Abstract
Background
To analyze data in terms of the glycaemic control and therapeutic regimens used for Type-2 Diabetes Mellitus (T2DM) management in Greece, identify factors that influence clinical decisions and determine the level of compliance of T2DM management with the latest international and local guidelines.
Methods
‘AGREEMENT’ was a national-multicenter, non-interventional, cross-sectional disease registry. A total of 1191 adult T2DM patients were enrolled consecutively from 59 sites of the National Health System (NHS) or University Hospitals, representing the majority of Diabetes centers or Diabetes outpatient clinics in Greece with a broad geographic distribution. Patients were stratified by gender and analysis was done according to 3 treatment strategies: A (lifestyle changes or use of one oral antidiabetic agent), B (up to 3 antidiabetic agents including injectables but not insulin) and C (any regimens with insulin).
Results
Mean (±SD) HbA1c % of the total population was 7.1 (±1.2) while mean (±SD) FPG (mg/dl) was measured at 136 (±42). The proportion of patients who achieved HbA1c < 7% was 53% and ranged from 74.2% for group A, to 60.6% for group B and 35.5% for group C. Median age of the studied population was 65.0 year old (Interquartile Range-IQR 14.0) with an equal distribution of genders between groups. Patients on insulin therapy (treatment strategy C) were older (median age: 67 years vs 63 or 65 for A and B, respectively) with longer diabetes duration (mean duration: 15.3 years vs 5.2 and 10.1 for A and B, respectively). Patients who received insulin presented poor compliance. There was a consensus for a series of decision criteria and factors that potentially influence clinical decisions, used by physicians for selection of the therapeutic strategy among the three groups. Compliance with international and Greek guidelines received a high score among groups A, B and C. No significant differences were presented as per sites’ geographic areas, NHS or University centers and physicians’ specialty (endocrinologists, diabetologists and internists).
Conclusions
The presented findings suggest the need for improvement of the glycaemic control rate, especially among insulin treated patients as this group seems to achieve low glycaemic control, by setting appropriate HbA1c targets along with timely and individualised intensification of treatment as well as post-therapy evaluation of the compliance with the proposed treatment.
Similar content being viewed by others
Background
Diabetes is a multifaceted disease which causes major morbidity and mortality due to micro- and macro- vascular complications [1]. Evidence from key studies established the importance of tight and sustained glycaemic control among type 1 and 2 diabetic patients [2, 3]. Metformin monotherapy should be started at diagnosis of Type-2 Diabetes Mellitus (T2DM) along with lifestyle modifications.
Current treatment guidelines therefore advocate a patient-centred approach, with treatment goals of HbA1c < 7.0% according to the American Diabetes Association (ADA), the European Diabetes Association (EASD) [4] and the Hellenic Diabetes Association (HDA) [5] and < 6.5% according to the International Diabetes Federation (IDF) [6] and the American Association of Clinical Endocrinologists (AACE) [7] while they stress the need to modify therapy if HbA1c goal is not met within 3 months.
Nevertheless, despite treatment guidelines, a large proportion of T2DM patients achieve suboptimal goals. The frequency of inadequate glycaemic control is commonly around 50% and even as high as 76% [8,9,10,11,12,13]. In the European Study on Cardiovascular Risk Prevention and Management in Usual Daily Practice (EURIKA), HbA1C < 6.5% was reached by 37% of treated patients. Particularly, 44% of patients from Greece achieved HbA1C < 6.5% [14]. Another recent retrospective observational study in Greece showed that the proportion of patients achieving the target of HbA1c < 7% was 53.9% in 2012 versus 56.1% in 2006 [15].
In PANORAMA, an observational study of T2DM patients, assessing glycaemic control and treatment patterns, 37.4% of patients enrolled, had an HbA1c ≥ 7% with a mean HbA1c of 6.9%. Particularly 32.9% of patients from Greece did not achieve HbA1c < 7%, with a mean HbA1c of 6.7% [16].
Limited data are currently available on T2DM management, daily clinical practice and real-life treatment in Greece to the best of our knowledge. Clearly, an update on glycaemic control in the general population of T2DM patients and a possible assessment of reasons why treatment goals are not achieved is needed. The Disease Registry ‘AGREEMENT’ was designed to provide a reliable picture of current T2DM management in the public healthcare sector in Greece, aiming to determine clinical approaches, therapeutic strategies, level of compliance with latest international and local guidelines in terms of glycaemic control and therapeutic regimens used [4, 5].
Methods
Study design
AGREEMENT was a national-multicentre, non-interventional, cross-sectional disease registry, conducted under real life conditions of daily clinical practice of T2DM in the public healthcare sector in Greece.
In close collaboration with the Hellenic Diabetes Association (HDA), the Northern Greece Diabetes Association (NGDA) and the Hellenic Endocrine Society (HES), a Steering Committee supervised the whole procedure, advised on scientific issues, determined the recruiting capacity of each site and contributed to the analysis of results and writing of the manuscript. The Steering Committee, was composed of the authors, 8 of whom (SA, KK, SL, GM, ZM, MN, EP, CS) were indicated by the three scientific medical societies involved and 1 Sanofi staff member (KM). The data were gathered by site investigators and the sponsor performed site monitoring and data collection. The data were analysed by ANTAEA Consulting.
