Abstract
Introduction
Despite the high prevalence of type 2 diabetes (T2D) and suboptimal glycemic control in the Middle East and Africa, comprehensive data on the management of T2D remain scarce. The main aim of this study is to describe the characteristics and treatment of patients with T2D initiating second-line glucose-lowering therapy in these regions.
Methods
DISCOVER is a global, 3-year, prospective observational study of patients with T2D enrolled at initiation of second-line glucose-lowering therapy. Baseline characteristics and treatments are presented for patients from 12 countries divided into three regions: Mediterranean, Gulf Cooperation Council, and South Africa.
Results
Among 3525 patients (52.5% male, mean age 54.3 years), mean time since T2D diagnosis was 6.2 years [across-region range (ARR) 5.8–7.5 years] and mean glycated hemoglobin levels were 8.7% (72.0 mmol/mol) [ARR 8.6–9.0% (68–75 mmol/mol)]. At first line, metformin was prescribed for 88.1% (ARR 85.4–90.3%) of patients and a sulfonylurea for 34.4% (ARR 12.7–45.4%). Sulfonylureas and dipeptidyl peptidase-4 inhibitors were prescribed at second line for 55.5% (ARR 48.6–82.5%) and 49.0% (ARR 3.7–73.8%) of patients, respectively. Main reasons for choice of second-line therapy were efficacy (73.2%; ARR 60.1–77.7%) and tolerability (26.8%; ARR 3.7–31.2%).
Conclusions
We demonstrate considerable inter-region variations in the management of T2D, likely affected by multiple factors (health system, physician behavior, and patient compliance), all of which should be addressed to optimize outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What is already known about this subject |
Global DISCOVER study has already described the characteristics and treatment of patients with type 2 diabetes mellitus, initiating a second-line glucose-lowering therapy worldwide. |
This international study outlined clinician therapeutic decisions for managing type 2 diabetes showing wide variety in different countries. |
Longitudinal data on glucose-lowering treatment patterns and factors behind the wide variability are scarce. |
What this study adds |
This study focused for the first time on the second-line treatment pattern for Middle East and Africa (MEA) region. |
It has shown that there is suboptimal glycemic control related to serious limitations in access to both diagnostic and therapeutic medical services in African countries in relation to other Mediterranean and GCC countries. |
Low- and middle-income countries had limited access to the new generations of oral hypoglycemic agents that are mostly costly compared with the old ones, which was reflected by failing to achieve the target glycemic control and increasing the risk of cardiovascular diseases. |
Countries from MEA region demonstrated a big variation in the prevalence of cardiovascular comorbidities that could reflect inter-region variations or lifestyle. |
Introduction
The escalating incidence of type 2 diabetes mellitus (T2D) is a global public health crisis [1] and is of particular concern in the Middle East and Africa. The 2019 International Diabetes Federation (IDF) Diabetes Atlas estimates that 12.8% of people aged 20–79 years have diabetes in the Middle East and North Africa, which represents the highest estimated age-adjusted diabetes prevalence of all IDF regions [2]. In the Middle East alone, 14.6% of adults (81 million individuals) are living with diabetes [3]. Similarly, in South Africa, the estimated prevalence of T2D in adults was 15.3% [4]. The number of patients with T2D in the countries of the Gulf Cooperation Council (GCC) has increased dramatically in the past two decades and is projected to double by 2035 [5].
Despite the high prevalence of T2D and suboptimal glycemic control in these regions [6,7,8,9,10], comprehensive data on the management of T2D remain scarce [11]. A clear understanding of the impact of patient characteristics, risk factors, and treatment patterns on disease progression and outcomes is needed to reduce the burden of diabetes and its impact on morbidity, mortality, and healthcare resource use.
DISCOVER is a 3-year observational study of people with T2D initiating second-line glucose-lowering therapy in 38 countries [12, 13]. Data from the DISCOVER study have demonstrated global variations in the treatment of T2D [14]. This current analysis describes the characteristics and treatment of patients in the DISCOVER study enrolled in 12 countries representing the Middle East and Africa cohort: Algeria, Bahrain, Egypt, Jordan, Kuwait, Lebanon, Oman, Saudi Arabia, South Africa, Tunisia, Turkey, and United Arab Emirates.
