoration, and post-pH cycling.
SEM preparation
One sample from each group was selected by random number sampling to use in the SEM analysis. The samples in each group were numbered from 1 to 10 and a number from 1 to 10 was randomly selected. The sample from each group with that number was used in the SEM analysis.
The 4 selected samples were cut in the horizontal plane with a slow speed cutting machine and were dried in a critical point dryer (Quarum Model K850). The samples were sputtered with a thin layer of gold and attached to aluminum stubs (JSM-IT300, JEOL, Japan). The surface morphology of the sample was observed using SEM (Quanta 250; FEI Company, Netherlands) at 60× and 5000×. The SEM results represented the morphology of the adjacent dentin beneath the GIC restoration with or without chlorhexidine gluconate treatment on each 5000× magnification SEM micrograph, 3 random tubule diameters were measured by SEM [37].
Statistical analysis
Descriptive statistics described the mineralization on the dentin carious lesions under the restoration from the SEM image of each group’s sample. The Shapiro–Wilk test and Levene’s test were performed to test the normality and homogeneity of variance of the MMD of carious dentin, respectively. The MMD was compared (1) between baseline/post-restoration or baseline/post-pH cycling in the same group using the paired t-test, (2) between groups using one way ANOVA with Post hoc (Tukey) test. The MMD gain was compared (1) between groups using one way ANOVA with Post hoc (Tukey) test, (2) between the EquiaTM/CHX-EquiaTM groups or KetacTM/CHX-KetacTM groups using the independent t-test. For all statistical analyses, the tests were performed at the 95% confidence level using SPSS statistic 22.
Results
Mean mineral density
We determined the MMD at baseline, post-restoration, and post-pH cycling (Table 1). There were no significant differences in MMD at baseline, post-restoration, or post-pH cycling between the groups (one way ANOVA with Post Hoc (Tukey) test, P = 0.356, P = 0.299, and P = 0.419, respectively) (Table 1, Fig. 2). The MMD in all groups (post-restoration and post-pH cycling) were significantly increased compared with baseline (Paired t-test, P < 0.001) (Fig. 2).
The MMD gain is shown in Table 2. The MMD gain post-restoration between the groups was significantly different (One way ANOVA, P = 0.045) (Table 2). The Post Hoc (Tukey) test revealed a significant difference between the Equia™ and CHX-Ketac™ groups (P = 0.049). In contrast, the MMD gain post-pH cycling between the 4 groups was not significantly different (oneway ANOVA, P = 0.065) (Table 2). However, there was a significant difference in MMD gain post-restoration between the Equia™ and CHX-Equia™ groups (Independent t-test, P = 0.046) (Fig. 3).
SEM
The morphology of the dentin surface in contact with the H-GIC restorations in each group was investigated by SEM at 60× and 5000× magnification (Fig. 4). The H-GIC restoration and adjacent dentin was seen in all groups, except for the CHX-Equia™ group because the restoration was dislodged during specimen preparation (Fig. 4A, C, E, F). The area of the dentinal tubules in the red rectangles are shown at 5000× magnification.
Dentin which contacted H-GIC restoration micrographs of SEM images at 60X magnification A Equia™ group, C CHX-Equia™ group, E Ketac™ group and G CHX-Ketac™ group and 5000X magnification B Equia™ group, D CHX-Equia™ group; the arrow represented inside surface of intratubular dentin, F Ketac™ group; the arrow represented peritubular dentin and (H.) CHX-Ketac™ group; the arrow represented particles on the dentin surface
In the Equia™ group, dentin conditioner was applied before restoring with H-GIC (Equia Forte™). The surface of the intertubular dentin appeared eroded with an uneven surface (Fig. 4B). The dentinal tubule orifices were visible, with little if any, peritubular dentin present. Furthermore, the margins of the dentinal tubule orifices were rounded. The dentinal tubules were approximately 5–7 m wide.