The study protocol was approved by all Hospitals’ Review Boards and conducted in accordance with Good Pharmacoepidemiology Practices (GCPs) and all applicable regulations. All patients provided written informed consent.
Data sources
All patients were recruited in one single visit and all relevant data from medical records were registered in an electronic CRF (e-CRF). The study collected data for two periods, the initial diagnosis period, concerning the medical and diabetes history as well as the status of the patients and the current period (inclusion visit). Patients’ data registered in e-CRF included demographics and living conditions, anthropometric measurements including physical activity, T2DM treatment at initial diagnosis and at current period, lab measurements regarding glycemia and lipid profile, blood pressure measurements, family history of T1DM and T2DM, diet assessment, smoking and alcohol consumption, co-morbidities and overall quality of life estimations through a non validated questionnaire based on simple questions and using a scale from 1 to 10 (worst to best).
Since 70% of participants had been initially diagnosed at another centre than that participating in this study, this piece of information was used only for benchmarking and not for statistical inference. The following data were collected: T2DM treatments used currently and in the past (depending on availability of data), factors that potentially influence clinical decisions, level of compliance with latest international and local clinical guidelines in terms of glycaemic control and therapeutic regimens used, glycaemic control expressed as HbA1c < 7% and 130 mg/dl > FPG > 70 mg/dl at current visit.
Site and patient selection
The sites selection process was performed following the response of 96 diabetic centers functioning in a public setting to a relative feasibility questionnaire. Of those, 69 sites accepted initially to participate and 59 were active at the end of the study.
Patients were included depending on the recruitment capacity of each site, in order to avoid any centers driving the results. The Steering Committee made a classification of the 69 diabetic centers, in 3 categories: high capacity (A), medium capacity (B) and low capacity (C) according to the number of patient monthly visits in each site, as recorded in the feasibility questionnaire. 18 out of the 69 sites were classified as high capacity (group A), 19 as medium capacity (group B) and 32 as low capacity (group C).
Patients were further stratified by treatment strategies using the information of a market survey as the main source of real life stratification, [regarding the therapeutic strategies for the diabetic patients]. 20% of patients with lifestyle changes or receiving up to one oral anti-diabetic agent (treatment strategy A), 40% of patients receiving 2 or 3 antidiabetic agents including injectables but not insulin (treatment strategy B) and 40% of patients receiving insulin with or without other anti-diabetic medication (treatment strategy C). Patients were finally stratified according to gender by 50%.
Stratification of patients took place according to the breakdown of Additional file 1: Table S1. Patients were recruited in a sequential manner, minimizing patient selection bias. Data on antidiabetics medication including mean dose in total and per treatment strategy are provided in Additional file 1: Table S2a-e.
Study population
A total of 1236 T2DM patients from 69 sites were planned to be enrolled in the study. T2DM patients ≥18 years old, were eligible. Exclusion criteria included T1DM patients, pregnant or lactating women, any clinically significant acute major organ or systemic disease and need for hospitalization occurring within 3 months before enrolment. All participants provided written informed consent.
Study objectives
The primary objectives were the collection and analysis of data on current clinical practice and relevant treatment strategies, the identification of factors that potentially influence clinical decisions, the level of compliance with latest international and local guidelines in terms of glycaemic control and use of therapeutic regimens based on answers to relevant questionnaires of the e-CRF. Secondary objectives included potential differentiation in T2DM management between geographic areas, National Health System- NHS and University centres and different medical specialties (endocrinologists, diabetologists and internists).
Sample population size
The study planned to include approximately 1200 patients. This would allow to calculate the percentage of patients with HbA1c < 7%, both in total and in different subgroups, with an acceptable precision, based on the fact that the expected prevalence of different therapeutic strategies would be close to relevant data for categorization of treatment approaches and the expected percentage of patients achieving HbA1c < 7%. The expected patients with HbA1c < 7% is close to 50% [11, 13,14,15].
Subgroups of interest
Study population was analysed according to three treatment strategies. In addition patients were grouped according to HbA1c < 7% or ≥ 7% or < 6.5, 6.5–7.0%, < 7.0, 7.0–7.5%, 7.5–8.0% and > 8.0% and FPG of 70-100 mg/dl, 100-130 mg/dl and ≥ 130 mg/dl.
Statistical analysis
Descriptive statistics, Chi-square test for testing differences between strategies and Kruskal – Wallis test to test hypotheses when distributions were not normal, were the main statistical methods used. In the descriptive analysis, the variables were presented as mean, median and the quartiles. Dispersants measures were calculated by variance and standard deviation and IQR. No sensitivity analysis was performed. Statistical analysis SAS Enterprise Guide was used. There was no record of current treatment strategy for 2 patients who were excluded from the analysis.
