Abstract
Background
Dimethyl fumarate (DMF) is an effective drug for multiple sclerosis and can improve the cognitive dysfunction caused by streptozotocin, but the effect on cognitive dysfunction caused by hypothyroidism is unclear.
Methods
After the hypothyroidism rat model induced by propylthiouracil, we gave rats 25 mg/kg DMF by gavage. The body weight during model building and administration was recorded. The levels of T4 and T3 in serum were detected by an automatic biochemical analyzer. Morris water maze test was used to detect the effect of DMF on cognitive learning ability. The effect of DMF on Nissl bodies in the brain tissue was evaluated by Nissl staining. The mRNA and protein levels of BDNF in brain tissue were detected by quantitative reverse transcription-polymerase chain reaction and Western blot. The degrees of p-AKT/AKT and p-CREB/CREB in brain tissue were detected by Western blot.
Results
After DMF treatment, the body weight of hypothyroid rats recovered, and the levels of T3 and T4 in the serum were ameliorated. DMF also reduced the escape latency and distance traveled, and increased the swim speed. The number of Nissl bodies and expression of BDNF, p-AKT/AKT, and p-CREB/CREB in the brain tissue were increased after DMF treatment.
Conclusion
DMF improved the cognitive dysfunction of hypothyroid rats by increasing the level of BDNF in the brain tissue of hypothyroid rats.
Similar content being viewed by others
Background
Hypothyroidism is a syndrome caused by insufficient thyroid hormone secretion [1]. Thyroid hormones have a vital impact on the development and normal work of the brain throughout life [2]. At the same time, thyroid hormones play an important role in cognition [3]. Clinical psychology studies have shown that the attention, mobility, memory and spatial ability of patients with hypothyroidism are significantly reduced, while the index of depression and anxiety is increased [4]. In addition, animal behavior studies have shown that the spatial memory ability of hypothyroid animals is impaired [5]. Although various degrees of cognitive impairment caused by hypothyroidism have been recognized by academia, the specific mechanism is still unclear.
Studies have suggested that cognitive dysfunction caused by hypothyroidism may be related to the down-regulation of Brain-derived Neurotrophic Factor (BDNF) [6, 7]. BDNF is a member of the neurotrophic factor, and it has a high content in the hippocampus and prefrontal cortex. BDNF can not only promote the growth and differentiation of neurons but also participate in the regulation of synaptic transmission and synaptic plasticity [8]. Animal experiments have shown that deleting BDNF in the broad forebrain regions of rats will cause damage to the hippocampus-dependent learning ability of rats [9]. And artificially increasing the level of BDNF can improve the spatial memory ability of rats [10, 11]. BDNF is also important for human brain function. Michael F Egan et al. found that BDNF val66met polymorphism is associated with human hippocampal-dependent memory impairment [12].
Fumaric acid esters (FAEs) are compounds that have antioxidant and anti-inflammatory effects in a variety of tissues and cells, and dimethyl fumarate (DMF) is the most biologically active compound in FAE [13]. DMF was used in the treatment of psoriasis [14]. In addition, DMF can also be used to treat multiple sclerosis [15]. DMF was first applied to the study of the nervous system. Isabel Lastres-Becker et al. reported the improvement of DMF on dyskinesia of neurodegenerative disease Parkinson’s mice [16]. Ludwig Kappos and his colleagues found that DMF inhibits the oxidative damage of nerve cells by activating the Nrf2 pathway, and maintains the integrity of nerve cell myelin [17]. It is worth noting that studies have shown that DMF reduces secondary degeneration after spinal cord injury by increasing the expression of BDNF [18]. In addition, DMF also has a certain alleviating effect on the spatial memory impairment of Alzheimer’s rats [19]. However, there is currently a lack of studies evaluating DMF on cognitive function after hypothyroidism. Considering the role of BDNF in hypothyroidism, we speculate that DMF may play a role in hypothyroidism by affecting the expression of BDNF.
