Abstract
Background
The association between the triglyceride-glucose (TyG) index and arterial stiffness in individuals with normoglycaemia remains unclear. We aimed to evaluate the relationship between the TyG index and arterial stiffness in Japanese individuals with normoglycaemia, providing additional evidence for predicting early arterial stiffness.
Methods
This study included 15,453 adults who participated in the NAGALA Physical Examination Project of the Murakami Memorial Hospital in Gifu, Japan, from 2004 to 2015. Data on clinical demographic characteristics and serum biomarker levels were collected. The TyG index was calculated from the logarithmic transformation of fasting triglycerides multiplied by fasting glucose, and arterial stiffness was measured using the estimated pulse wave velocity calculated based on age and mean blood pressure. The association between the TyG index and arterial stiffness was analysed using a logistic regression model.
Results
The prevalence of arterial stiffness was 3.2% (500/15,453). After adjusting for all covariates, the TyG index was positively associated with arterial stiffness as a continuous variable (adjusted odds ratio (OR) = 1.86; 95% Confidence Interval = 1.45–2.39; P<0.001). Using the quartile as the cutoff point, a regression analysis was performed for arterial stiffness when the TyG index was converted into a categorical variable. After adjusting for all covariates, the OR showed an upward trend; the trend test was P<0.001. Subgroup analysis revealed a positive association between the TyG index and arterial stiffness in Japanese individuals with normoglycaemia and different characteristics.
Conclusion
The TyG index in Japanese individuals with normoglycaemia is significantly correlated with arterial stiffness, and the TyG index may be a predictor of early arterial stiffness.
Similar content being viewed by others
Background
Cardiovascular diseases (CVDs) are a major cause of disability and mortality worldwide [1]. Arterial stiffness is one of the earliest functional impairments of the vascular aging process that directly affects the cardiovascular system through loss of vascular reactivity and an increase in vascular thickness and stiffness [2]. Arterial stiffness is an independent predictor of adverse cardiovascular events [3]. However, the biological mechanisms underlying arterial stiffness remain unclear. Early identification of predictors in patients allows appropriate preventive measures, thereby reducing the incidence of CVDs and the global healthcare economic burden.
There are various methods for assessing arterial stiffness, such as the pulse wave velocity (PWV), dynamic arterial stiffness index (AASI), arterial pulse waveform (enhanced index), and magnetic resonance imaging. However, their measurement has limitations, such as high technical requirements, specialised equipment, and high cost, and it is not widely used in clinical practice [4]. Recent studies have shown that estimated pulse wave velocity (ePWV) calculated based on age and mean blood pressure (MBP) is a new indicator that can reliably reflect the degree of arterial stiffness [5], is low-cost, easy to implement, and has cardiovascular risk predictive value [6, 7].
Insulin resistance (IR) is defined as a decrease in insulin responsiveness in tissues, and is an important factor in the glycolipid metabolic pathway [8]. Due to the production of vascular inflammatory factors and impairment of vascular endothelial smooth muscle cell function, IR is considered a major risk factor for the development of arterial stiffness and CVDs [9]. Currently, the hyperinsulinemic-euglycemic clamp (HEC) is the gold standard for assessing IR [10]. However, this traditional evaluation method is time-consuming, expensive, complicated, and labour-intensive, making it unsuitable for clinical use. Recently, various studies have shown that the TyG index, calculated from fasting triglyceride (TG) and fasting blood glucose (FBG) levels, can be used as a reliable and inexpensive surrogate biomarker of IR, with better performance than the homeostatic modelling assessment of IR (HOMA-IR) [11,12,13].
