Abstract
Background
We aimed to assess potential associations of habitual dietary pattern scores in relation to the risk of pre-diabetes (Pre-DM) progression to type 2 diabetes mellitus (T2DM) or the chance of returning to normal glycemia.
Methods
This cohort study included 334 Pre-DM individuals (mean age of 49.4 years, and 51.5% men) who participated in the third phase of the Tehran Lipid and Glucose Study (2006–2008) and followed up for a median of 9 years. A validated food frequency questionnaire at baseline assessed usual intakes of the participants. Major dietary patterns were identified using principal component analysis. The DASH score and Mediterranean diet score (MDS) were also calculated. Multinomial logistic regression analysis was used to estimate the odds ratios (95% confidence intervals (CIs)) of developing T2DM and returning to normal glycemia in relation to dietary pattern scores.
Results
During the study follow-up, 39.8% progressed to T2DM, and 39.8% returned to normal glycemia. Three following major dietary patterns, including Western-style (with a higher load of red meats, hydrogenated fats, sodium, and total fat intakes), healthy pattern (with a higher load of whole grains, vegetables, and dairy products), and processed-foods pattern (with a higher load of processed-meats, fast-foods, salty snakes, and sweets and candies) were identified. The Western-style dietary pattern increased the risk of progressing to T2DM by 38% (OR = 1.38; 95% CI = 1.00 to 1.89, P = 0.050). Other dietary pattern scores were not related to regression or progression from Pre-DM.
Conclusion
The Western-style dietary pattern (characterized by higher load of red meats, hydrogenated fats, sodium intake, and high-GI foods) may accelerate the progression of Pre-DM to T2DM.
Similar content being viewed by others
Background
Prediabetes (Pre-DM) is a transitional glycemic state (between normal glucose regulation and type 2 diabetes mellitus (T2DM)) which may progress to T2DM or regress to normal glycemia [1]. The Pre-DM state is associated with a significantly higher risk of developing T2DM [1, 2]. The global prevalence of Pre-DM is increasing quickly [3, 4], and it is estimated that 587 million individuals will live with Pre-DM by 2045 [5], with an annual progression rate of 5–10% to T2DM [6]. Previous studies show that lifestyle modifications (i.e., diet and physical activity) can prevent or even revert the Pre-DM progression [6, 7].
In addition to medical treatments [8] and intensive training programs [9, 10], dietary modifications have been effective to attenuate the risk of Pre-DM progression to T2DM [11,12,13]. Dietary patterns like Mediterranean Diet (Med Diet) [14] affect the risk of Pre-DM [15, 16]. However, the effect of dietary intervention per se on the progression towards T2DM is still controversial [9]; a combined-approach (dietary intervention + lifestyle modifications) was reported to be successful to preventing or delaying the progression from Pre-DM to T2DM [17,18,19,20]. Observational studies also reported adherence to Med Diet [21, 22], higher intake of low-fat dairy [23], and a reduced dietary glycemic load (GL) [24] may reduce risk of progressing to T2DM among subjects with Pre-DM. The possible association of habitual dietary patterns with Pre-DM regression to normal glycemic regulation (NGR) is less investigated; some evidence indicate that lifestyle interventions might have the power to convert back the Pre-DM state to NGR [25, 26], especially in women [7].
Here, we aimed to examine the association of dietary patterns scores with Pre-DM progression and regression in an Iranian population. Using dietary patterns to assess the diet-disease relationship is a more comprehensive and holistic approach which gives a better view of interactive effects of dietary components [27].
Methods
Study population
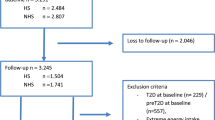
This longitudinal study was conducted in the framework of an ongoing community-based prospective study (the Tehran Lipid and Glucose Study, TLGS), which started in 1999 with 15,005 individuals, aged ≥ 3 years, to investigate and prevent non-communicable diseases [28]. For the current study, we recruited adult (age ≥ 21 y) Pre-DM men and women (n = 334) with completed data (usual diet, demographics, anthropometrics, and biochemical measurements) participated in the third phase of the TLGS (2006–2008) and followed up to the sixth phase of the TLGS (2015–2017). The study protocol was carried out according to the relevant guidelines expressed in the Declaration of Helsinki.