Therefore, we constructed a rat model of hypothyroidism induced by propylthiouracil (PTU) to observe the effects of DMF on the behavior of hypothyroidism rats in the Morris water maze, serum thyroid hormone levels and BDNF, p-AKT/AKT, p-CREB/CREB expression in the hippocampus, in order to clarify the effect of DMF on the learning and memory ability of hypothyroid rats and explore its possible mechanism.
Methods
Animals
Thirty male Sprague–Dawley rats (8 weeks old, 215 ± 20 g) were purchased from Shanghai Jihui Laboratory Animal Care Co., Ltd. (SCXK (Hu) 2017–0012). All rats were kept in an environment where the ambient temperature is maintained at 22–23 °C, the relative humidity is 45–50%, and the light cycle is 12/12 hours. All rats were fed adaptively for 1 week, and they were free to eat and drink. Those with normal drinking and eating were included in the experiment.
The construction of the PTU rat model referred to the previous literature [20]. Rats were randomly divided into the control group (n = 10) and the model group (n = 20). The rats in the control group drank water normally, while the rats in the PTU group drank tap water containing 0.05% PTU [21] (IP0420, Solarbio, China). After 28 days, the rats in the model group were randomly divided into the PTU group (n = 10) and the PTU + DMF25 group (n = 10). The rats in the control group and the PTU group were given saline once a day, while the rats in the PTU + DMF25 group were given 25 mg/kg DMF (ID0320, Solarbio, China) once a day for 14 days [22]. The body weight of each group of rats was measured weekly.
Morris water maze test
The learning and memory abilities of rats are tested through the Morris water maze test [23]. The diameter of the maze was 1.6 m and the height was 50 cm. The pool water was dyed black with food coloring. The water depth was 30 cm, and the water temperature was 22–23 °C. The experiment was divided into two parts: positioning navigation and space search. In the positioning navigation experiment, the rats received 4 days of training, 4 times a day. The specific training was as follows: each time before entering the water, put the rat on the underwater platform to adapt for 30 seconds, record the swimming distance of the rat from the four quadrants and different entry points to the platform within 60 seconds, and the average of the 4 results was the final grade. On the fifth day, the space search experiment was carried out, the platform was removed, and the rats were placed into the water from the entry point in the opposite quadrant of the platform, facing the wall of the pool, and the swimming trajectory of the rats within 60 seconds was recorded and analyzed.
Specimen collection and preparation
After the water maze experiment, the rats were fasted for 12 hours but were allowed to drink water freely. After the rats were anesthetized, blood was taken from the abdominal aorta, and the collected blood was centrifuged to obtain serum. The content of T3 and T4 in serum was measured by an automatic biochemical analyzer (C1600, Abbott, USA) within 48 hours. The rats were euthanized with an overdose of sodium pentobarbital, the brain tissues of the rats were separated, a part of the brain tissue was fixed in 4% paraformaldehyde, paraffin-embedded and sectioned, and the rest was stored in a refrigerator at − 80 °C for Western blot and quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Nissl staining
After the paraffin sections (4 μm) were conventionally deparaffinized and hydrated, they were reacted with Nissl staining solution (G1036, Wuhan Google Biotechnology Co., Ltd., China) in an oven at 60 °C for 20 min. After washing the sections with distilled water, the sections were dried in an oven at 60 °C. Finally, the sections were routinely dehydrated, transparent, and sealed [24]. The number of Nissl bodies at the same site in each group of rats under a field of view was counted. And the average thickness of the granular layer was measured at five random positions in CA1 of each slice in each group under the same field of view [25] (Per × 400 field).
qRT-PCR
Total RNA from rat brain tissue was extracted by a total RNA extraction kit (R1200, Solarbio, China). Total RNA was reverse transcribed into cDNA with the help of a reverse transcription kit (CW2569, cwbiotech, China). Then the SYBR Green qPCR kit (CW2601, cwbiotech, China) was used for qPCR. β-actin was employed as an internal control. The primers were listed as follows: BDNF, forward: 5′- GGCAGGCTTTGATGAGACCG-3′ and reverse: 5′-TCACCTGGTGGAACTCAGGGT-3′; β-actin, forward: 5′-AACCTTCTTGCAGCTCCTCC-3′ and reverse: 5′-TACCCACCATCACACCCTGG-3′. Relative expressions of BDNF were analyzed by a Real-Time PCR Detection system (CFX96, Bio-rad, USA) with the 2-ΔΔCt method [26].