Results
Patient characteristics
Overall 1189 patients (out of 1191 initially consented) from 59 sites, were recruited between 13 June 2014 (first subject in) and 04 June 2015 (last subject in). Patients’ flowchart at current visit is shown in Fig. 1. Median age of the studied population was 65.0 year old (IQR 14.0) with an equal gender distribution between the groups. Demographics analyses by treatment strategies A, B, and C revealed statistical significant differences as indicated in Table 1. The difference in patients’ age and in years under treatment between groups were statistically significant (K-W test, p = 0.002, K-W test, p < 0.001). Patients that received insulin (treatment strategy C) were older (median age 67 years vs 63 or 65 for A and B, respectively) and their median length of time under treatment was longer (15 years vs. 4 or 9 for A and B, respectively).
Basal insulin regimens were prescribed for 80% of patients, with 51% on basal insulin and OADs and 28% on basal-plus regimens. Premixes were prescribed for 19% of patients with any regimens with insulin.
There were no differences in vital signs or lab measurements such as cholesterol, triglycerides etc. among treatment groups. Living condintion characteristics were the same between treatment strategies, except education (chi square test, p = 0.032) and self –care capability (chi square test, p = 0.009). More patients of treatment strategy C (53.8%) had received only basic education as compared to those of groups A (47.7%) and B (46.9%) (p = 0.032) and needed help sometimes in terms of self-care capability (Table 1).
Considering risk factors by treatment strategy at current period (Additional file 1: Table S3), statistical significant differences were found for limited physical exercise (chi square test, p = 0.012) and overall poor diet (chi square test, p = 0.002). Patients under treatment strategy C had limited physical exercise and followed an overall poorer diet when compared to groups A and B.
Table 2 presents the reported diabetes complications by treatment strategy. Patients following treatment strategy C had more chronic complications than A and B. Acute complications and hospitalizations were rare in all therapeutic strategies.
70% of patients declared that the initial diagnosis was made at another centre and 67% of patients by a public health sector physician.
Collection and analysis of data on current clinical practice in T2DM management and relevant treatment patterns in public sector in Greece
Laboratory measurements of glycaemic control by treatment strategy at the time of diagnosis (initial period) and at the inclusion visit (current period) are shown in Table 3. HbA1c and FPG values were available for both periods while PPG values were recorded only at the inclusion visit.
Approximately 30% of patients under treatment strategy A, 50% of patients under B and 90% of patients under C at the time of diagnosis continued the same treatment regimen at current period, as shown in Fig. 2.
Mean (±SD) HbA1c % and FPG (mg/dl) of the total population decreased from 8.7 ± 7.5, 201 ± 85 at the time of diagnosis (initial period) to 7.1 ± 1.2, 136 ± 42 at the inclusion visit (current period).
At the inclusion visit (current period), mean (±SD) HbA1c % and FPG (mg/dl) values were higher among patients under treatment strategy C 7.5 ± 1.2, 141 ± 46 followed by patients under B 6.9 ± 1.1, 135 ± 41 and A 6.6 ± 1.0, 126 ± 32 (Chi square test, p < 0.001). PPG values were higher among patients under treatment strategy B followed by patients under A and C. However, after adjustment for covariates that are important predictors of T2DM progression, such as BMI, age, duration of DM and family history of DM, the difference in HbA1c and FPG between treatment groups were attenuated, not reaching any more statistical significance (Table 3).
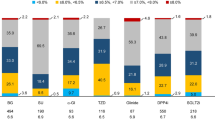
Overall, 53% (95% CI: 50.2–55.8%) of patients had HbA1c < 7% at the inclusion visit (current period). The proportion of patients with HbA1c < 7% ranged from 74.2% (95% CI: 68.7–79.7%) for A, to 60.6% (95% CI: 56.2–65.0%) for B and 35.4% (95% CI: 31.2–39.2%) for C. (Table 3, Fig.3). Distribution of HbA1c values across < 6.5, 6.5–7.0%, 7.0–7.5%, 7.5–8.0%, > 8.0% between different treatment strategies are presented in Fig. 4 (Chi square test, p < 0.001).
Considering the targets of HbA1C for each patient the time of diagnosis (initial period) and the goal achievement at the inclusion visit (current period), 58.1% of patients under treatment strategy A achieved the goal set compared to 52.5% of B and 40.2% of C (Pearson Chi-Square test, p = 0.000).
Distribution of FPG values across < 70 mg/dl, 70-100 mg/dl, 100-130 mg/dl and ≥ 130 mg/dl between different treatment strategies are also presented in Additional file 1: Table S4.
The improvement of laboratory measurements of HbA1c and FPG values at the inclusion visit (current period) compared to their respective measurements at the time of diagnosis (initial period) is presented in Additional file 1: Table S5. Treatment strategy C had the greatest improvement for both HbA1c and FPG.