Written informed consent was obtained from all participants. The ethics research council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved the study protocol (Ethics code: IR.SBMU.ENDOCRINE.REC.1401.020).
Demographic, anthropometric and biochemical measurements
Details of data collection and measurements of the variables in the TLGS have been reported elsewhere [28]. In brief, anthropometric data, including body weight, height, and waist circumference (WC) were collected using standard methods. Body mass index (BMI) was calculated as weight (kg) divided by square of height in meters (m2). Systolic (SBP) and diastolic (DBP) blood pressures were measured using a standard mercury sphygmomanometer calibrated by the Institute of Standards and Industrial Research of Iran [29]. Blood pressure was measured twice on participants' right arm, after a 15-min rest in a sitting position, with at least a 30-s interval between two measurements. The mean of the two measurements was considered as the participant’s blood pressure.
Details of biochemical measurements in the TLGS samples have been described elsewhere [30]. In brief, measurements of fasting serum glucose (FSG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) levels were all done after a 12- to 14-h overnight fasting. The standard oral glucose tolerance test (OGTT) was performed for all adults (age ≥ 21 y) who were not on glucose-lowering medications.
The physical activity was assessed using the Modifiable Activity Questionnaire (MAQ); the frequency and time spent on light, moderate, hard, and very hard intensity activities according to the list of everyday activities of daily life over the past year were documented and physical activity levels expressed as metabolic equivalent hours per week (MET-hour/week) [31]. Reliability and validity of the Persian version of the MAQ have previously been investigated [32].
Dietary assessment and dietary pattern scores calculation
The usual dietary intakes of the participants over the previous year were assessed using a validated semi-quantitative 168-item Food Frequency Questionnaire (FFQ) (supplementary file 1). Details of dietary assessment in the TLGS were described elsewhere [33]. In brief, the frequency of food items consumed during the past year was asked daily, weekly, or monthly. Portion sizes of consumed foods reported in household measures were converted to the gram. Since the Iranian Food Composition Table is incomplete and has limited data on raw foods and beverages' nutrient content, the US Department of Agriculture Food Composition Table was used [34]. To remove confounding effect of under- or over-report of energy intakes on estimated-intakes of food groups and nutrients, a residual adjustment was performed using a regression model (with total caloric intake as the independent variable and food/nutrients intake as the dependent variable) [35].
The Dietary Approaches to Stop Hypertension (DASH) diet score was calculated based on the index developed by Fung et al. [36], comprised of 8 components (7 food groups, i.e., fruit + fruit juice, non-starchy vegetables, low-fat dairy products, whole grains, and nuts + seeds + legumes, and one nutrient, i.e., sodium). The DASH score was calculated for each participant; component score for fruit, vegetables, nuts and legumes, low-fat dairy products, and whole grains was the intakes’ quintile ranking (e.g., quintile 1 is assigned 1 point and quintile 5.5 points). For sodium, red and processed meats, and sweetened beverages, where their lower intakes was desired, the lowest quintile was given a score of 5 points and the highest quintile, 1 point. Finally, the component scores were summed up to obtain an overall DASH score ranging from 8 to 40.
Adherence to Med Diet was assessed using a modified version of Trichopoulou’s Mediterranean diet score (MDS) [37, 38], calculated based on nine items, i.e., dietary monounsaturated-to-saturated fats ratio (MUFAs to SFAs ratio), and intakes of whole grains, fruits, legumes, nuts, fish, processed and red meats, and vegetables; for religious reasons, alcohol was excluded from the final score in our study. For each of the components which were hypothesized to be beneficial to health, 1 point was given to a participant if intake was above the median, 0 otherwise. Scores for all eight items were hence summed up to calculate MDS (ranged from 0 to 8).