In the CHX-Equia™ group, 2% CHX was applied for 1 min followed by the dentin conditioner and restored with H-GIC (Equia Forte™). In contrast to the group treated only with the dentin conditioner, the dentin surface was flat, with wide intertubular dentin present (Fig. 4D). The dentinal tubule orifices were irregularly shaped and were approximately 4–6 µm wide. Peritubular dentin was not always present at the tubule orifice. The inner surface of the peritubular dentin was rough with some particles in the deep part of the dentinal tubules (arrow).
In the Ketac™ group, the cavity was directly restored with H-GIC (Ketac Universal™) without prior treatment. Wide, flat intertubular dentin was observed (Fig. 4F). Deposited material was seen in the intertubular dentin surface. Peritubular dentin was present (arrow) in round dentinal tubules. The dentinal tubules were approximately 6–8 µm wide.
In the CHX-Ketac™ group, the dentin was applied with 2% CHX for 1 min and restored with H-GIC (Ketac Universal™). The intertubular dentin was flat and was the widest among the 4 groups (Fig. 4H). Many single and clustered particles were scattered on the dentin surface (arrow). Peritubular dentin was evident in all dentinal tubules. The dentinal tubules orifices in this group had the smallest diameter. The tubules were approximately 2–4 µm wide.
Discussion
The present study evaluated the remineralization effect of CHX used as a cavity disinfectant on dentin carious lesions restored with H-GIC using micro-CT. Our results demonstrated that applying CHX disinfectant prior to restoration improved the MMD gain in the CHX-EquiaTM and CHX-KetacTM groups compared with the non-CHX–treated groups. A previous study, found that cavity conditioner partially demineralized dentin and caused microporosities [41]. Therefore, CHX might neutralize the dentin surface before it is applied with acid conditioner [33].
Sealing carious lesions and bacteria after selective removal and ART is based on the concept of changing the ecological environment by depriving the bacteria of nutrition and reducing or inhibiting bacterial proliferation and activity [42, 43]. To increase the antibacterial effect of sealing by reducing the remaining bacteria, several studies have investigated using an antibacterial agent as a cavity disinfectant or restorative material [2, 29, 42,43,44]. CHX is a well-known antimicrobial agent used as a cavity disinfectant in ART [27]. CHX is commonly used in dentistry, such as oral surgery, endodontics, prevention, and prophylaxis. CHX is used in different formulations, such as mouthwashes, gels, galenic preparations, solutions, creams, or dentifrices [29]. CHX solutions are typically used in ART. CHX disrupts the cell membrane leading to cell death. A 2% CHX solution killed oral microbes, such as S. aureas, E. faecalis, C. albicans, P. endodontalis, P. gingivalis, and P. intermedia within 60 s [45].
The effect of CHX on promoting remineralization might be explained via two mechanisms. CHX inhibits two collagen-degrading enzymes present in dentin, matrix metalloproteinases (MMPs) and cysteine cathepsins [46, 47]. The MMPs are inactive when the dentin matrix structure is mineralized [48]. Acid production from cariogenic bacteria or acid etching demineralizes the dentin, which activates matrix metalloproteinases and cathepsins that degrade the dentin [36]. The exposed collagen network after acid etching can be degraded by endogenous metalloproteinases, which results in the degradation of the adhesive/dentin interface [49,50,51]. CHX has been shown to prevent the cross-linked collagen in dentin from degrading by inhibiting matrix metalloproteinase (MMP) activity through a cation-chelating mechanism [37, 51, 52]. The inhibitory effect of CHX on MMPs tended to be dose-dependent and remained active at low concentration 6 months after application [52].
Dentin remineralization is encouraged from the remaining scaffold collagen fibrils that contain minerals [37, 53]. Extra-fibrillar mineral, intra-fibrillar mineral are inorganic structures surrounding the collagen fibrils. Intra-fibrillar mineral affects the elastic behavior in collagen fibrils and resist demineralization. Intra- and extra-fibrillar mineral regrowth in partially demineralized dentin should allow for the recovery of its mechanical properties. Reincorporating collagen fibrils with mineral promotes remineralization [54].