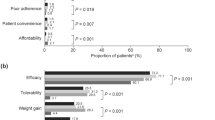
The assessment of patients’ compliance, interest and active participation per therapeutic strategy is presented in Additional file 1: Table S6. 7% of all patients declared poor compliance with the prescribed medication. 9.1% of patients who followed treatment strategy C were less compliant while differences between treatment strategies were statistically significant (p < 0.001). 71.9% of patients overall had a satisfactory interest and an active participation in the treatment of the disease.
Using a scale from 1 to 10 (Worst to Best), patients assessed subjectively their Quality of Life (QoL), at each period (Additional file 1: Table S7). QoL score was improved for treatment strategies A and B and remained the same for C. Using Kruskal – Wallis test to examine whether there are statistically significant differences between the three treatment groups, the hypothesis was rejected (p < 0.001).
Identification of factors that potentially influence clinical decisions and guide management strategies in T2DM
Using a scale from 1 to 10 (completely disagree to completely agree), Table 4 presents the score for decision criteria used for selection of therapeutic strategy. For the three treatment strategies there was a consensus (8–10) for all decision criteria while the differences in responses between the therapeutic strategies were statistically significant. Factors such as compliance with international and local guidelines, physician’s clinical experience and familiarity, compliance with drug indications and prescribing guidelines, efficacy and safety of each treatment strategy with minimization of adverse reactions received the highest score among groups A, B and C.
Level of compliance of current clinical management of T2DM patients in Greece with latest international and local clinical practice guidelines in terms of glycaemic control and therapeutic regimens used
Compliance with international and Greek guidelines for the management of T2DM received a high score among groups A, B and C as presented in Table 4.
Identification of any differentiations in clinical practice in T2DM management between different geographic areas in Greece, between NHS and university centres and between physicians of different medical specialties
No significant differences between groups were identified when analysing each treatment strategy as per sites’ geographic areas, management between NHS or University centres and physicians’ specialty (endocrinologists, diabetologists, and internists) as presented in Additional file 1: Table S8.
Safety data collection
Spontaneous reporting of Adverse Events (AEs) and safety data collection were made. Investigators were advised to report any AE or Serious AEs (SAE) to the Marketing Authorization Holder of the suspected pharmaceutical product or to the National Organization of Medicines via the completion of the yellow card. No AEs were reported during the study.
Discussion
The AGREEMENT study provides an important update and snapshot of real world glycaemic control in Greece. HbA1c values of 1189 T2DM patients receiving initial treatment have decreased substantially during the time interval between the time of diagnosis (initial period) and the inclusion visit (current period).
An overall mean decrease of HbA1c of 1.6% from 8.7 to 7.1% was observed. The rate of overall glycaemic control defined as HbA1c < 7% was 53% at the inclusion visit (current period) with differences between treatment groups. 74.2% of patients of treatment strategy A had HbA1c < 7%, compared to 60.6% of B and 35.4% of C.
The poorer glycaemic control with insulin treatment was possibly related to the disease progression and the fact that clinicians prescribe insulin to patients with most advanced diabetes. There is a number of barriers that may prevent initiation and optimization of insulin therapy including fear/risk of hypoglycaemia, lack of dose titration and flexibility, poor adherence and persistence, weight gain and treatment satisfaction [17,18,19,20,21,22]. These barriers must be mitigated to improve diabetes management. Patients with most advanced diabetes may need an additional treatment such as rapid acting insulin (RAI) on top of basal insulin.
An age effect was also identified with insulin treated patients since these were older and with longer time under treatment. 83.8% of elderly insulin treated patients above 65 years old reached HbA1c ≤ 8%. Moreover they had limited physical exercise, followed a poorer diet and presented poorer compliance with treatment. Older age and presence of comorbidities may influence the target for less stringent glucose control as reflected by HbA1c according to current ADA/EASD treatment guidelines [4]. QoL score was improved for treatment strategies A and B and remained the same for C. This is in contrast with Mellita study, a 6 month observational study in everyday clinical practice in Greece, that showed high compliance rate with the addition of insulin glargine on inadequately controlled T2DM patients with oral antidiabetic drugs (OADs) and benefits in both glycaemic control and health related QoL [23]. Population characteristics and changes in clinical outcomes among insulin treated patients may explain the differences in QoL score between Agreement and Mellita studies.
In this study, the percentage of patients not achieving the cut-off value of HbA1c < 7% was approximately 47% of the participants. Our results are in agreement with other similar observational studies [14,15,16, 24].
The reported 60.6% of patients in AGREEMENT study receiving up to 3 antidiabetic agents including injectables but not insulin and achieving HbA1C < 7%, was consistent with the results of another study, where 59% of patients with different non-insulin drugs achieved HbA1c < 7% followed by HbA1c reduction of approximately 1% within 6 months [25].
Compliance with international and Greek guidelines received a high score among groups A, B and C in terms of glycaemic control and therapeutic regimens used. No significant differences were presented when analysing as per sites’ geographic areas, management between NHS or University centres and physicians’ specialty.
Our real life observational study has both strengths and weaknesses. The strength of this observational study is that it examined every day clinical practice in a large sample of patients, indicating a degree of representativeness and extrapolation of available management of T2DM to the entire population in the public sector.