To obtain major dietary patterns among our population and estimate dietary pattern scores for study participants, principal component analysis (PCA, i.e., a commonly used method for deriving major dietary patterns in population based studies) was used with Varimax rotation based on 15 food items (i.e., whole grains, refined grains, legumes, red meats, fruits and dried fruits, vegetables, total dairy products, fish and poultry, salty snacks, sweets and candies, hydrogenated fats, tea and coffee, total fat and sodium intakes). Three factors were created regarding the scree plot and eigenvalues more than 1. To include in the dietary patterns, we selected the food items with factor loading ≥ 0.30. In our models, all food items contributed to the calculation of dietary pattern scores. To assess our sampling adequacy, we used the Kaiser-Mayer-Olkin statistic test which its result was 0.74 indicating a good appropriateness of factor analysis.
The PCA considers the correlations between food-groups intakes to identify underlying patterns in the data. PCA-derived dietary patterns were named using both quantitative and qualitative approaches [39]; in quantitative approach, the variable with the highest factor loading (e.g., fruits, vegetables, cereals, meat) or quantitative descriptions of dietary composition (e.g., high-fat or high-energy density) are considered, while in qualitative approach specific combinations of foods and/or descriptions of nutritional composition are considered [39]. Patterns that contained a variety of different foods or food groups that combined together in “more” and “less” healthy combinations are often given qualitative labels to denote healthfulness [39]. Accordingly, three following major dietary patterns were derived: 1) western-style dietary pattern: (high-fat/high-sodium/high-glycemic-index(GI) with a higher load of red meats, hydrogenated fats, sodium, and total fat intakes), 2) healthy pattern (with a higher load of whole grains, vegetables, and dairy products), and 3) processed-foods pattern (with a higher load of processed-meats, fast-foods, salty snakes, and sweets and candies). These dietary patterns explained 43.2% of the total variance in food intake (24.9, 9.6, and 8.7% of the patterns, respectively).
Factor scores of the participants were calculated using sum of multiplying the intake of the standardized food items by their respective factor loadings on each pattern.
Definition of terms
Participants were categorized into different groups of glycemic status as follows [40, 41]: normal glycemia [i.e., normal fasting glucose (NFG) and normal glucose tolerance (NGT)], as FSG < 100 and 2-h serum glucose (2 h-SG) < 140; Pre-DM, as having at least one of the Impaired Fasting Glucose (IFG) (100 ≤ FSG < 126 mg/dL) or Impaired Glucose Tolerance (IGT) (140 ≤ 2 h-SG < 200 mg/dL); T2DM as FSG ≥ 126 mg/dL or 2 h-SG ≥ 200 mg/dL, or using glucose-lowering medications. A positive family history of T2DM was defined as having at least one parent or sibling with T2DM.
The T2DM-risk score was calculated as follows: SBP (mm Hg) < 120 (0 point), 120 < SBP < 140 (3 point), SBP ≥ 140 (7 point); family history of T2DM (5 point); waist-to-height ratio (WHR): < 0.54 (0 point), 0.54–0.59 (6 point), ≥ 0.59 (11 point); TG-to-HDL-C ratio: < 3.5 (0 point), ≥ 3.5 (3 point); FSG: < 90 mg/dL (0 point), ~ 90–99 mg/dL (12 point), ~ 100–125 mg/dL (33 point) [42].