Methods
Study Design
Selection Criteria, Study Cohorts, and Subgroups
The methods for the DISCOVER study program have been reported in detail elsewhere and are briefly summarized below [12, 13]. DISCOVER was a 3-year, non-interventional, prospective observational study conducted in a real-world setting in 38 countries (ClinicalTrials.gov identifier: NCT02322762 in 37 countries and NCT02226822 in Japan). In the present analysis, the 12 countries from the Middle East and Africa region were divided into three regions according to geographical location: Mediterranean countries (Algeria, Egypt, Jordan, Lebanon, Tunisia, and Turkey), GCC countries (Bahrain, Kuwait, Oman, Saudi Arabia, and United Arab Emirates,) and South Africa. Study protocols were approved by the appropriate clinical research ethics committee in each country and the relevant institutional review board at each site. The protocols complied with the Declaration of Helsinki, the International Conference on Harmonisation of Good Clinical Practice, and local regulations for clinical research.
Adult patients with T2D initiating a second-line glucose-lowering therapy (add-on or switching) after first-line oral therapy were invited by physicians to participate in the study from September 2014 to June 2016 [12]. Exclusion criteria included being pregnant, undergoing dialysis, having a history of renal transplant, and receiving first-line therapy with an injectable agent or an herbal remedy/natural medicine alone. All participating patients provided informed consent.
Data were collected using a standardized electronic case report form and included patient sociodemographics, laboratory test results, first- and second-line glucose-lowering therapies, preexisting comorbidities, and comedications. In line with the observational nature of the study, data were recorded according to routine clinical practice at each site during each noncompulsory study visit. To assess health-related quality of life (HRQoL), patients completed local-language versions of the 36-item Short-Form Health Survey version 2.0 (SF-36v2). The SF-36v2 is a generic health status measure that provides physical component summary (PCS) scores and mental component summary (MCS) scores. PCS and MCS scores are scaled to overall US norms of 50 and a standard deviation of 10, with higher scores indicating better HRQoL. A minimum clinically important difference is ~ 2 points [15]. SF-36v2 data were not collected from patients in Bahrain, Kuwait, and Oman owing to site preferences or limited availability of validated translated versions of the questionnaire.
Compliance with Ethics Guidelines
The study protocols were approved by the relevant clinical research ethics committees in each country and institutional review boards at each site, and complied with the Declaration of Helsinki, the International Conference on Harmonization of Good Clinical Practice, and the local regulations for clinical research.
Statistical Analysis
Patient data were compared among regions using one-way analysis of variance for continuous variables and the chi-squared test for categorical variables. P value < 0.05 was considered statistically significant. Statistical analyses were conducted using the SAS version 9.4 software (SAS Institute, Inc., Cary, NC, USA).
Results
Patient Characteristics
A total of 3525 patients were enrolled in DISCOVER from 152 sites across the 12 countries included in this analysis: 2240 patients (63.5%) from 113 sites in the Mediterranean, 766 patients (21.7%) from 21 sites in GCC countries, and 519 patients (14.7%) from 18 sites in South Africa (Table 1). The most common enrolling sites were primary care centers (37/113) and university/teaching hospitals (37/113) in Mediterranean countries, general hospitals (12/21) in GCC countries, and primary care centers (4/18) in South Africa. Nearly all sites (147/152) were located in urban areas.
The mean age of patients at enrollment was 54.3 ± 10.8 years, 1850 (52.5%) were men, 974 (29.7%) had no or only primary education, and 487 (14.4%) had no medical insurance (Table 2). The proportion of men was lowest in South Africa (Mediterranean versus GCC versus South Africa: 54.9% versus 59.7% versus 31.4%; P < 0.001), as was the proportion with governmental insurance (Mediterranean versus GCC versus South Africa: 61.4% versus 86.2% versus 43.7%; P < 0.001). Hypertension and hyperlipidemia were both common, with the lowest rates found in Mediterranean countries. Mean SF-36v2 PCS and MCS scores were highest in GCC countries (51.3 ± 7.1 and 48.2 ± 9.4, respectively) versus Mediterranean countries (47.6 ± 8.0 and 42.5 ± 10.2, respectively) versus South Africa (47.0 ± 8.1 and 47.8 ± 9.9, respectively).