Western blot
Western blot was performed as previously described [27]. The protein sample was collected from rat brain tissue by RIPA lysate (P0013D, Beyotime, China), PMSF (ST506, Beyotime, China) and protease inhibitors (60,237, Beyotime, China). Then the protein was sequentially quantified by the BCA kit (pc0020, Solarbio, China). The protein was separated with 10% separating gel, and then the protein was transferred to the PVDF membrane (10,600,023, GE Healthcare Life, USA). Before incubating the primary antibody, the membrane needed to be blocked with 5% skimmed milk. Primary antibodies include anti-BDNF (ab108319, Abcam, UK), p-AKT (20 t9742, Affinity, China), AKT (59z1942, Affinity, China), CREB (36v1551, Affinity, China), p-CREB (58y21722, Affinity, China) antibody and anti-β-actin antibody (ab8226, Abcam, UK). After incubating with the primary antibody overnight at 4 °C, the membrane reacted with the secondary antibody goat anti-rabbit (ab205718, Abcam, UK) or goat anti-mouse (ab6789, Abcam, UK) at room temperature for 2 hours. The membrane was developed on a chemiluminescence instrument (610020-9Q, Qinxiang, China) with an ECL luminescence reagent (C510045, Sangon, China). β-actin was used as the internal control.
Statistical analysis
Data were analyzed by SPSS 16.0 (SPSS, Chicago, USA) and represented as mean ± standard deviation. One-way analysis of variance was used for measurement data among multiple groups, and Tukey test was used for comparison between groups. Kruskal-Wallis H test was used for results of uneven variance. P < 0.05 was accepted to be statistically significant.
Results
Effect of DMF treatment on body weight and thyroid level in rats with hypothyroidism
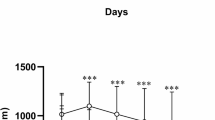
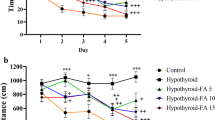
After the SD rats were experimentally fed for 1 week, the rats were divided into groups. At this time, there was no significant difference in the body weight of rats in each group (Fig. 1). During the 4 weeks when the rats were treated with 0.05% PTU, the weight of the rats was significantly lower than that of the control rats (Fig. 1, P < 0.01). However, after DMF treatment, the trend of weight loss in PTU group rats was reversed (Fig. 1, P < 0.05). In addition, the levels of T3 and T4 in the serum of rats with PTU-induced hypothyroidism were also significantly lower than those in the control group (Fig. 2, P < 0.01). However, the treatment of DMF restored the levels of T3 and T4 in the serum of rats in the PTU group to normal (Fig. 2, P < 0.01).
Effect of DMF on the learning ability of hypothyroid rats
We evaluated the effect of DMF on the learning and memory abilities of hypothyroid rats through the Morris water maze. As shown in Fig. 3, the escape latency of rats in the PTU group was much higher than that of the control group, while DMF treatment can effectively reduce the escape latency of hypothyroid rats to a normal level (P < 0.01). Not only that, compared with the control group, rats in the PTU group swam longer and had a slower swimming speed, but these problems can be improved by DMF treatment (P < 0.01).
DMF increased the number of Nissl bodies in the hippocampal CA1 area of hypothyroidism rats
Nissl body is a small triangular or elliptical mass distributed in the cytoplasm of nerve cells, which can be stained blue-purple by Nissl staining solution. The disappearance of the Nissl body is an important indicator of nerve cell damage. From Fig. 4, we can see that the Nissl body in the control group is very complete and the neurons were closely aligned, while the number of Nissl bodies in the PTU group is significantly less than that in the control group (P < 0.01). Compared with the PTU group, the number of Nissl bodies in the PTU + DMF25 group was increased (P < 0.01), and the neurons were arranged relatively neat and close. Besides, the granular layer was significantly thickened in the PTU group than it was in the control group (P < 0.05). After DMF treatment, the thickness of the granular layer was reduced but not significantly different compared to the PTU group (P > 0.05).