Statistical methods
Statistical analyses were conducted using the SPSS for Windows version 20 (SPSS Inc., Chicago, IL, USA). Baseline characteristics of the participants were compared across the groups using analysis of variance (ANOVA). The odds ratios (95% confidence intervals (CIs)) of Pre-DM regression to normal glycemia or progression to T2DM in relation to dietary pattern scores were estimated using multinomial logistic regression analysis. Potential covariates were selected based on both statistical and scientific evidence. A univariate analysis was performed for potential confounding variables, and those with PE < 0.2 were selected for the final multivariable model; PE (P-value for entry) determines which variables should be included in the multivariable model [43]. Finally, three logistic models, including crude model, adjusted-model 1 (adjusted for sex, age and T2DM-risk score), and adjusted-model 2 (additionally adjusted for body weight changes, smoking and physical activity) were conducted.
Results
The mean age of the study participants was 49.4 ± 12.8 y, and 51.5% were men. During a median of 8.9 years of follow-up (inter-quartile range: 7.0-9.6 years), 133 cases (39.8%) of T2DM were diagnosed, 68 participants (20.4%) remained in Pre-DM state, and 133 participants (39.8%) backed into normal glycemia. The baseline characteristics of the study participants are summarized in Table 1. Participants who reversed to the normal glycemic state were significantly younger than those who remained at Pre-DM and or developed T2DM. The participants who developed T2DM had significantly higher FSG and 2 h-SG at baseline compared to other groups. Compared to T2DM patients, participants who backed into normal glycemia also had lower BMI and TG-to-HDL-C ratio at baseline. Other variables, i.e., blood pressures, smoking status, and physical activity levels, were comparable.
The associations between dietary pattern scores and nutrient intakes are shown in Table 2. The Western-style dietary pattern was positively correlated with dietary fats and sodium, and negatively correlated with dietary fiber, protein and potassium intakes. Table 3 represents the odds ratio (95% CI) of Pre-DM regression to normal glycemia and progression to T2DM in relation to dietary pattern scores. Despite other patterns being not related to the chance of progression or regression of Pre-DM, the Western-style dietary pattern increased the risk of progressing to T2DM by 38% (OR = 1.36; 95% CI: 1.00 to 1.89, P = 0.050) independent of the well-known risk factors of T2DM.
Discussion
In this 9-year follow-up of subjects with Pre-DM, the Western-style dietary pattern characterized by high amounts of red meats, hydrogenated fats, sodium intake, and high-GI food, increased the risk of Pre-DM progressing to T2DM by 38%. However, it did not show any significant relationship with returning to NGR. Other dietary pattern scores, i.e., DASH-style pattern, MDS, healthy-style pattern, and processed-foods pattern showed no significant association with either regression or progression of Pre-DM.
Dietary factors affecting the risk of developing T2DM are well documented [44,45,46]. However, the association of diet with Pre-DM regression and progression has been less investigated, and their results are inconclusive. Primary evidence suggested that low-calorie diets decreased the incidence of T2DM in people with IGT over six years [20], and a 40-g increase in dietary fats increased the risk of progression from IGT to T2DM to sixfold [47]. Other observational studies have concluded that high-GL diet accelerates the progression of Pre-DM by 85% [24] and 64% [48]. All of the mentioned studies have assessed the components of our western-style pattern (fats and high-GL diets). Our results support the previous findings: there is a positive association between the western-style pattern and risk of Pre-DM progression. Furthermore, in the present study, western-style pattern negatively correlates with protein and calcium, which are the main content of dairy products. In accordance with our result, a recent cohort has shown that decreasing low-fat dairy product consumption increased the risk of progression from Pre-DM to T2DM after 9 years of follow-up [23]. Also, contrary to our hypothesis, the western-style pattern did not affect the chance of returning to NGR.