Complications and Risk Factors
At enrollment, 17.7% of patients had known microvascular complications and 11.5% had known macrovascular complications (Table 3). Microvascular complications were least common in South Africa (Mediterranean versus GCC versus South Africa: 18.7% versus 20.5% versus 9.2%; P < 0.001), whereas the prevalence of macrovascular complications was similar among regions (Mediterranean versus GCC versus South Africa: 12.0% versus 10.4% versus 10.8%; P = 0.434). Use of medications for cardiovascular risk reduction was suboptimal across regions, with 33.6% of patients receiving either an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker (Mediterranean versus GCC versus South Africa: 31.8% versus 35.5% versus 38.5%; P = 0.006) and 42.2% of patients receiving a statin, the use of which was notably low in patients from Mediterranean countries (Mediterranean versus GCC versus South Africa: 33.8% versus 59.7% versus 52.8%; P < 0.001).
Glycemic Factors
The mean time since T2D diagnosis was higher in South Africa than in Mediterranean or GCC countries (Mediterranean versus GCC versus South Africa: 5.8 years versus 6.5 years versus 7.5 years; P < 0.001 (Table 3). Patients in South Africa were more likely to have missing glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) measurements than those in Mediterranean or GCC countries (Mediterranean versus GCC versus South Africa: missing HbA1c: 6.7% versus 5.0% versus 64.4%; missing FPG: 14.1% versus 19.6% versus 84.8%). Among patients with HbA1c measurements, the baseline mean HbA1c level was 8.7% (72.0 mmol/mol). HbA1c levels were highest in South Africa (Mediterranean versus GCC versus South Africa: 8.6% (71 mmol/mol) versus 8.8% (68 mmol/mol) versus 9.0% (75 mmol/mol); P < 0.001).
First- and Second-Line Glucose-Lowering Therapies
First-line therapy with a single agent was the most common glucose-lowering strategy across regions and was highest in South Africa (Mediterranean versus GCC versus South Africa: 67.0% versus 51.2% versus 90.2%; P < 0.001) (Table 4). Metformin was the most commonly prescribed first-line medication (Mediterranean versus GCC versus South Africa: 85.4% versus 90.3% versus 96.5%; P < 0.001), and sulfonylureas were also commonly used, particularly in GCC countries (Mediterranean versus GCC versus South Africa: 35.6% versus 45.4% versus 12.7%; P < 0.001). At second line, the majority of patients received two or three glucose-lowering medications, with most patients continuing to receive metformin. Sulfonylureas were commonly prescribed, particularly in South Africa (Mediterranean versus GCC versus South Africa: 48.6% versus 57.6% versus 82.5%; P < 0.001), whereas dipeptidyl peptidase-4 inhibitors were often added in Mediterranean and GCC countries (Mediterranean versus GCC versus South Africa: 51.0% versus 73.8% versus 3.7%; P < 0.001). Across all three regions, the overall use of sodium–glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide receptor-1 (GLP-1) receptor agonists was low (3.2% and 2.7%, respectively).
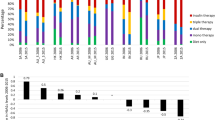
The main reason for changing from first- to second-line therapy reported by investigators was lack of efficacy of first-line therapy (92.3%, Fig. 1). Second-line therapies were mostly chosen on the basis of efficacy (73.2%), tolerability (26.8%), and low risk of weight gain (20.5%). However, in South Africa, tolerability and risk of weight gain were uncommon reasons for second-line medication selection (3.7% and 4.4%, respectively), while access to medications and cost were more commonly cited (21.8% and 13.3%) than in Mediterranean and GCC countries.