A meta regression study also found that the effect of CHX on inhibiting MMPs might depend on the adhesive system used [55]. For resin restorations, the sequential application of phosphoric acid, CHX, and an etch-and-rinse adhesive may more effectively inhibit MMP activity than the self-etching adhesives because CHX acts better on exposed collagen fibrils [55]. This observation corresponds with our results that CHX had a greater remineralization effect in the Equia™ group, which had a dentin conditioner step that contains a mild polyacrylic acid that can expose the collagen fibrils similar to etch-and-rinse adhesive systems. In contrast, Ketac™ does not require a dentin conditioner step; therefore, the dentin is not demineralized and the MMP inhibitor effect of CHX does not occur. This likely explains why no significant difference in MMD gain was observed between the KetacTM and CHX-KetacTM groups.
Another possible mechanism by which CHX might promote remineralization is via electrostatic attraction [37]. The interaction between CHX and its target results from a cationic-anionic reaction. The cationic part of the CHX molecule can bind to the negatively charged area of the target substrate. CHX bound to dentin collagen might strongly attract the negatively charged phosphate from hydroxyapatite and H-GIC via the electrostatic interaction between the protonated amine groups of CHX and the mineral phosphates that promote mineral growth and deposition in demineralized dentin [56, 57].
The Ketac™ and CHX-Ketac™ group were not treated with dentin conditioner, thus, dentin demineralization by the mild acid conditioner did not occur in these groups. The collagen fibrils were undegraded and maintained for remineralization as observed in the micrographs of the Ketac™ and CHX-Ketac™ groups. In the Ketac™ group, the dentinal tubule orifices were usually visible and rounded. Peritubular dentin was present on the dentinal tubule orifices. Among the 4 groups, the CHX-Ketac™ group exhibited the thickest intertubular dentin with the smallest dentinal tubule diameters indicating mineral deposition [37]. Our SEM results corresponded with the micro-CT results where the MMD gain post-restoration was highest in the CHX-Ketac™ group.
In the Equia™ group, the intertubular and peritubular dentin were removed by the dentin conditioner. The SEM image of this group demonstrated an irregular eroded surface with very little peritubular dentin that had been highly demineralized. In contrast, the CHX-Equia™ group had a flat surface with peritubular dentin typically seen in the tubules. Interestingly, the intertubular dentin was thicker compared with the Equia™ group. Moreover, precipitates were present on the dentin surface and peritubular dentin in the dentinal tubules. Our results were in accordance with previous studies that found a dense granular deposition of nanoparticles after applying CHX [37, 58]. Based on the SEM images and the micro-CT results, the dentin conditioner demineralized dentin, while the CHX neutralized the action of the dentin conditioner.
To date, our study is the first report to quantify the remineralization effect of CHX on demineralized dentin post-restoration with H-GIC using micro-CT analysis. In this study, we chose to analyze the remineralization in actual dentin carious lesions to mimic the clinical scenario as much as possible. However, the naturally occurring cavities were different in size, shape, and baseline mineral content, which may influence the remineralization quantity at the H-GIC-dentin interface. A large multi-surface cavity with a large H-GIC contact area might exhibit more mineral gain compared with a small cavity. Furthermore, the chemical pH cycling model used to imitate the oral environment [40] had a minimal effect in our study. This might be because most of the cavities were deep class I cavities. The pH cycling solution could not reach bottom of the restored cavities, therefore no significant differences in MMD were observed in any group post-pH cycling. Moreover, the slight increase in MMD post-pH cycling might be due to the exchange of charged ions between the H-GIC and tooth structure.
Our results corresponded with a previous study that investigated the remineralization of dentin as shown by elastic modulus [37]. This study found that the elastic modulus of the demineralized dentin block in the CHX-treated group was higher compared with the non-CHX group. Moreover, the higher the concentration of CHX, the higher an elastic modulus was found. Therefore, applying CHX on demineralized dentin is effective in promoting the remineralization of deep residual caries.