Certain weaknesses can be raised since the exact time elapsed from the time of diagnosis till current period was not predetermined from the study protocol. Also there was no information recorded on medical records concerning hypoglycaemia episodes per treatment strategy. This emphasises the need for better registration of hypoglycaemic episodes in this patient population by sites.
Limitations also concerned the non-randomised study design, which does not allow to establish any causal relationship between exposure and outcomes. Insulin titration and diet modification were left to the clinical judgement of the physicians and blood samples were measured locally. Improvement of the QoL Score and compliance was based on patients’ answer to simple questions and not to a validated questionnaire thus limiting the comparability of patient reported outcomes to the results of other studies. Another limitation of the current study is that it reflects clinical practice 3–4 years ago as patients were recruited between June 2014 and June 2015.
Conclusion
In conclusion, the present nation-wide observational study extended our understanding of the T2DM management in the public sector in Greece.
Still a substantial proportion of T2DM patients (47%) did not achieve recommended HbA1c targets and this was particularly true in the most advanced and older patients. Clinical inertia exists in diabetes care resulting in suboptimal glycaemic control. Among different treatment strategies, the proportion of patients with HbA1c < 7% ranged from 74.2% for A, to 60.6% for B and 35.4% for C. Moreover it seems that there was a failure to achieve individual targets set for each patient by treatment strategies. 41.9% of patients under treatment strategy A failed to achieve the target set compared to 47.5% of B and 59.8% of C. Insulin treated patients were older, had more complications, limited physical exercise and lower overall adherence to diet.
There was a consensus of factors that potentially influence clinical decisions and treatment strategies, a high degree of compliance with international and local guidelines and no differentiations were noted in T2DM management between geographic areas, National Health System- NHS and University centres and different medical specialties.
Our study findings suggest there is a room for improvement of glycaemic control rate and call for further activities and educational awareness campaigns by all parties involved in diabetes management in the public domain in Greece to help patients to achieve better glycaemic control, to optimize the timing of add-on therapies and eventually minimize the risk of complications in diabetic population. Particular attention must be given among insulin treated patients to improve their clinical outcomes as this group seems to achieve low glycaemic control. This can be done by setting appropriate HbA1c targets along with timely and individualised intensification of treatment to ameliorate disease outcomes as well as post-therapy evaluation of the compliance with the proposed treatment. Further studies to explore the level of glycaemic control and its associated factors in the private sector will further contribute to evaluate disease management strategies.
Abbreviations
- AACE:
-
American Association of Clinical Endocrinologists
- ADA:
-
American Diabetes Association
- AEs:
-
Adverse Events
- EASD:
-
European Diabetes Association
- GCPs:
-
Good Pharmacoepidemiology Practices
- HAD:
-
Hellenic Diabetes Association
- HDL-C:
-
High Density Lipoprotein-Cholesterol
- HES:
-
Hellenic Endocrine Society
- IDF:
-
International Diabetes Federation
- IQR:
-
Interquartile Range
- LDL-C:
-
Low Density Lipoprotein-Cholesterol
- NGDA:
-
Northern Greece Diabetes Association
- NHS:
-
National Health System
- OADs:
-
Oral antidiabetic drugs
- QoL:
-
Quality of Life
- SD:
-
Standard Deviation
- T2DM:
-
Type-2 Diabetes Mellitus
References
Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7.
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986.
UK prospective diabetes study (UKPDS) group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of Hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care. 2015;38(1):140–9.
Hellenic Diabetes Association (EDE). 2016 [cited 2016 Jan 15]. Available from: http://www.ede.gr/wp-content/uploads/2012/odigies.pdf
IDF Diabetes Atlas 7th edition. 2016 [cited 2016 Nov 27]. Available from www.idf.org/diabetesatlas
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Henry RR, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE) Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm--2016 executive summary. Endocr Pract. 2016;22:84–113.
Fox KM, Gerber RA, Bolinder B, Chen J, Kumar S. Prevalence of inadequate glycemic control among patients with type 2 diabetes in the United Kingdom general practice research database: a series of retrospective analyses of data from 1998 through 2002. Clin Ther. 2002;28:388–95.
Yurgin N, Secnik K, Lage MJ. Antidiabetic prescriptions and glycemic control in German patients with type 2 diabetes mellitus: a retrospective database study. Clin Ther. 2007;29:316–25.
Eliasson B, Eeg-Olofsson K, Cederholm J, Nilsson PM, Gudbjörnsdóttir S, Steering Committee of the Swedish National Diabetes Register (NDR). Antihyperglycaemic treatment of type 2 diabetes: results from a national diabetes register. Diabetes Metab. 2007;33:269–76.
Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The Prevalence of Meeting A1C, Blood Pressure, and LDL Goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–9.
Wilke T, Groth A, Fuchs A, Seitz L, Kienhöfer J, Lundershausen R, Maywald U. Real life treatment of diabetes mellitus type 2 patients: an analysis based on a large sample of 394,828 German patients. Diabetes Res Clin Pract. 2014;106(2):275–84.