Studies have indicated that elevated TyG index levels are associated with an increased risk of arterial stiffness [14,15,16]. However, most studies were conducted in populations with underlying conditions (e.g. hypertension, insulin resistance, and diabetes mellitus) and involved small sample sizes. Therefore, there is a need for studies with large sample sizes to further validate this association. Limited knowledge exists regarding the role of the TyG index in assessing the risk of arterial stiffness in individuals with normoglycaemia, and previous studies have not used ePWV to measure arterial stiffness. Therefore, the present study used relevant databases to conduct an association analysis, aiming to elucidate whether the TyG index can be used as an independent biomarker for predicting the risk of arterial stiffness in individuals with normoglycaemia. The objective is to identify individuals at risk of arterial stiffness at an early stage, aiding clinicians in formulating more targeted preventive strategies and clinical interventions.
Methods
Study design and population
This study used data from the DATADRYAD, of which a detailed description has been previously provided [17]. All researcher data are freely available from the website: http://www.Datadryad.org/. We used a standardised questionnaire to collect the following variables: sociodemographic information, health behaviours, medical history, and substance use. Subsequently, we measured physical parameters using standardised equipment, and collected serum specimens from the study participants after they fasted for 8 h. The biochemical parameters included ALT, AST, GGT, FPG, HDL-C, TC, TG, and HbA1c. A total of 20,944 participants were recruited between 2004 and 2015. The inclusion criteria comprised individuals with normal blood glucose levels (no history of diabetes or with a fasting blood glucose < 6.1 mmol/L at baseline examination) and with a complete blood pressure measurement data. The exclusion criteria were as follows: (1) missing recorded data, (2) known history of liver disease, (3) alcohol abuse (> 60 g/day for men and > 40 g/day for women), (4) history of oral medication use, and (5) history of diabetes or fasting blood glucose > 6.1 mmol/L at baseline. In total, 15,453 participants (8,419 women and 7,034 men) were included in the analysis (Fig. 1). The project was approved by the Ethics Committee of the Murakami Memorial Hospital and all participants signed a written informed consent form.
Triglyceride-glucose (TyG) index
The TyG index was calculated as follows: Ln [fasting triglycerides (mg/dL) × fasting blood glucose (mg/dL)/2] [18].
Estimating pulse wave velocity (ePWV)
It was calculated as follows: ePWV = 9.587 − 0.402 × age + 4.560 × 10 − 3 × age 2 − 2.621 × 10 − 5 × age 2 × MBP + 3.176 × 10 − 3 × age × MBP − 1.832 × 10 − 2 × MBP, and MBP was calculated as DBP + 0.4 (SBP−DBP) [19]. An ePWV ≥ 10 m/s was defined as arterial stiffness [20].
Covariate assessment
We considered both sociodemographic characteristics and health-related factors. Sociodemographic characteristics included age and sex. Health-related factors included: history of alcohol consumption, as measured by alcohol breakdown and average weekly alcohol consumption in the previous month (none or minimal drinking (40 g/week), light drinking (40–140 g/week), moderate drinking (140–280 g/week), and heavy drinking (> 280 g/week)) [21]; smoking history (no smoking, smoking cessation, or current smoking); regular exercise, defined as regular participation in any type of sport more than once per week [22]; liver disease, meeting the diagnostic criteria in abdominal ultrasound [23]; obesity, defined as a body mass index (BMI) of ≥ 25 kg/m2 [24]; and visceral fat obesity, defined as a waist circumference ≥ 90 cm in men or ≥ 80 cm in women [25].
Statistical analysis
We processed and analysed the data using SPSS 25.0 software and expressed continuous variables with normal distribution by mean ± standard deviation (SD). Comparisons between groups was made using t-test and continuous variables with skewed distribution by median ± interquartile range (IQR) and Wilcoxon rank sum test. Categorical variables were expressed as numbers (percentages, %), and compared between groups using the chi-square test. To study the significance of intergroup differences stratified by TyG index quartiles, the Kruskal–Wallis test or ANOVA was used. Finally, the correlation between the TyG index and arterial stiffness was analysed using logistic regression.