Although using CHX could be beneficial in the ART method due to its antimicrobial and remineralization effect, the effect of CHX on GI bond strength is unclear. Recent studies found that there was no significant difference in bond H-GIC bond strength after applying CHX [33,34,35]. Given that new H-GICs are being developed, it will be beneficial to see how CHX affects the bond strength and stability. Moreover, the antimicrobial effect of CHX might reduce pulpal pathology from developing in deep dentin caries cases post-restoration. Thus, clinical studies on the survival rate of ART-treated teeth when using CHX with an H-GIC restoration are of particular interest to improve the clinical success of ART in the future.
Conclusion
Our results indicated that the groups that used 2% CHX as a cavity disinfectant with H-GIC restoration had a higher mean mineral density gain compared with the groups with H-GIC restoration alone. When the dentin was demineralized, CHX increased remineralization by neutralizing the acid effects of the dentin conditioner, maintaining the collagen fibrils, and mineral phosphate attraction. Thus, 2% CHX enhances the remineralization of the dentin adjacent to the H-GIC restoration. Using CHX as a cavity disinfectant is beneficial to ART due to its antimicrobial and remineralization effects. The limitation of this study is that it was an in vitro study. Our results need to be confirmed by clinical studies of the remineralizing effects of CHX used as a cavity disinfectant. Furthermore, clinical studies on the survival rate of ART-treated teeth when using CHX with an H-GIC restoration are of particular interest to improve the clinical success of ART in the future.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to Chulalongkorn University thesis copyright but are available from the corresponding author on reasonable request.
Abbreviations
- ART:
-
Atraumatic restorative treatment
- CHX:
-
Chlorhexidine gluconate
- °C:
-
Celsius
- gr:
-
Gram
- H-GIC:
-
High viscosity glass ionomer cement
- h:
-
Hours
- kVp:
-
Kilovoltage peak
- MMD:
-
Mean mineral density
- Micro-CT:
-
Micro-computed topography
- min:
-
Minute
- mm:
-
Millimeter
- mm2 :
-
Square millimeter
- mm3 :
-
Cubic millimeter
- mg/cm3 :
-
Milligram per cubic centimeter
- mM:
-
Millimolar
- μA:
-
Microampere
- μm:
-
Micrometer
- psi:
-
Pound per square inch
- sec:
-
Second
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
References
FDI World Dental Federation. FDI policy statement on Minimal Intervention Dentistry (MID) for managing dental caries: adopted by the General Assembly: September 2016, Poznan, Poland. Int Dent J. 2017;67(1):6–7.
Cicciù M, Fiorillo L, Cervino G. Chitosan use in dentistry: a systematic review of recent clinical studies. Mar Drugs. 2019;17(7):417.
Cervino G, Fiorillo L, Spagnuolo G, Bramanti E, Laino L, Lauritano F, Cicciù M. Interface between MTA and dental bonding agents: scanning electron microscope evaluation. J Int Soc Prev Community Dent. 2017;7(1):64.
Innes NP, Chu C, Fontana M, Lo EC, Thomson WM, Uribe S, Heiland M, Jepsen S, Schwendicke F. A century of change towards prevention and minimal intervention in cariology. J Dent Res. 2019;98(6):611–7.
Freitas M, Santos J, Fuks A, Bezerra A, Azevedo T. Minimal intervention dentistry procedures: a ten year retrospective study. J Clin Pediatr Dent. 2014;39(1):64–7.
Dentistry AAoP. Pulp therapy for primary and immature permanent teeth. American Academy of Pediatric Dentistry; 2021. p. 399–407.
Giacaman RA, Munoz-Sandoval C, Neuhaus KW, Fontana M, Chalas R. Evidence-based strategies for the minimally invasive treatment of carious lesions: review of the literature. Adv Clin Exp Med. 2018;27(7):1009–16.
Dorri M, Martinez-Zapata MJ, Walsh T, Marinho VCC, Sheiham A, Zaror C. Atraumatic restorative treatment versus conventional restorative treatment for managing dental caries. Cochrane Database Syst Rev. 2017;12(12):CD008072.