Stone M, Charpentier G, Doggen K, Kuss O, Lindblad U, Kellner C, Nolan J, Pazderska A, Rutten G, Trento M, Khunti K, GUIDANCE Study Group. Quality of Care of People with Type 2 diabetes in eight European countries. Diabetes Care. 2013;36:2628–38.
Banegas JR, Lopez-Garcia E, Dallongeville J, Guallar E, Halcox JP, Borghi C, Massó-González EL, Jiménez FJ, Perk J, Steg PG, De Backer G, Rodríguez-Artalejo F. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J. 2011;32:2143–52.
Liatis S, Papaoikonomou S, Ganotopoulou A, Papazafiropoulou A, Dinos C, Michail M, Xilomenos A, Melidonis A, Pappas S. Management of type 2 diabetes and its prescription drug cost before and during the economic crisis in Greece: an observational study. BMC Endocr Disord. 2014;23:1–8.
De Pablos-Velasco P, Parhofer KG, Bradley C, Eschwège E, Gönder-Frederick L, Maheux P, Wood I, Simon D. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol. 2014;80:47–56.
Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA. 2014;311:2315–25.
Garber AJ. The importance of titrating starting insulin regimens in patients with type 2 diabetes. Diabetes Obes Metab. 2009;11(5):10–3.
Peyrot M, Rubin R, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR, Landgraf R, Kleinebreil L, International DAWN Advisory Panel. Resistance to insulin therapy among patients and providers: results of the cross-national diabetes attitudes, wishes, and needs (DAWN) study. Diabetes Care. 2005;28(11):2673–9.
Ahrén B. Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Man. 2013;9:155–63.
Peyrot M, Rubin RR, Knunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes. 2010;4(1):S11–8.
Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–9.
Koukoulis G, Melidonis A, Milios K, Mellita Investigators. Quality of life of insulin-Naïve people with type 2 diabetes mellitus inadequately controlled on Oral antidiabetic drugs after the addition of insulin glargine, in every day clinical practice in Greece. J Diabetes Metab Disord Control. 2015;2(2):00037.
Thomsen RW, Baggesen LM, Svensson E, Pedersen L, Nørrelund H, Buhl ES, Haase CL, Johnsen SP. Early glycaemic control among patients with type 2 diabetes and initial glucose lowering treatment: a 13-year population based cohort study. Diabetes Obes Metab. 2015;17:771–80.
Thomsen RW, Baggesen LM, Sogaard M, Pedersen L, Nørrelund H, Buhl ES, Haase CL, Johnsen S. Early glycaemic control in metformin users receiving their first add-on therapy: a population-based study of 4,734 people with type 2 diabetes. Diabetologia. 2015;58:2247–53.
Acknowledgements
The authors would like to thank all investigator teams for the conduct of the study.
-
I.
Alexiou Zoi, Second Department of Internal Medicine, General Hospital of Elefsina Thriasio, Elefsina, Greece.
-
II.
Almanidou-Kougioumtzidi Olga, Second Department of Internal Medicine, General Hospital of Karditsa, Karditsa, Greece.
-
III.
Anastasiou Eleni, Department of Endocrinology & Diabetes Center, General Hospital of Athens “Alexandra”, Athens, Greece.
-
IV.
Avramidis Iakovos, First Department of Internal Medicine, General Hospital of Thessaloniki “G.Papanikolaou”, Thessaloniki, Greece.
-
V.
Βakidis Sofoklis, Department of Internal Medicine, General Hospital of Lakonia – Molaoi, Molaoi, Greece.
-
VI.
Bargiota Alexandra, Department of Endocrinology and Metabolic Diseases, University Hospital of Larisa, Larisa, Greece.
-
VII.
Bikas Christos, Department of Internal Medicine, General Hospital of Pyrgos, Pyrgos, Greece.
-
VIII.
Bousboulas Stavros, Diabetes Centre, General Hospital of Nikea-Piraeus “Agios Panteleimon”, Piraeus, Greece.
-
IX.
Boutel Dimitrios, Department of Internal Medicine, General Hospital of Giannitsa, Giannitsa, Greece.
-
X.
Chaliotis Georgios, Department of Internal Medicine, General Hospital of Chalkida, Chalkida, Greece.
Chrisoulidou Alexandra, Department of Endocrinology, Theagenio Cancer Hospital, Thessaloniki, Greece.
Didangelos Triantafyllos, Diabetes Center, First Propaedeutic Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, AHEPA Hospital, Thessaloniki, Greece.
Dimitriadis George, Second Department of Internal Medicine and Research Institute and Diabetes Center, Attikon University Hospital, Athens, Greece.
Dimou Eftihia, Department of Internal Medicine, General Hospital of Samos “Agios Panteleimon”, Samos , Greece.
Douitsis Petros, Department of Internal Medicine, “Eleni Dimitriou” General Hospital of Florina, Florina, Greece.
Doupis John, Internal Medicine and Diabetes Department, Salamis Naval Hospital, Salamis, Greece.