To assess the influence of potential effect confounders, we conducted subgroup analyses according to: age (< 65 versus ≥ 65 years); sex (female versus male); BMI (< 25 versus ≥ 25 kg/cm 2), WC (< 90 versus ≥ 90 cm for men and < 80 versus ≥ 80 cm for women); alcohol consumption history (no drinking or minimal drinking (40 g/week) versus light drinking (40–140 g/week) versus moderate drinking (140–280 g/week) versus heavy drinking (> 280 g/week); and smoking history (no smoking versus abstaining versus current smoking).
In all analyses, statistical significance was achieved at p < 0.05.
Results
Baseline characteristics of participants
As observed in the flowchart (Figs. 1), 15,453 participants were included in this study, of whom 7,034 (45.5%) were men and 8,419 (54.5%) were women. The mean ± SD age of all participants was 43.7 ± 8.9 years and the mean ± SD TyG index was 8.0 ± 0.6.
The clinical and biological characteristics of participants in the TyG index quartiles are listed in Table 1. Participants with a higher TyG index were older and exhibited elevated BMI and WC values. Additionally, a greater proportion of individuals in this category were currently smoking or consuming alcohol compared to those in the lowest quartile group. Significant differences in biological parameters were observed between groups. Participants in the highest TyG index quartile had significantly higher ALT, AST, GGT, TC, TG, FPG, SBP, and DBP levels than those in the lowest quartile. The TyG index was significantly lower in women and regular exercisers.
Univariate and multivariate analyses of arterial stiffness
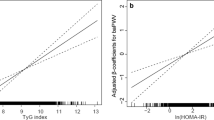
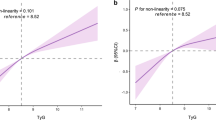
Age, sex, alcohol consumption, smoking history, regular exercise, BMI, WC, ALT, AST, GGT, TC, HDL-c, TG, FPG, and HbA1c levels were significantly associated with arterial stiffness (Table 2). After adjusting for the covariates included in Table 2, the risk of developing arterial stiffness steadily increased when the TyG index was used as a continuous variable (Fig. 2).
We used various linear regression models to analyse the association between the TyG index and arterial stiffness. After adjusting for the two variables of age and sex (model 1), the OR of the linear regression between the two was 1.92 (95% Confidence Interval (CI): 1.59–2.33; p < 0.001), and after continuing to add multiple variables of smoking history, alcohol consumption, regular exercise, waist circumference, BMI, ALT, TC, HDL-C, and HbA1 to the models (models 2–3), the corresponding ORs were 1.46 (95% CI: 1.18–1.79; p < 0.001) and 1.86 (95% CI: 1.45–2.39; p < 0.001). This suggests that the TyG index is independently and positively associated with arterial stiffness in all three models after adjusting for different covariates (Table 3).
The TyG index was transformed into categorical variables using quartiles as cutoffs, as follows: Q1 (< 7.95), Q2 (7.59–8.01), Q3 (8.01–8.45), and Q4 (≥ 8.45) for use in regression analyses with arterial stiffness. The results revealed that in the unadjusted model and the model adjusted for all variables of age, sex, smoking, alcohol consumption, regular exercise, waist circumference, BMI, ALT, TC, HDL-c, and HbA1 (model 3), the OR for Q2 was 3.02 (95% CI: 2.06–4.41; p < 0.001), 1.61 (95% CI: 1–2.59; p = 0.049), and 3.99 (95% CI: 2.76–5.77; p < 0.001) for Q3, compared to Q1, respectively. Additionally, the OR was 1.72 (95% CI: 1.07–2.75; p = 0.025) for Q4 in the fully adjusted model, and ORs of 6.31 (95% CI: 4.42–9; p < 0.001) and 2.37 (95% CI: 1.44–3.9; p = 0.001) in the unadjusted and fully unadjusted models, respectively. The trend test was significant in both models (p < 0.001), indicating a stable linear association between TyG index and arterial stiffness (Table 3).