Schwendicke F, Leal S, Schlattmann P, Paris S, Dias Ribeiro AP, Gomes Marques M, Hilgert LA. Selective carious tissue removal using subjective criteria or polymer bur: study protocol for a randomised controlled trial (SelecCT). BMJ Open. 2018;8(12):e022952–e022952.
Luz PB, Barata JS, Meller CR, Slavutzky SMB, Araujo FB. ART acceptability in children: a randomized clinical trial. Revista da Faculdade de Odontologia de Porto Alegre Porto Alegre. 2012;53(1):27–31.
van Gemert-Schriks MCM, van Amerongen WE, ten Cate JM, Aartman IHA. Three-year survival of single- and two-surface ART restorations in a high-caries child population. Clin Oral Investig. 2007;11(4):337–43.
Croll TP, Nicholson J. Glass ionomer cements in pediatric dentistry: review of the literature. Pediatr Dent. 2002;24(5):423–9.
Bonifácio C, Kleverlaan C, Raggio DP, Werner A, De Carvalho R, Van Amerongen W. Physical–mechanical properties of glass ionomer cements indicated for atraumatic restorative treatment. Aust Dent J. 2009;54(3):233–7.
Caro TER, Aguilar AAA, Saavedra JH, Alfaya TA, Franca CM, Fernandes KP, Mesquita-Ferrari RA, Bussadori SK. Comparison of operative time, costs and self-reported pain in children treated with atraumatic restorative treatment and conventional restorative treatment. Med Sci Technol. 2012;53:159–63.
de Amorim RG, Frencken JE, Raggio DP, Chen X, Hu X, Leal SC. Survival percentages of atraumatic restorative treatment (ART) restorations and sealants in posterior teeth: an updated systematic review and meta-analysis. Clin Oral Investig. 2018;22(8):2703–25.
Chadwick BL, Evans DJ. Restoration of class II cavities in primary molar teeth with conventional and resin modified glass ionomer cements: a systematic review of the literature. Eur Arch Paediatr Dent. 2007;8(1):14–21.
Moshaverinia M, Navas A, Jahedmanesh N, Shah KC, Moshaverinia A, Ansari S. Comparative evaluation of the physical properties of a reinforced glass ionomer dental restorative material. J Prosthet Dent. 2019;122(2):154–9.
Wang L, D’Alpino PHP, Lopes LG, Pereira JC. Mechanical properties of dental restorative materials: relative contribution of laboratory tests. J Appl Oral Sci. 2003;11(3):162–7.
Hicks M, Flaitz C, Silverstone L. Secondary caries formation in vitro around glass ionomer restorations. Quintessence Int. 1986;17(9):527.
Ten Cate J, Van Duinen R. Hypermineralization of dentinal lesions adjacent to glass-ionomer cement restorations. J Dent Res. 1995;74(6):1266–71.
Ngo HC, Mount G, Mc Intyre J, Tuisuva J, Von Doussa RJ. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent. 2006;34(8):608–13.
Koruyucu M, Topcuoglu N, Tuna EB, Ozel S, Gencay K, Kulekci G, Seymen F. An assessment of antibacterial activity of three pulp capping materials on Enterococcus faecalis by a direct contact test: an in vitro study. Eur J Dent. 2015;9(2):240–5.
Fiorillo L, Cervino G, Herford AS, Laino L, Cicciù M. Stannous fluoride effects on enamel: a systematic review. Biomimetics. 2020;5(3):41.
Prada I, Micó-Muñoz P, Giner-Lluesma T, Micó-Martínez P, Muwaquet-Rodríguez S, Albero-Monteagudo A. Update of the therapeutic planning of irrigation and intracanal medication in root canal treatment. A literature review. J Clin Exp Dent. 2019;11(2):e185–93.
Bin-Shuwaish MS. Effects and effectiveness of cavity disinfectants in operative dentistry: a literature review. J Contemp Dent Pract. 2016;17(10):867–79.