Effect of DMF on hippocampal neurons of rats (n = 5). A Representative images showing Nissl bodies in the hippocampal CA1 (× 100, Scale bar = 100 mm; × 400, Scale bar = 50 mm.); B Quantitation of pyramidal cells in the CA1 hippocampal region (\(\overline{x}\) ±s). The number of Nissl bodies at the same site in each group of rats under a field of view was counted; C The thickness of granular layer of CA1 region of the hippocampus; The average thickness of granular layer was measured at five random positions in CA1 of each slice in each group under the same field of view. **P < 0.01 compared with the control group; ##P < 0.01 compared with the PTU group
DMF increased the mRNA and protein level of BDNF in the hippocampal CA1 area of hypothyroid rats
By detecting the expression of BDNF mRNA and protein in the brain tissue of each group of rats (Figs. 5, 6A, and B P< 0.01), we found that PTU-induced hypothyroidism rats express less BDNF in the brain tissue (P < 0.01). But the decrease of BDNF expression in rat brain tissue caused by PTU can be reversed by DMF treatment (P < 0.05, P < 0.01).
Effect of DMF on BDNF, p-AKT/AKT, p-CREB/CREB protein expression levels in hippocampal CA1 region of hypothyroidism rats (\(\overline{x}\) ±s, n = 3). A The protein expression of BDNF; B the relative expression of BDNF protein; C the protein expression of p-AKT/AKT and p-CREB/CREB; D the relative expression of p-AKT/AKT; E the relative expression of p-CREB/CREB. **P < 0.01 compared with the control group. ##P < 0.01 compared with the PTU group. Corresponding uncropped full-length gels and blot can be saw in the supplementary information
DMF increased the levels of p-AKT/AKT and p-CREB/CREB in the hippocampal CA1 area of hypothyroid rats.
Western blot was used to measure the levels of p-AKT/AKT and p-CREB/CREB (Fig. 6C). In the PTU group. The level of p-AKT/AKT was not significantly different compared to the control group (P > 0.05), and DEM treatment was significantly upregulated compared to the PTU group (P < 0.01) (Fig. 6D). The degree of p-CREB/CREB was markedly decreased in the PTU group compared to the control group (P < 0.01), while it was notably improved in the PTU + DEM25 group (P < 0.01) (Fig. 6E).
Discussion
Thyroid hormones are essential for the normal development of the mammalian brain [28]. Mounting studies have shown that thyroid hormone deficiency during brain development can lead to abnormal brain structure and dysfunction, which seriously affects learning and memory functions [29, 30]. At present, the treatment of hypothyroidism mostly uses thyroid hormone replacement therapy [31]. Thyroid hormones mainly include T3, T4, and TSH. Clinically, hypothyroidism is usually diagnosed by detecting the levels of T3 and T4. The body weight, serum T3 and T4 levels decreased significantly in PTU-induced rats, and after DMF treatment, they were reversed. It suggested that DMF can improve hypothyroidism. Graciela Freitas Zarbato et al. found that the anti-inflammatory and antioxidant effects of DMF were exerted in experimental sepsis rats. In addition, they also found that DMF can improve cognitive impairment after bacterial sepsis [32]. Coincidentally, Sofia P das Neves and others also found that DMF can enhance the cognitive ability of mice with experimental autoimmune encephalomyelitis [33]. Therefore, our study used the classic Morris water maze experiment to evaluate the cognitive function of rats in each group, and found that DMF has the same alleviating effect on the cognitive impairment of PTU-induced hypothyroid rats. Besides, we found through Nissl staining that DMF can significantly improve the pathological morphology of neurons in the CA1 region of the hippocampus of hypothyroid rats. Similar to our conclusion, Xiaowen Hou et al. also found that DMF has a repairing effect on neuronal damage in the hippocampus CA1 area of rats with middle cerebral artery occlusion [34].