Subgroup analyses by adjusted potential effect confounders
Subgroup analyses were performed to assess the effect of the TyG index (per 1-unit increment) on arterial stiffness in the different subgroups (Fig. 3). As observed in the forest plot, the positive association between the TyG index and arterial stiffness was stronger in the WC and alcohol consumption groups (P interaction = 0.033 and 0.005, respectively). In contrast, the association between the TyG index and arterial stiffness was not significantly altered in the age, sex, BMI, and smoking groups.
Discussion
In this large cross-sectional survey based on a Japanese population with normoglycaemia, after rigorous adjustment for covariates, we found that the TyG index was positively associated with arterial stiffness, both as a continuous and categorical variable with quartile cutoff points. The higher the TyG index, the higher the risk of arterial stiffness.
To the best of our knowledge, few studies have examined the association between TyG index and arterial stiffness in populations with normoglycaemia, and even fewer have evaluated arterial stiffness using ePWV. However, assessment of arterial stiffness using ePWV suggests that an association between the TyG index and arterial stiffness has been reported in several studies [11, 26,27,28]. Lambrinoudaki et al. [29] found an association between the TyG index and arterial stiffness, assessed using PWV between the common carotid and common femoral arteries. However, this study has limitations due to its small sample size and inclusion of only women during post-menopause and without diabetes. Another study by Lee et al. [11] found an association between the TyG index and an increased risk of arterial stiffness, independently assessed using PWV in healthy Korean adults. Nonetheless, this study did not exclude individuals with diabetes and an unquantifiable history of alcohol consumption. Similarly, Wu et al. [14] discovered a positive association between the TyG index and the risk of arterial stiffness progression, as assessed by brachial-ankle PWV. Despite indicating that a higher TyG index predicted an increased risk and faster progression of arterial stiffness, this study, like Lee et al.‘s [11], did not exclude individuals with diabetes and had a small sample size. Since diabetes mellitus is a major risk factor of arterial stiffness, the results of these studies may be influenced by the presence of diabetes mellitus. In our study, we report, for the first time, the correlation between the TyG index and arterial stiffness in a population with normal blood glucose levels. Our findings clearly demonstrate that the TyG index is independently and positively correlated with ePWV, further confirming its potential as a novel and simple non-invasive biomarker for predicting the risk of arterial stiffness.
Some researchers have suggested that risk factors such as age, sex, BMI, WC, alcohol consumption, and smoking history may confound the association between the TyG index and atherosclerosis [11,12,13,14,15]. However, we performed subgroup analyses adjusting for potential effect confounders, and the results indicated that only the inclusion of WC and alcohol consumption significantly altered the association between TyG index and arterial stiffness. In contrast, factors such as age, gender, BMI, and smoking history showed no significant impact. The conflicting results may be attributed to differences in age and body composition, which require further validation.
The underlying biological mechanisms of the association between TyG index and arterial stiffness remain unclear. We postulate that these mechanisms may be related to metabolic pathways, inflammation, endothelial dysfunction, or other factors influenced by TyG dysregulation. Prolonged impairment of glucose metabolism has been associated with increased aortic stiffness [30], and IR and IR-related metabolic disorders have been linked to the development of arterial stiffness. Studies have demonstrated that IR contributes to the development of arterial stiffness through the following pathways: (1) IR can disrupt insulin signalling at the level of endothelial cells, vascular smooth muscle cells, and macrophages leading to varying degrees of oxidative stress and impaired endothelial cell function; this, in turn reduces nitric oxide bioavailability [31], causes vascular functional and structural damage, and ultimately reduces arterial wall dilatancy, leading to arterial stiffness [32]; (2) IR promotes the development of atherosclerotic dyslipidaemia, increases the vascular inflammatory response, disrupts endothelial function, and influences the prethrombotic state and arterial stiffness [33]; and (3) IR accelerates the accumulation of advanced glycosylation end products (AGEs), alters collagen and elastin contents, and remodels the arrangement and structure of the extracellular matrix, which in turn induces changes in arterial stiffness [33, 34].