Reasons cited by investigators for (a) changing first-line therapy and (b) choosing a second-line therapy for patients in the Middle East and Africa cohort of the DISCOVER study according to subregion. Multiple reasons could be selected. Reasons for changing first-line therapy appearing in less than 2% of cases are not shown (developed acute disease, developed chronic disease, inability to self-administer, prescriber access, and drug interactions)
Discussion
This analysis of baseline DISCOVER study data provides a unique insight into the demographic and clinical characteristics of patients with T2D moving from first- to second-line glucose-lowering therapy in Mediterranean countries, GCC countries, and South Africa. Data are presented on contemporary strategies used for glycemic control and cardiovascular risk reduction, as well as variations in care. Importantly, a number of potential gaps in care and the influence of different healthcare systems and socioeconomic structures across the regions are highlighted. Patient access to appropriate testing and medications can have a marked impact on the ability of clinicians to provide quality care. While the extent to which differences in treatment observed across regions are due to deficiencies in clinician understanding or patient access is unknown, improving quality of care within the regions must address both these issues.
One key finding of our analysis was the suboptimal monitoring of glycemic control. Both HbA1c and FPG measurements were missing for a substantial number of patients, particularly in South Africa, indicating serious limitations in access to this important measurement. Inadequate glycemic testing may lead to treatment inertia and suboptimal glucose control, putting these patients at higher risk of developing micro- and macrovascular complications [16]. Access to medications and costs were much more likely to influence the choice of second-line glucose-lowering medication in South Africa than in the other regions, whereas clinical reasons more often had an impact on the decision in Mediterranean and GCC countries. The proportion of patients prescribed sulfonylureas as second-line glucose-lowering therapy was particularly high in South Africa (82.5% of patients), most likely reflecting limited access to newer and potentially more costly medications at the time of study enrollment. In addition, the use of glucose-lowering medications with cardiovascular risk reduction (i.e., SGLT-2 inhibitors[17] and GLP-1 receptor agonists[18]) was low across regions. This was in contrast to the more frequent use of both insulin and sulfonylureas, possibly reflecting a glucose-centric approach to disease management within these countries and a lack of understanding of the potential cardioprotective benefits of these drugs [19]. Indeed, results from the recent CAPTURE observational study also highlighted deficits in the uptake of cardioprotective glucose-lowering drugs, despite the markedly high prevalence of cardiovascular complications in those patients [20]. Despite the cost, another explanation of such low rates of prescribing these two classes, mainly SGLT-2 inhibitors, is the fact that it was approved by the US Food and Drug Administration (FDA) in March 2013, which means that it was available in the markets of the study regions just before, or even after, the commencement of the DISCOVER study in 2014. For other classes, such as thiazolidinediones, the main limiting factor for choosing it as second-line therapy could be in accordance with the decreased worldwide trend of the use of thiazolidinediones, especially after the global withdrawal of rosiglitazone in 2010, and subsequent negative impression about the whole class.
Another key finding was the variation in the prevalence of cardiovascular comorbidities, such as hypertension and hyperlipidemia, across regions. This could represent the effect of inter-region variations in ethnicity or lifestyle (e.g., the Mediterranean diet) [21]. The lower prevalence of microvascular disease in South Africa could be explained by the lower proportion of men or current smokers. However, given other gaps in care noted in this country, reduced access to screening for such complications may potentially be a major contributor to this finding. Use of medications that reduce the risk of diabetes complications (e.g., angiotensin-converting enzyme inhibitors, angiotensin-II receptor blockers, and statins) was suboptimal across regions, despite the high rates of cardiovascular risk factors and evident diabetes complications at enrollment. Interestingly, these drugs were used in South Africa at similar or higher rates than in the other regions; quality of care may therefore be driven more by access issues (i.e., these classes of medications are inexpensive across regions) than by gaps in clinician education. While clinician understanding of guideline-directed medical care is important, our data demonstrate the effect the structure of the health system can have on both diagnostic and therapeutic management for such chronic diseases.