Some possible mechanisms might underlie between western-style pattern characterized by high fat, high sodium, and high-GI foods in our study and the risk of T2DM. First, sodium intake influences the renin-angiotensin system activity [49], insulin resistance [50], catecholamine levels and lipids [51]. Each of these factors could be a potential mediator in the development of T2DM. Moreover, excessive sodium intake activates the sympathetic nervous system, which causes an increase in the peripheral vascular resistance, then promoting hypertension [52]. Second, dysregulation of fat metabolism happens in the primary steps of insulin resistance development. Free fatty acids (FFA) are independent predictors of progression to T2DM. There is a general agreement that elevated FFA flux from an expanded adipose tissue to non-adipose tissues has a deleterious effect on insulin regulation of carbohydrate metabolism. It is an important cause of the hypertriglyceridemia of T2DM, aggravates cytosolic triglyceride accumulation in non-adipose tissues, and may have other direct adverse effects, such as effects on endothelium, myocardium, and cell proliferation [53]. Last but not least, higher GI diets are positively associated with HbA1c concentrations, a result of higher plasma glucose levels, and in turn associated with lower plasma adiponectin concentrations. Adiponectin is associated with a lower risk of T2DM by several proposed mechanisms, including increasing insulin sensitivity and anti-inflammatory effects [54].
In previous studies, high adherence to the Med Diet has been associated with a 44% to 85% reduction in the risk of progression from Pre-DM to T2DM [21, 22]. The null association observed in our study may be due to the different approaches to calculate MDS [55], and the diverse characteristics of study populations, as well as the different study designs and follow-up time.
The relationship between diet and returning to NGR is less investigated by observational studies. Clinical trials have consistently shown the possibility of returning to NGR through combined drug therapy and lifestyle intervention (both diet and physical activity) [7] although most of them have not focused on the diet alone [26]. In contrast to a recent cohort which has found a significant relationship between the lower dietary GL, but not GI, and increased incidence of NGR [24], we did not find any significant relationship with neither of the patterns, even after controlling for confounders.
This study had some strength. First, this study is a longitudinal cohort of Pre-DM subjects among an Asian population with a higher prevalence of T2DM. Second, we used a valid and reliable collection of the dietary data using a semi-quantitative 168-FFQ that reduced the possibility of reporting biases. Third, the well-known risk factors of T2DM were detected and controlled in our analyses, however, due to existing of other possible unknown risk factors, complete controlling for confounders was not possible in our models. Using PCA to derived dietary pattern scores, as a holistic analytical approach that reflects the complexity of the human diet and measures cumulative and interactive effects dietary exposures in a sample population, was also strength. Using multinomial logistic regression, enabled us to include the exposure and three-categorized outcome variables (i.e., normal glycemia, Pre-DM, and T2DM), improves the study power and provided us the chance of comparing Odds of the outcomes simultaneously. Finally, previous studies have defined Pre-DM by just one factor such as IGT, however our study included both IGT and IFG criteria.
Conclusions
In conclusion, we found a positive association between western-style dietary pattern (with higher load of red meats, hydrogenated fats, sodium and high-GI foods intakes) with progressive Pre-DM to T2DM over a 9-years of follow-up.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the ethical concerns but are available from the corresponding author on reasonable request.
Abbreviations
- Pre-DM:
-
Pre-diabetes
- T2DM:
-
Type2 Diabetes Mellitus
- CI:
-
Confidence Interval
- OR:
-
Odds Ratio
- Med Diet:
-
Mediterranean diet
- GL:
-
Glycemic Load
- GI:
-
Glycemic Index
- NGR:
-
Normal Glucose Regulation
- MDS:
-
Mediterranean Diet Score
- TLGS:
-
Tehran Lipid and Glucose Study
- WC:
-
Waist Circumference
- BMI:
-
Body Mass Index
- SBP:
-
Systolic Blood Pressure
- DBP:
-
Diastolic Blood Pressure
- FSG:
-
Fasting Serum Glucose
- 2 h-SG:
-
2-Hour Serum Glucose
- IFG:
-
Impaired Fasting Glucose
- IGT:
-
Impaired Glucose Tolerance
- NFG:
-
Normal Fasting Glucose
- NGT:
-
Normal Glucose Tolerance
- TG:
-
Triglyceride
- HDL-c:
-
High-Density Lipoprotein cholesterol
- OGTT:
-
Oral Glucose Tolerance Test
- MAQ:
-
Modifiable Activity Questionnaire
- FFQ:
-
Food Frequency Questionnaire
- DASH:
-
Dietary Approaches to Stop Hypertension
- MUFAs to SFAs ratio:
-
Dietary Monounsaturated-to-Saturated fats ratio
- PCA:
-
Principal Component Analysis
References
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes care. 2019;42(Suppl 1):S13-S28.