Vergara-Buenaventura A, Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg. 2020;58(8):924–7.
Joshi JS, Roshan NM, Sakeenabi B, Poornima P, Nagaveni NB, Subbareddy VV. Inhibition of residual cariogenic bacteria in atraumatic restorative treatment by chlorhexidine: disinfection or incorporation. Pediatr Dent. 2017;39(4):308–12.
Takahashi Y, Imazato S, Kaneshiro AV, Ebisu S, Frencken JE, Tay FR. Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent Mater. 2006;22(7):647–52.
Fiorillo L. Chlorhexidine gel use in the oral district: a systematic review. Gels. 2019;5(2):31.
Radman IK, Djeri A, Arbutina A, Milašin J. Microbiological findings in deep caries lesions. Serb Dent J. 2016;63(1):7–14.
Ruiz M, Baca P, Pardo-Ridao MM, Arias-Moliz MT, Ferrer-Luque CM. Ex vivo study of bacterial coronal leakage in indirect pulp treatment. Medicina oral patologia oral y cirugia bucal. 2013;18(2):e319.
Jaidka S, Somani R, Singh DJ, Shafat S. Comparative evaluation of compressive strength, diametral tensile strength and shear bond strength of GIC type IX, chlorhexidine-incorporated GIC and triclosan-incorporated GIC: An in vitro study. J Int Soc Prev Community Dent. 2016;6(Suppl 1):S64–9.
Sheikh Hasani Y, Paryab M, Saffarpour A, Kharazifard MJ, Shahrabi M. The effect of disinfection with chlorhexidine on the shear bond strength of equia resin-modified glass ionomer cement to dentin in permanent teeth after two thermocycling protocols. J Dent (Shiraz). 2017;18(4):265–71.
Wadenya R, Menon S, Mante F. Effect of chlorhexidine disinfectant on bond strength of glass ionomer cement to dentin using atraumatic restorative treatment. N Y State Dent J. 2011;77(1):23–6.
Lugassy D, Segal P, Blumer S, Eger M, Shely A, Matalon S. Effect of two traditional polyacrylic acid conditioners and 2% chlorhexidine digluconate on cavosurface microleakage of glass ionomer restorations. J Clin Pediatr Dent. 2018;42(4):287–91.
Nawrocka A, Łukomska-Szymańska M. Extracted human teeth and their utility in dental research. Recommendations on proper preservation: a literature review Zastosowanie usuniętych zębów ludzkich w badaniach naukowych. Wytyczne dotyczące przechowywania próbek–przegląd piśmiennictwa. Dent Med Probl. 2019;56(2):185–90.
Kim D-S, Kim J, Choi K-K, Kim S-Y. The influence of chlorhexidine on the remineralization of demineralized dentine. J Dent. 2011;39(12):855–62.
Ersin NK, Uzel A, Aykut A, Candan U, Eronat C. Inhibition of cultivable bacteria by chlorhexidine treatment of dentin lesions treated with the ART technique. Caries Res. 2006;40(2):172–7.
Dias GF, Chibinski ACR, Santos FA, Hass V, Alves FBT, Wambier DS. The hardness and chemical changes in demineralized primary dentin treated by fluoride and glass ionomer cement. Rev Odontol UNESP. 2016;45(1):33–40.
Ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16(3):201–10.
Hajizadeh H, Ghavamnasiri M, Namazikhah MS, Majidinia S, Bagheri M. Effect of different conditioning protocols on the adhesion of a glass ionomer cement to dentin. J Contemp Dent Pract. 2009;10(4):9–16.
Fiorillo L. We do not eat alone: formation and maturation of the oral microbiota, vol. 9. Multidisciplinary Digital Publishing Institute; 2020. p. 17.
Elkady DM, Khater AG, Schwendicke F. Chlorhexidine to improve the survival of ART restorations: a systematic review and meta-analysis. J Dent. 2020;103:103491.