This subanalysis of the DISCOVER study contains data from over 3000 patients from many countries in the Middle East and Africa for which recent information on the management and outcomes of patients with T2D is lacking. By enrolling patients at the point of initiation of second-line therapy, the DISCOVER study provides insights into the treatment and management of T2D at an early stage in disease progression. However, these data are therefore not strictly representative of all patients with T2D, such as those with a longer disease history or those managing their disease through diet and exercise alone. Although sites were selected to be as representative as possible of the management of T2D within each country, urban sites are over-represented within this region, with only two countries (Saudi Arabia and South Africa) including rural sites. Per-country recruitment was very low in some countries, limiting the generalizability of the data on disease management in each region. Data on the use of glucose-lowering drugs were limited to clinician records, as patient adherence to treatment was not assessed. Additionally, the variability between South Africa and other regions in terms of the rate of governmental insurance coverage and main investigator specialty could have made the comparison of the diabetes medication perception pattern somewhat complicated. Finally, South Africa was the only country in DISCOVER to represent the South and Central Africa regions, and is geographically distinct from Mediterranean and GCC countries. However, we believe its inclusion in this analysis provides important insight into differences in practice patterns in the overall region.
Conclusions
Using baseline data from the Middle East and Africa region of the DISCOVER study program, substantial inter-region variations were highlighted in the management of T2D when switching from first- to second-line therapy. Most patients received metformin as first-line therapy, either as monotherapy or in combination with another agent, while sulfonylureas (SUs) and dipeptidyl peptidase-4 (DPP-4) inhibitors were commonly prescribed as second line. Despite these differences, glycemic control was often poor, owing to suboptimal monitoring and potential delays in treatment intensification. The use of newer glucose-lowering drugs with cardioprotective effects, such as SGLT-2 inhibitors and GLP-1 receptor agonists, was uncommon, despite the high prevalence of cardiovascular risk factors and existing vascular complications. Improved access to and government support for reimbursement of these newer but more expensive glucose-lowering medications may be of substantial benefit to patients in these regions. Improved monitoring of blood glucose levels, as well as timely and appropriate treatment intensification, may reduce the risk of patients developing future complications in regions known to have a high prevalence of T2D.
References
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
International Diabetes Federation. IDF diabetes atlas, 9th edition. Brussels, Belgium: International Diabetes Federation; 2019.
Kalan Farmanfarma KH, Ansari-Moghaddam A, Zareban I, Adineh HA. Prevalence of type 2 diabetes in Middle-East: systematic review & meta-analysis. Prim Care Diabetes. 2020;14:297–304.
Pheiffer C, Pillay-van Wyk V, Turawa E, Levitt N, Kengne AP, Bradshaw D. Prevalence of type 2 diabetes in South Africa: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:5868.
Abuyassin B, Laher I. Diabetes epidemic sweeping the Arab world. World J Diabetes. 2016;7:165–74.
Sonmez A, Haymana C, Bayram F, Salman S, Dizdar OS, Gurkan E, et al. Turkish nationwide survEy of glycemic and other metabolic parameters of patients with diabetes mellitus (TEMD study). Diabetes Res Clin Pract. 2018;146:138–47.
Alramadan MJ, Magliano DJ, Almigbal TH, Batais MA, Afroz A, Alramadhan HJ, et al. Glycaemic control for people with type 2 diabetes in Saudi Arabia—an urgent need for a review of management plan. BMC Endocr Disord. 2018;18:62.
Masilela C, Pearce B, Ongole JJ, Adeniyi OV, Benjeddou M. Factors associated with glycemic control among South African adult residents of Mkhondo municipality living with diabetes mellitus. Medicine (Baltimore). 2020;99: e23467.
Alramadan MJ, Afroz A, Hussain SM, Batais MA, Almigbal TH, Al-Humrani HA, et al. Patient-related determinants of glycaemic control in people with type 2 diabetes in the Gulf Cooperation Council countries: a systematic review. J Diabetes Res. 2018;2:9389265.
Al-Rasheedi AA. Glycemic control among patients with type 2 diabetes mellitus in countries of Arabic Gulf. Int J Health Sci (Qassim). 2015;9:345–50.
Zabetian A, Keli HM, Echouffo-Tcheugui JB, Narayan KMV, Ali MK. Diabetes in the Middle East and North Africa. Diabetes Res Clin Pract. 2013;101:106–22.
Ji L, Bonnet F, Charbonnel B, Gomes MB, Kosiborod M, Khunti K, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Complications. 2017;31:1188–96.
Katakami N, Mita T, Takahara M, Hashigami K, Kawashima M, Shimomura I, et al. Rationale and design for the J-DISCOVER study: DISCOVERing the treatment reality of type 2 diabetes in a real-world setting in Japan—a protocol. Diabetes Ther. 2018;9:165–75.