Xu XY, Leung AYM, Smith R, Wong JYH, Chau PH, Fong DYT. The relative risk of developing type 2 diabetes among individuals with prediabetes compared with individuals with normoglycaemia: Meta-analysis and meta-regression. J Adv Nurs. 2020;76(12):3329–45.
Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clinical diabetes and endocrinology. 2019;5:5.
Hadaegh F, Derakhshan A, Zafari N, Khalili D, Mirbolouk M, Saadat N, et al. Pre-diabetes tsunami: incidence rates and risk factors of pre-diabetes and its different phenotypes over 9 years of follow-up. Diabetic medicine : a journal of the British Diabetic Association. 2017;34(1):69–78.
International diabetes federation: IDF diabetes atlas. Eighth Edition 2017. https://diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf. Accessed 15 June 2022.
Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. The Lancet. 2012;379(9833):2279–90.
Liu Y, Guo H, Wang Q, Chen J, Xuan Y, Xu J, et al. Short-term effects of lifestyle intervention in the reversion to normoglycemia in people with prediabetes. Prim Care Diabetes. 2022;16(1):168–72.
Zhang Y, Fu Y, Mu YM, Huang Y, Xuan J. Network Meta-analysis of the Therapeutic Effects of Hypoglycemic Drugs and Intensive Lifestyle Modification on Impaired Glucose Tolerance. Clin Ther. 2021;43(9):1524–56.
Hemmingsen B, Gimenez-Perez G, Mauricio D, Roque IFM, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. The Cochrane database of systematic reviews. 2017;12:CD003054.
Slentz CA, Bateman LA, Willis LH, Granville EO, Piner LW, Samsa GP, et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia. 2016;59(10):2088–98.
Toi PL, Anothaisintawee T, Chaikledkaew U, Briones JR, Reutrakul S, Thakkinstian A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients. 2020;12(9).
Galaviz KI, Weber MB, Suvada KB, Gujral UP, Wei J, Merchant R, et al. Interventions for Reversing Prediabetes: A Systematic Review and Meta-Analysis. Am J Prev Med. 2022;62(4):614–25.
Lindstrom J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinanen-Kiukaanniemi S, et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013;56(2):284–93.
Viscogliosi G, Cipriani E, Liguori ML, Marigliano B, Saliola M, Ettorre E, et al. Mediterranean dietary pattern adherence: associations with prediabetes, metabolic syndrome, and related microinflammation. Metab Syndr Relat Disord. 2013;11(3):210–6.
Shen X-M, Huang Y-Q, Zhang X-Y, Tong X-Q, Zheng P-F, Shu L. Association between dietary patterns and prediabetes risk in a middle-aged Chinese population. Nutrition J. 2020;19(1):1.
Pestoni G, Riedl A, Breuninger TA, Wawro N, Krieger JP, Meisinger C, et al. Association between dietary patterns and prediabetes, undetected diabetes or clinically diagnosed diabetes: results from the KORA FF4 study. Eur J Nutr. 2021;60(5):2331–41.
Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019;7(6):452–61.
Kerrison G, Gillis RB, Jiwani SI, Alzahrani Q, Kok S, Harding SE, et al. The Effectiveness of Lifestyle Adaptation for the Prevention of Prediabetes in Adults: A Systematic Review. J Diabetes Res. 2017;2017:8493145.
Yoon U, Kwok LL, Magkidis A. Efficacy of lifestyle interventions in reducing diabetes incidence in patients with impaired glucose tolerance: a systematic review of randomized controlled trials. Metabolism: clinical and experimental. 2013;62(2):303–14.
Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes care. 1997;20(4):537–44.
Cea‐Soriano L, Pulido J, Franch‐Nadal J, Santos JM, Mata‐Cases M, Díez‐Espino J, et al. Mediterranean diet and diabetes risk in a cohort study of individuals with prediabetes: propensity score analyses. Diabet Med. 2021;00(e14768).
Filippatos TD, Panagiotakos DB, Georgousopoulou EN, Pitaraki E, Kouli GM, Chrysohoou C, et al. Mediterranean Diet and 10-year (2002–2012) Incidence of Diabetes and Cardiovascular Disease in Participants with Prediabetes: The ATTICA study. The review of diabetic studies : RDS. 2016;13(4):226–35.
Yuzbashian E, Asghari G, Mirmiran P, Chan CB, Azizi F. Changes in dairy product consumption and subsequent type 2 diabetes among individuals with prediabetes: Tehran Lipid and Glucose Study. Nutrition Journal. 2021;20(1).
He F. Diets with a low glycaemic load have favourable effects on prediabetes progression and regression: a prospective cohort study. J Hum Nutr Diet. 2018;31(3):292–300.
Dagogo-Jack S, Umekwe N, Brewer AA, Owei I, Mupparaju V, Rosenthal R, et al. Outcome of lifestyle intervention in relation to duration of pre-diabetes: the Pathobiology and Reversibility of Prediabetes in a Biracial Cohort (PROP-ABC) study. BMJ open diabetes research & care. 2022;10(2).
Sallar A, Dagogo-Jack S. Regression from prediabetes to normal glucose regulation: State of the science. Exp Biol Med. 2020;245(10):889–96.
Agnoli C, Pounis G, Krogh V. Chapter 4 - Dietary Pattern Analysis. In: Pounis G, editor. Analysis in Nutrition Research: Academic Press; 2019. p. 75–101.
Azizi F, Zadeh-Vakili A, Takyar M. Review of Rationale, Design, and Initial Findings: Tehran Lipid and Glucose Study. International journal of endocrinology and metabolism. 2018;16(4 Suppl): e84777.
Askari S, Asghari G, Ghanbarian A, Khazan M, Alamdari S, Azizi F. Seasonal variations of blood pressure in adults: Tehran lipid and glucose study. Arch Iran Med. 2014;17(6):441–3.
Tohidi M, Ghasemi A, Hadaegh F, Derakhshan A, Chary A, Azizi F. Age- and sex-specific reference values for fasting serum insulin levels and insulin resistance/sensitivity indices in healthy Iranian adults: Tehran Lipid and Glucose Study. Clin Biochem. 2014;47(6):432–8.
Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80.
Momenan AA, Delshad M, Sarbazi N, Rezaei Ghaleh N, Ghanbarian A, Azizi F. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012;15(5):279–82.
Hosseini-Esfahani F, Hosseinpour-Niazi S, Asghari G, Bahadoran Z, Moslehi N, Golzarand M, et al. Nutrition and Cardio-Metabolic Risk Factors: Findings from 20 Years of the Tehran Lipid and Glucose Study. Int J Endocrinol Metab. 2018;16(4 (Suppl)):e84772.
Bodner-Montville, Janice, Jaspreet KC Ahuja, Linda A. Ingwersen, Etta Susanne Haggerty, Cecilia Wilkinson Enns, and Betty P. Perloff. USDA food and nutrient database for dietary studies: released on the web. J Food Compost Anal. 2006;19:S100–7.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. The American journal of clinical nutrition. 1997;65(4 Suppl):1220S-8S; discussion 9S-31S.
Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20.
Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, et al. Mediterranean diet and cognitive function in older age. Epidemiology. 2013;24(4):490–9.
Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, et al. Diet and overall survival in elderly people. BMJ (Clinical research ed). 1995;311(7018):1457–60.
Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62(5):177–203.
Diagnosis TECot, Mellitus* CoD. Follow-up Report on the Diagnosis of Diabetes Mellitus. Diabetes Care. 2003;26(11):3160–7.
Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired Fasting Glucose and Impaired Glucose Tolerance. Implications Care. 2007;30(3):753–9.
Bozorgmanesh M, Hadaegh F, Ghaffari S, Harati H, Azizi F. A simple risk score effectively predicted type 2 diabetes in Iranian adult population: population-based cohort study. Eur J Public Health. 2011;21(5):554–9.
Hosmer DW, Lemeshow S. Applied logistic regression. 2nd Ed. New York: Wiley; 2000.
Martín-Peláez S, Fito M, Castaner O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review Nutrients. 2020;12(8):2236.
Jannasch F, Kröger J. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. 2017;147(6):1174–82.
Breuninger TA, Riedl A, Wawro N, Rathmann W, Strauch K, Quante A, et al. Differential associations between diet and prediabetes or diabetes in the KORA FF4 study. Journal of nutritional science. 2018;7: e34.
Marshall JA, Hoag S, Shetterly S, Hamman RF. Dietary Fat Predicts Conversion From Impaired Glucose Tolerance to NIPPM. Diabetes Care. 1994;17(1):50–6.
He FY, Chen CG, Lin DZ, Lin XH, Qi YQ, Yan L. A greater glycemic load reduction was associated with a lower diabetes risk in pre-diabetic patients who consume a high glycemic load diet. Nutrition research (New York, NY). 2018;53:77–84.
Ribeiro-Oliveira A, Jr., Nogueira AI, Pereira RM, Boas WWV, Dos Santos RAS, Simões e Silva AC. The renin-angiotensin system and diabetes: an update. Vasc Health Risk Manag. 2008;4(4):787–803.
Petrie JR, Morris AD, Minamisawa K, Hilditch TE, Elliott HL, Small M, et al. Dietary sodium restriction impairs insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(5):1552–7.
Graudal NA, Galløe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA. 1998;279(17):1383–91.
Feldstein C. Salt intake, hypertension and diabetes mellitus. J Hum Hypertens. 2002;16:S48–51.
Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23(2):201–29.
AlEssa HB, Ley SH, Rosner B. High Fiber and Low Starch Intakes Are Associated with Circulating Intermediate Biomarkers of Type 2 Diabetes among Women. 2016;146(2):306–17.
Aoun C, Papazian T, Helou K, El Osta N, Khabbaz LR. Comparison of five international indices of adherence to the Mediterranean diet among healthy adults: similarities and differences. Nutr Res Pract. 2019;13(4):333–43.
Acknowledgements
We thank the Tehran Lipid and Glucose Study participants and the field investigators of the Tehran Lipid and Glucose Study for their cooperation and assistance in physical examinations, biochemical evaluation and database management.
Funding
This work was not supported by any funding agency.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. P.M designed the study. Sh.H, Z.B and F.A analyzed the data from TLGS population, Sh.H and P.M wrote the manuscript, Z.B revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was carried out according to the relevant guidelines expressed in the Declaration of Helsinki. Written informed consent was obtained from all participants. The ethics research council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved the study protocol (Ethics code: IR.SBMU.ENDOCRINE.REC.1401.020).
Consent for publication
Not applicable.
Competing interests
Parvin Mirmiran, Shabnam Hosseini, and Fereidoun Azizi declare that they have no competing interests. Dr. Zahra Bahadoran is a member of the editorial board of BMC Endocrine Disorders.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mirmiran, P., Hosseini, S., Bahadoran, Z. et al. Dietary pattern scores in relation to pre-diabetes regression to normal glycemia or progression to type 2 diabetes: a 9-year follow-up. BMC Endocr Disord 23, 20 (2023). https://doi.org/10.1186/s12902-023-01275-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01275-9