In addition, systemic inflammation plays a role in atherosclerotic process [35]. C-reactive proteins have direct pro-inflammatory effects on human endothelial cells [36] and can induce endothelial dysfunction [37], and endothelial-derived nitric oxide is important for the functional regulation of arterial stiffness in large arteries in vivo [38, 39]. Alternatively, inflammation may induce structural changes in the arterial wall by altering the balance between elastin breakdown and synthesis [35]. In conclusion, the specific mechanisms of TyG index and arterial stiffness needs to be investigated further.
Our study has the following advantages: first, the data analysis was based on a large sample size, and the results of the study are stable and reliable; second, our study population included a wide range of adults with normal glycaemia, which is more representative of the population; and lastly, our assessment method is simple, low-cost, and can be widely used in clinical practice. However, our study has some limitations: first, this study has cross-sectional design, which does not allow us to verify the causal relationship; second, the study data were from the Japanese population, which has ethnic group specificity; and third, we were limited by the database and could not compare the TyG index with the HOMA-IR and HEC trials. Despite these limitations, our study provides reliable evidence of the association between the TyG index and arterial stiffness, further deepens the understanding of the TyG index in predicting arterial stiffness in individuals with normoglycaemia and provides a direction to guide the development of more targeted preventive strategies and clinical interventions.
Conclusion
The TyG index significantly correlated with arterial stiffness, suggesting that it can be used as a predictor of early arterial stiffness. The TyG index should generally be applied to screen for arterial stiffness in clinical practice and to perform individualised prevention and management at an early stage, which will help reduce the incidence of CVDs.
Data availability
The datasets used and/or analysed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- TyG:
-
Triglyceride-glucose
- CVDs:
-
Cardiovascular diseases
- IR:
-
Insulin resistance
- SMC:
-
Smooth muscle cell
- HEC:
-
High insulin normoglycaemic clamp
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- FBG:
-
Fasting blood glucose
- TG:
-
Triglyceride
- PWV:
-
Pulse wave velocity
- AASI:
-
Ambulatory arterial stiffness index
- ePWV:
-
Estimated pulse wave velocity
- MBP:
-
Mean blood pressure
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- ALT:
-
Alanine aminotransferase
- ASL:
-
Aspartate aminotransferase
- GGT:
-
Gamma glutamyl
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- HbA1c:
-
Hemoglobin A1c
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- AGEs:
-
Advanced glycosylation end products
References
Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395:795–808.
Pierce GL, Coutinho TA, DuBose LE, Donato AJ. Is it good to have a stiff aorta with aging? Causes and consequences. Physiol (Bethesda). 2022;37:154–73.
Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11.
Wang X, Keith JC Jr, Struthers AD, Feuerstein GZ. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther. 2008;26:214–23.
Greve SV, Blicher MK, Kruger R, Sehestedt T, Gram-Kampmann E, Rasmussen S, et al. Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. J Hypertens. 2016;34:1279–89.
Ji C, Gao J, Huang Z, Chen S, Wang G, Wu S, et al. Estimated pulse wave velocity and cardiovascular events in Chinese. Int J Cardiol Hypertens. 2020;7:100063.
Vishram-Nielsen JKK, Laurent S, Nilsson PM, Linneberg A, Sehested TSG, Greve SV, et al. Does estimated pulse wave velocity add prognostic information? MORGAM prospective cohort project. Hypertension. 2020;75:1420–8.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302.
Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26.
Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17:41.
Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16:108.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51.
Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20:146.
Li M, Zhan A, Huang X, Hu L, Zhou W, Wang T, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China H-type hypertension Registry Study. Cardiovasc Diabetol. 2020;19:139.
Poon AK, Meyer ML, Tanaka H, Selvin E, Pankow J, Zeng D, et al. Association of insulin resistance, from mid-life to late-life, with aortic stiffness in late-life: the arterial stiffness risk in communities Study. Cardiovasc Diabetol. 2020;19:11.
Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond). 2019;43:139–48.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304.
Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–50.
Heffernan KS, Stoner L, London AS, Augustine JA, Lefferts WK. Estimated pulse wave velocity as a measure of vascular aging. PLoS ONE. 2023;18:e0280896.
Hashimoto Y, Hamaguchi M, Kojima T, Ohshima Y, Ohbora A, Kato T, et al. Modest alcohol consumption reduces the incidence of fatty liver in men: a population-based large-scale cohort study. J Gastroenterol Hepatol. 2015;30:546–52.
Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. Gamma-glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71–7.
Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–15.
Hashimoto Y, Hamaguchi M, Fukuda T, Obora A, Kojima T, Fukui M. Weight gain since age of 20 as risk of metabolic syndrome even in non-overweight individuals. Endocrine. 2017;58:253–61.
Misra A, Vikram NK, Gupta R, Pandey RM, Wasir JS, Gupta VP. Waist circumference cutoff points and action levels for Asian indians for identification of abdominal obesity. Int J Obes (Lond). 2006;30:106–11.
Won KB, Park EJ, Han D, Lee JH, Choi SY, Chun EJ, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19:34.
Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18:95.
Won KB, Park GM, Lee SE, Cho IJ, Kim HC, Lee BK, et al. Relationship of insulin resistance estimated by triglyceride glucose index to arterial stiffness. Lipids Health Dis. 2018;17:268.
Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, et al. The TyG Index as a marker of subclinical arterial stiffness and arterial stiffness in lean and overweight Postmenopausal women. Heart Lung Circ. 2018;27:716–24.
McEniery CM, Wilkinson IB, Johansen NB, Witte DR, Singh-Manoux A, Kivimaki M, et al. Nondiabetic glucometabolic status and progression of aortic stiffness: the Whitehall II study. Diabetes Care. 2017;40:599–606.
Jia G, Aroor AR, DeMarco VG, Martinez-Lemus LA, Meininger GA, Sowers JR. Vascular stiffness in insulin resistance and obesity. Front Physiol. 2015;6:231.
Adeva-Andany MM, Ameneiros-Rodríguez E, Fernández-Fernández C, Domínguez-Montero A, Funcasta-Calderón R. Insulin resistance is associated with subclinical vascular disease in humans. World J Diabetes. 2019;10:63–77.
Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123:825–48.
Llauradó G, Ceperuelo-Mallafré V, Vilardell C, Simó R, Gil P, Cano A, et al. Advanced glycation end products are associated with arterial stiffness in type 1 diabetes. J Endocrinol. 2014;221:405–13.
Yasmin MECM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–74.
Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–8.
Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–41.
Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–7.
Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–53.
Acknowledgements
We thank all the study participants and Takuro Okamura (Department of Endocrinology and Metabolism, Kyoto Prefectural University of Medicine, Graduate School of Medical Science, Kyoto, Japan) for providing the original data.
Funding
This study was supported by the Guangdong Provincial Science and Technology Department “Overseas Famous Teachers"’ Special Fund Project (No.2020A1414010318).
Author information
Authors and Affiliations
Contributions
CYY and SWY made substantial contributions to the conception and design of the study, data collection and analysis, and manuscript drafting and served as equal contributing first authors. DHL, ZTM, YLL, YY, and FDH contributed substantially to the study design, intellectual direction, and manuscript revision. LJH, LGY, and WYY contributed to data collection. FDH is the guarantor of this work and takes responsibility for the integrity and accuracy of the data analysis. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Studies involving human participants were reviewed and approved by the Ethics Committee of the Murakami Memorial Hospital, and all participants provided written informed consent. All the experiments were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cai, Y., Sha, W., Deng, H. et al. Correlation between the triglyceride-glucose index and arterial stiffness in Japanese individuals with normoglycaemia: a cross-sectional study. BMC Endocr Disord 24, 30 (2024). https://doi.org/10.1186/s12902-024-01551-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01551-2