BDNF plays an important role in the survival, differentiation, migration of neurons, the development of axons and dendrites of new neurons, and the formation of synapses, in particular, it shows advantages for protecting memory functions [35]. When simulating learning-related signals, the expression of BDNF in the pre- and post-synaptic membranes will increase, which means that BDNF is one of the key proteins in learning and memory [36]. And BDNF plays a neuroprotective and growth-promoting role in damaged neuronal [37]. Wu’s study suggested that BDNF could inhibit autophagy to play a neuroprotection function [38]. Scientists working on neuronal damage in intracerebral hemorrhage found the TrkB/Akt signaling was important in neuronal protection [39]. Moreover, Liu’s team studied n-3 docosapentaenoic acid’s neuroprotection and found that it could activate BDNF/TrkB-PI3K/AKT signaling to protect neurons from neuroinflammation [40].
At present, the research on the regulation of cognitive function by DMF has mostly focused on the Nrf2 pathway and other oxidative stress pathways [22, 32, 41]. This is because neurons are extremely sensitive to the damage of reactive oxygen species. When reactive oxygen species are generated excessively, the oxidative stress response is enhanced, which can damage the nucleic acids, proteins, and lipids on the inner membrane of neuronal mitochondria, causing cell damage or death, and causing cognition disfunction [42, 43]. However, our study found that DMF treatment was able to promote the expression level of BDNF in the hippocampus of hypothyroid rats, which may be one of the biological mechanisms by which DMF improves memory function. In the study of Mohammad Saied Salehi and others, DMF can promote the expression of BDNF in epidermal neural crest stem cells [44]. The regulatory effect of DMF on BDNF has also been confirmed to improve the depressive behavior of rats and the secondary degeneration after spinal cord injury [18, 45]. In addition, a study found that DMF can enhance p-Akt and its downstream targets CREB and BDNF that they are neuroprotective [46]. In our study, DMF treatment can increase the levels of p-AKT, p-CREB and BDNF in the brain tissue of hypothyroid rats, which suggested that the regulation of DMF on p-AKT/p-CREB/BDNF can also improve the cognitive dysfunction of hypothyroid rats. Pallavi Mishra’s team found that oral T4 significantly increased p-Akt/Akt levels in rat hearts [47]. It suggested that the improvement of cognitive dysfunction by DMF might be associated with the up-regulation of T4, which warrants further exploration in future studies. Certainly, this study investigated the effects of DMF on improving memory ability and neuronal damage in hypothyroid rats, and made a preliminary exploration of its biological mechanism, about the biological action mechanism of DMF on promoting BDNF secretion will be deeply studied in the future.
Conclusion
In general, we constructed a hypothyroid rat model, which proved that DMF can advance the levels of T3 and T4 in serum, improve cognitive behavior of hypothyroid rats to a certain extent, ameliorate hippocampal neuronal injury, raise the levels of BDNF, p-AKT/AKT, and p-CREB/CREB protein. However, we have only initially studied the effect of DMF on the expression of BDNF, and the regulation of its specific signaling pathway has not yet been clarified.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- BDNF:
-
Brain-derived Neurotrophic Factor
- FAEs:
-
Fumaric acid esters
- DMF:
-
dimethyl fumarate
- PTU:
-
propylthiouracil
References
Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390(10101):1550–62.
Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123–40.
Ritchie M, Yeap BB. Thyroid hormone: influences on mood and cognition in adults. Maturitas. 2015;81(2):266–75.
Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuro-Psychopharmacol Biol Psychiatry. 1997;21(6):925–35.
Zhang F, Chen J, Lin X, et al. Subclinical hypothyroidism in pregnant rats impaired learning and memory of their offspring by promoting the p75(NTR) signal pathway. Endocr Connect. 2018;7(5):688–97.
Chakraborty G, Magagna-Poveda A, Parratt C, et al. Reduced hippocampal brain-derived neurotrophic factor (BDNF) in neonatal rats after prenatal exposure to propylthiouracil (PTU). Endocrinology. 2012;153(3):1311–6.
Cortés C, Eugenin E, Aliaga E, et al. Hypothyroidism in the adult rat causes incremental changes in brain-derived neurotrophic factor, neuronal and astrocyte apoptosis, gliosis, and deterioration of postsynaptic density. Thyroid. 2012;22(9):951–63.