Gomes MB, Rathmann W, Charbonnel B, Khunti K, Kosiborod M, Nicolucci A, et al. Treatment of type 2 diabetes mellitus worldwide: baseline patient characteristics in the global DISCOVER study. Diabetes Res Clin Pract. 2019;151:20–32.
Maruish M, Kosinski M, Bjorner J, Gandek B, Turner-Bowker D, Ware J. User’s manual for the SF-36v2 health survey. Lincoln: QualityMetric. Inc; 2011.
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2011;29:116.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9.
Tsapas A, Karagiannis T, Avgerinos I, Liakos A, Bekiari E. GLP-1 receptor agonists for cardiovascular outcomes with and without metformin. A systematic review and meta-analysis of cardiovascular outcomes trials. Diabetes Res Clin Pract. 2021;177:108921.
Ahmed HM, Khraishah H, Cho L. Cardioprotective anti-hyperglycaemic medications: a review of clinical trials. Eur Heart J. 2017;39:2368–75.
Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20:154.
Martín-Peláez S, Fito M, Castaner O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients. 2020;12:2236.
Acknowledgements
The authors would like to thank all investigators and patients who participated in the DISCOVER study program. Medical writing support was provided by Bobby Thompson, MSc(Res), of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
Funding
The DISCOVER study program and rapid service fee were funded by AstraZeneca. DISCOVER is a non-interventional study and no drugs were supplied or funded.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility of the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Consept and design: KAR, MAS, AH and VR. Data collection: KAR, ABN, FB, AE, KH, KK, AK, RM, SVA. Statistical anlysis: ABN, AH and MAS. Manuscript writing: The general content of the manuscript was agreed upon by all authors, and all authors contributed to manuscript development. all authors approved the final version of the manuscript before its submission. An AstraZeneca team reviewed the manuscript during its development and was allowed to make suggestions. However, the final content was determined by the authors.
Compliance with Ethics Guidelines
The study protocols were approved by the relevant clinical research ethics committees in each country and institutional review boards at each site, and complied with the Declaration of Helsinki, the International Conference on Harmonization of Good Clinical Practice, and the local regulations for clinical research. Written consent form was obtained from each participant in DISCOVER study.
Disclosures
Khalid Al-Rubeaan, Abdullah Ben-Nakhi, Rachid Malek, and, Suzanne V. Arnold have nothing to disclose. Mohamed Alsayed, Ahmed Hadaoui, and Viraj Rajadhyaksha are employees of AstraZeneca. Kevin Kennedy is an employee of Saint Luke’s Mid America Heart Institute, which has received funding from AstraZeneca. Fahri Bayram reports speaker honoraria and travel sponsorship from Bilim İlaç, Abbott, Novo Nordisk, Sanofi Genzyme, Pfizer, and Eczacıbaşı İlaç, and advisory board and consultancy fees from Novo Nordisk, Sanofi Genzyme, Abbott, Pfizer, and Bilim İlaç. Akram Echtay has received advisory board fees from AstraZeneca, Boehringer Ingelheim, Merck, and Novo Nordisk, and speaker fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Novo Nordisk, and Sanofi. Khadija Hafidh has received speaker fees and research support from AstraZeneca, Boehringer Ingelheim, Servier, Sanofi, Merck & Co., Novartis, Novo Nordisk, Pfizer, and Eli Lilly. Adri Kok has received speaker and advisory board fees from Novo Nordisk, AstraZeneca, Merck & Co., Eli Lilly, Aspen Pharma, Sanofi, Boehringer Ingelheim, Cipla, Mylan, Pharmadynamics, Adcock Ingram, and Pfizer.
Data Availability
The data sets generated during and/or the current study are available in AstraZeneca's repository and may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Al-Rubeaan, K., Alsayed, M., Ben-Nakhi, A. et al. Characteristics and Treatment Patterns of Patients with Type 2 Diabetes Mellitus in the Middle East and Africa Cohort of the DISCOVER Study Program: a Prospective Study. Diabetes Ther 13, 1339–1352 (2022). https://doi.org/10.1007/s13300-022-01272-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01272-6