Coelho A, Amaro I, Apolónio A, Paula A, Saraiva J, Ferreira MM, Marto CM, Carrilho E. Effect of cavity disinfectants on adhesion to primary teeth—a systematic review. Int J Mol Sci. 2021;22(9):4398.
Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CCR, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2004;97(1):79–84.
Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, Carrilho MR, Pashley DH, Tay FR, Salo T. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36(3):475–81.
Scaffa PMC, Vidal CMP, Barros N, Gesteira TF, Carmona AK, Breschi L, Pashley DH, Tjäderhane L, Tersariol ILS, Nascimento FD. Chlorhexidine inhibits the activity of dental cysteine cathepsins. J Dent Res. 2012;91(4):420–5.
Müller S, Rupf S, Umanskaja N, Hannig M. Detection of matrix metalloproteinases (MMPs) in the root dentin of human teeth. Dtsch Zahnärztl Z Int. 2019;1:161–7.
Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27(25):4470–6.
Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114(2):160–6.
Carrilho M, Geraldeli S, Tay F, De Goes M, Carvalho RM, Tjäderhane L, Reis A, Hebling J, Mazzoni A, Breschi L. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86(6):529–33.
Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6(3):437–9.
Bertassoni LE, Habelitz S, Marshall SJ, Marshall GW. Mechanical recovery of dentin following remineralization in vitro—an indentation study. J Biomech. 2011;44(1):176–81.
Bertassoni LE, Habelitz S, Kinney J, Marshall SJ, Marshall GW Jr. Biomechanical perspective on the remineralization of dentin. Caries Res. 2009;43(1):70–7.
Collares FM, Rodrigues SB, Leitune VC, Celeste RK, Borba de Araújo F, Samuel SM. Chlorhexidine application in adhesive procedures: a meta-regression analysis. J Adhes Dent. 2013;15(1):11–8.
Sodhi R, Grad H, Smith D. Examination by X-ray photoelectron spectroscopy of the adsorption of chlorhexidine on hydroxyapatite. J Dent Res. 1992;71(8):1493–7.
Misra D. Interaction of chlorhexidine digluconate with and adsorption of chlorhexidine on hydroxyapatite. J Biomed Mater Res. 1994;28(11):1375–81.
Lapinska B, Klimek L, Sokolowski J, Lukomska-Szymanska M. Dentine surface morphology after chlorhexidine application—SEM study. Polymers. 2018;10(8):905.
Acknowledgements
The authors acknowledge Oral biology research center, Dental Material Science Research Center, Department of Biochemistry and Department of Pediatric dentistry, Faculty of Dentistry, Chulalongkorn University for supporting facilities and equipment. The authors are grateful to Dr. Kevin Tompkins for critical revision and language editing.
Funding
This study was supported by the Chulalongkorn University Graduate School Thesis Grant (Grant #GCUGR1225642005M).
Author information
Authors and Affiliations
Contributions
PB: Methodology, investigation, formal analysis, and writing-original draft preparation, BP: software, resources, SL: software, resources, WS: conceptualization, methodology, writing-review and editing, supervision, corresponding author OT: conceptualization, methodology, writing-review and editing, supervision, corresponding author. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The 40 human primary molar teeth in this study were collected from the Pediatric Dentistry Department Clinic, Faculty of Dentistry, Chulalongkorn University. All included teeth were extracted for reasons unrelated to the study and are so-called excess material. All donors were children and gave written informed consent to parent or legal guardian for research purpose. All methods have been performed in accordance with the Declaration of Helsinki. The study protocols were approved by the Human Research Ethics Committee of the Faculty of Dentistry, Chulalongkorn University (HREC-DCU2020-043) and were approved by the Institutional Biosafety Committee of the Faculty of Dentistry, Chulalongkorn University (DENT CU-IBC 032/2020).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Borompiyasawat, P., Putraphan, B., Luangworakhun, S. et al. Chlorhexidine gluconate enhances the remineralization effect of high viscosity glass ionomer cement on dentin carious lesions in vitro. BMC Oral Health 22, 60 (2022). https://doi.org/10.1186/s12903-022-02098-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-022-02098-1