Song M, Martinowich K, Lee FS. BDNF at the synapse: why location matters. Mol Psychiatry. 2017;22(10):1370–5.
Monteggia LM, Barrot M, Powell CM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101(29):10827–32.
Jakubowska-Doğru E, Gümüşbaş U. Chronic intracerebroventricular NGF administration improves working memory in young adult memory deficient rats. Neurosci Lett. 2005;382(1–2):45–50.
Pizzo DP, Thal LJ. Intraparenchymal nerve growth factor improves behavioral deficits while minimizing the adverse effects of intracerebroventricular delivery. Neuroscience. 2004;124(4):743–55.
Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69.
Wollina U. Fumaric acid esters in dermatology. Indian Dermatol Online J. 2011;2(2):111–9.
Blair HA. Dimethyl Fumarate: a review in moderate to severe plaque psoriasis. Drugs. 2018;78(1):123–30.
Gold R, Linker RA, Stangel M. Fumaric acid and its esters: an emerging treatment for multiple sclerosis with antioxidative mechanism of action. Clin Immunol. 2012;142(1):44–8.
Lastres-Becker I, García-Yagüe AJ, Scannevin RH, et al. Repurposing the NRF2 activator dimethyl Fumarate as therapy against Synucleinopathy in Parkinson's disease. Antioxid Redox Signal. 2016;25(2):61–77.
Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372(9648):1463–72.
Cordaro M, Casili G, Paterniti I, et al. Fumaric acid esters attenuate secondary degeneration after spinal cord injury. J Neurotrauma. 2017;34(21):3027–40.
Majkutewicz I, Kurowska E, Podlacha M, et al. Dimethyl fumarate attenuates intracerebroventricular streptozotocin-induced spatial memory impairment and hippocampal neurodegeneration in rats. Behav Brain Res. 2016;308:24–37.
Sun J, Hui C, Xia T, et al. Effect of hypothyroidism on the hypothalamic-pituitary-ovarian axis and reproductive function of pregnant rats. BMC Endocr Disord. 2018;18(1):30.
Branvold DJ, Allred DR, Beckstead DJ, et al. Thyroid hormone effects on LKB1, MO25, phospho-AMPK, phospho-CREB, and PGC-1alpha in rat muscle. J Appl Physiol (1985). 2008;105(4):1218–1227.
Liu Y, Qiu J, Wang Z, et al. Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of Keap1-Nrf2-ARE system. J Neurosurg. 2015;123(4):915–23.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60.
Shan Y, Sun S, Yang F, et al. Dexmedetomidine protects the developing rat brain against the neurotoxicity wrought by sevoflurane: role of autophagy and Drp1-Bax signaling. Drug Des Devel Ther. 2018;12:3617–24.
Wang Z, Zhou F, Dou Y, et al. Melatonin alleviates Intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl Stroke Res. 2018;9(1):74–91.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Alegria-Schaffer A, Lodge A, Vattem K. Chapter 33 Performing and Optimizing Western Blots with an Emphasis on Chemiluminescent Detection. In: Guide to Protein Purification. 2nd ed; 2009. p. 573–99.
Dussault JH, Ruel J. Thyroid hormones and brain development. Annu Rev Physiol. 1987;49:321–34.
Liu YY, Brent GA. Thyroid hormone and the brain: mechanisms of action in development and role in protection and promotion of recovery after brain injury. Pharmacol Ther. 2018;186:176–85.
Prezioso G, Giannini C, Chiarelli F. Effect of thyroid hormones on neurons and neurodevelopment. Horm Res Paediatr. 2018;90(2):73–81.
Sapin R, Schlienger JL. Thyroxine (T4) and tri-iodothyronine (T3) determinations: techniques and value in the assessment of thyroid function. J Ann Biol Clin (Paris). 2003;61(4):411–20.
Zarbato GF, de Souza Goldim MP, Giustina AD, et al. Dimethyl Fumarate limits Neuroinflammation and oxidative stress and improves cognitive impairment after Polymicrobial Sepsis. Neurotox Res. 2018;34(3):418–30.
das Neves S.P., G. Santos, C. Barros, et al., Enhanced cognitive performance in experimental autoimmune encephalomyelitis mice treated with dimethyl fumarate after the appearance of disease symptoms. J Neuroimmunol. 2020;340:577163.