Exiara Triada, Department of Internal Medicine, General Hospital of Komotini, Komotini, Greece.
Gkioka Maria, Department of Internal Medicine, General Hospital of Patras “Agios Andreas”, Patras, Greece.
Gkioulos Nikolaos, Health Center, Ag. Nikolaos, General Hospital of Kalamata, Kalamata, Greece.
Grigoropoulou Pinelopi, Department of Internal Medicine, General Hospital of Athens “Elpis”, Athens, Greece.
Ioannidis Ioannis, Diabetes and Obesity Outpatient Clinics, General Hospital of Nea Ionia “Konstantopouleio-Patission”, Athens, Greece.
Kirlaki Evridiki, Diabetes Center, General Hospital of Heraklio “Venizeleio-Pananio”, Heraklio, Greece.
Kitsios Kostas, Diabetes Outpatient Clinics, General Hospital of Thessaloniki “G.Gennimatas - Ag.Dimitrios”, Thessaloniki, Greece.
Kokkoris Panagiotis, Department of Endocrinology and Diabetes, 251 Hellenic Air Force & VA General Hospital, Athens, Greece.
Kotsa Kalliopi, First Department of Internal Medicine, Division of Endocrinology and Metabolism, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Kouroglou Maria, Department of Internal Medicine, General Hospital of Mytilene “Vostanio”, Mytilene, Greece.
Lanaras Leonidas, Department of Internal Medicine, General Hospital of Lamia, Lamia, Greece.
Magiannis Konstantinos, Department of Internal Medicine, General Hospital of Grevena, Grevena, Greece.
Manes Christos, Diabetes Center, Papageorgiou General Hospital, Thessaloniki, Greece.
Marathonitis Georgios, First Department of Internal Medicine, General Hospital of Attiki “Sismanoglio - Amalia Fleming” - Hospital Unit “Amalia Fleming”, Melissia, Greece.
Melidonis Andreas, Diabetes Center, Tzanio General Hospital, Piraeus, Greece.
Migdalis Ilias, Second Medical Department and Diabetes Centre, NIMTS Hospital, Athens, Greece.
Mitrakou Asimina, Department of Clinical Therapeutics, Athens University Medical School, General Hospital of Athens “Alexandra”, Athens, Greece.
Papantoniou Stefanos, Diabetes Clinic, First Department of Internal Medicine, General Hospital of Kavala, Kavala, Athens, Greece.
Papazoglou Dimitrios, Diabetes Centre, Second Department of Internal Medicine, University General Hospital of Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece.
Piaditis Georgios, Department of Endocrinology and Diabetes Center, “G. Gennimatas”, General Hospital of Athens, Athens, Greece.
Potolidis Evangelos, Department of Internal Medicine, General Hospital of Volos “Achillopoulio”, Volos, Greece.
Prokovas Ioannis, Endocrinology Clinic, General Hospital of Nikea-Piraeus “Agios Panteleimon”, Piraeus, Greece.
Rizos Evangelos, Second Department of Internal Medicine, University Hospital of Ioannina, Ioannina, Greece.
Rogkoti Maria, Department of Internal Medicine, General Hospital of Thessaloniki “G.Gennimatas - Ag.Dimitrios” - Unified Hospital “Agios Dimitrios”, Thessaloniki, Greece.
Romanidou Alexandra, First Department of Internal Medicine, General Hospital of Serres, Serres, Greece.
Sampanis Christos, Diabetes Centre, Second Propaedeutic Department of Internal Medicine, General Hospital of Thessaloniki – “Hippokration”, Thessaloniki, Greece.
Satsoglou Aimilios, Department of Internal Medicine, General Hospital of Goumenissa, Kilkis, Greece.
Simelidis Dimitrios, Department of Internal Medicine, Mamatseio General Hospital of Kozani, Kozani, Greece.
Taisir El Hasban, Health Center of Elefsina, General Hospital of Elefsina Thriasio, Elefsina, Greece.
Tentolouris Nikolaos, First Department of Propaedeutic Medicine, Diabetes Center, Athens University Medical School, Laiko Hospital, Athens, Greece.
Thanopoulou Anastasia, Second Department of Internal Medicine, General Hospital “Hippocratio”, National and Kapodistrian University, Athens, Greece.
Tolis Apostolos, Second Department of Internal Medicine, “G. Gennimatas”, General Hospital of Athens, Athens, Greece.
Tsanikidis Iraklis, Department of Internal Medicine, General Hospital of Katerini, Katerini, Greece.
Tsapas Apostolos, Clinical Research and Evidence Based Medicine Unit, Second Medical Department, Aristotle University of Thessaloniki, General Hospital of Thessaloniki “Hippokration”, Thessaloniki, Greece.
Tsapogas Panagiotis, Department of Internal Medicine, General Hospital of Corfu, Corfu, Greece.
Tsatsoulis Agathoklis, Department of Endocrinology, University Hospital of Ioannina, Ioannina, Greece.
Tsotoulidis Stefanos, Department of Internal Medicine, General Hospital of Chalkidiki – Kassandria, Kassandria, Greece.
Tzatzagou Glykeria, First Department of Internal Medicine, Papageorgiou General Hospital, Thessaloniki, Greece.
Vasiliadis Panagiotis, Department of Internal Medicine, General Hospital of Thessaloniki “G. Papanikolaou” - Interconnected Psychiatric Hospital of Thessaloniki, Thessaloniki, Greece.
Vasilopoulos Charalampos, Department of Endocrinology, Diabetes and Metabolism, “Evangelismos” Hospital, Athens, Greece.
Vlachogiannis Anestis, Department of Internal Medicine, General Hospital of Drama, Drama, Greece.
Vryonidou Andromachi, Department of Endocrinology and Diabetes, Hellenic Red Cross Hospital, Athens, Greece.
Xilomenos Apostolos, Department of Internal Medicine, General Hospital of Agrinio, Agrinio, Greece.
Funding
The study was funded by Sanofi, Greece. Writing/Editorial support was provided by Kostas Milios, Medical department of Sanofi, Greece. The Sponsor contributed to the study protocol conception and design, interpretation of the data and writing of the manuscript in close collaboration with the Steering Committee that was composed of the authors that were indicated by the three national medical societies, actively involved in Diabetes management in Greece; the Hellenic Diabetes Association (HDA), the Northern Greece Diabetes Association (NGDA) and the Hellenic Endocrine Society (HES). Data collection and management and statistical analysis were carried out by ANTAEA Medical Services Ltd. under the supervision of the Sponsor and the steering committee.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to institutional policies but can be available from the corresponding author after reasonable request to the study sponsor.
Author information
Authors and Affiliations
Consortia
Contributions
SL, SI, KK, GM, ZM, MN and EP contributed to the study protocol conception and design and reviewed the manuscript. CS was a study investigator, contributed to the study protocol conception and design and reviewed the manuscript. KM contributed to the study protocol conception and design and writing of the manuscript. All authors read and approved the final manuscript. All other study investigators conducted the study and collected the data.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by all Hospitals’ Review Boards and conducted in accordance with Good Pharmacoepidemiology Practices (GCPs) and all applicable regulations. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
Dr. Stavros Liatis has received research support and/or consulting honoraria from Astra-Zeneca, Boeringer Ingelheim, MSD, Novartis, Novo Nordisk, Sanofi, Pharmaserve Lilly and ELPEN and is a member of the Editorial Board of BMC Endocrine Disorders.
Dr. Styliani Iraklianou has received research support and consulting honoraria from Sanofi.
Dr. Kyriakos Kazakos has received research support and/or consulting honoraria from Astra Zeneca, Sanofi Aventis, Novo Nordisk and Lilly.
Pr. George Mastorakos declares no conflict of interest.
Kostas Milios is an employee of Sanofi working as Field Medical for Greece.
Dr. Zadalla Mouslech has received research support and/or consulting honoraria from Sanofi and Lilly.
Dr. Marina Noutsou has received research support and/or consulting honoraria from Novo Nordisk, Sanofi Aventis, Lilly, Boehringer Ingelheim, ELPEN and Astra Zeneca.
Dr. Emmanouil Pagkalos has received research support and/or consulting honoraria from Astra Zeneca, Bayer, ΒΙΑΝΕΧ, Boehringer Ingelheim, ELPEN, Galenica, GSK, Jansen, Lilly, MSD, Novartis, Novo Nordisk, Roche, Sanofi, Takeda and Winmedica.
Dr. Christos Sampanis has received research support and /or consulting honoraria from Novo Nordisk, Sanofi and Lilly Co.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
AGREEMENT Investigators. Table S1. Stratification of patients according to sites capacity, treatment strategy and gender. Table S2a. Percentage % of patients received Oral antidiabetic agents OADs. b Percentage % of patients received any Injectable treatments, not including Insulin. c Percentage % of patients received Insulin treatment. d Daily dose of treatment (total). e Daily dose per treatment strategy. Table S3. Risk Factors per treatment strategy at current period. Table S4. Distribution of FPG values across < 70 mg/dl, 70-100 mg/dl, 100-130 mg/dl and ≥ 130 mg/dl at initial diagnosis and current period per treatment strategy. Table S5. Difference of laboratory measurements (HbA1c & FPG) between initial diagnosis and current period. Table S6. Assessment of patients’ compliance, interest and active participation per treatment strategy at current period. Table S7. QoL score -scale from 1 to 10 (Worst to Best) at initial diagnosis and current period per treatment strategy (p < 0.001). Table S8. Doctors’ specialty, geographical region and hospital management per treatment strategy at current period. (DOCX 68 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liatis, S., Iraklianou, S., Kazakos, K. et al. A Greek registry of current type 2 diabetes management, aiming to determine core clinical approaches, patterns and strategies. BMC Endocr Disord 19, 39 (2019). https://doi.org/10.1186/s12902-019-0364-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-019-0364-5