Hou X, Xu H, Chen W, et al. Neuroprotective effect of dimethyl fumarate on cognitive impairment induced by ischemic stroke. Ann Transl Med. 2020;8(6):375.
Konishi K, Cherkerzian S, Aroner S, et al. Impact of BDNF and sex on maintaining intact memory function in early midlife. Neurobiol Aging. 2020;88:137–49.
Geist PA, Dulka BN, Barnes A, et al. BNDF heterozygosity is associated with memory deficits and alterations in cortical and hippocampal EEG power. Behav Brain Res. 2017;332:154–63.
Keefe KM, Sheikh IS, Smith GM. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int J Mol Sci. 2017;18(3):548.
Wu CL, Chen CH, Hwang CS, et al. Roles of p62 in BDNF-dependent autophagy suppression and neuroprotection against mitochondrial dysfunction in rat cortical neurons. J Neurochem. 2017;140(6):845–61.
Wu CH, Chen CC, Hung TH, et al. Activation of TrkB/Akt signaling by a TrkB receptor agonist improves long-term histological and functional outcomes in experimental intracerebral hemorrhage. J Biomed Sci. 2019;26(1):53.
Liu B, Zhang Y, Yang Z, et al. ω-3 DPA Protected Neurons from Neuroinflammation by Balancing Microglia M1/M2 Polarizations through Inhibiting NF-κB/MAPK p38 Signaling and Activating Neuron-BDNF-PI3K/AKT Pathways. Mar Drugs. 2021;19(11):587.
Gill AJ, Kovacsics CE, Cross SA, et al. Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest. 2014;124(10):4459–72.
Cano-Europa E, Pérez-Severiano F, Vergara P, et al. Hypothyroidism induces selective oxidative stress in amygdala and hippocampus of rat. Metab Brain Dis. 2008;23(3):275–87.
Sahoo DK, Roy A, Bhanja S, et al. Hypothyroidism impairs antioxidant defence system and testicular physiology during development and maturation. Gen Comp Endocrinol. 2008;156(1):63–70.
Salehi MS, Borhani-Haghighi A, Pandamooz S, et al. Dimethyl fumarate up-regulates expression of major neurotrophic factors in the epidermal neural crest stem cells. Tissue Cell. 2019;56:114–20.
Abd El-Fattah AA, Fahim AT, Sadik NAH, et al. Resveratrol and dimethyl fumarate ameliorate depression-like behaviour in a rat model of chronic unpredictable mild stress. Brain Res. 2018;1701:227–36.
Abd El-Fatah IM, Abdelrazek HMA, Ibrahim SM, et al. Dimethyl fumarate abridged tauo−/amyloidopathy in a D-Galactose/ovariectomy-induced Alzheimer's-like disease: modulation of AMPK/SIRT-1, AKT/CREB/BDNF, AKT/GSK-3β, adiponectin/Adipo1R, and NF-κB/IL-1β/ROS trajectories. Neurochem Int. 2021;148:105082.
Mishra P, Paital B, Jena S, et al. Possible activation of NRF2 by vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signalling in rat heart. Sci Rep. 2019;9(1):7408.
Acknowledgments
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Haiyan Pan acquired data and drafted the manuscript; Yanbo Wang analyzed and interpreted the data; Xiaowei Wang Statistical analyzed these data; Ci Yan designed this study and revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal experiments were in accordance with the guidelines of laboratory animal care and were approved by the Animal Experimentation Ethics Committee of Hangzhou Eyong Biotechnological Co., Ltd. Animal Experiment Center (SYXK, (Zhe)2020–0024). ALL methods are reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pan, H., Wang, Y., Wang, X. et al. Dimethyl fumarate improves cognitive impairment by enhancing hippocampal brain-derived neurotrophic factor levels in hypothyroid rats. BMC Endocr Disord 22, 188 (2022). https://doi.org/10.1186/s12902-022-01086-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01086-4