Abstract
Background
To evaluate the accuracy of screw placement using the TiRobot surgical robot in the Harms procedure and to assess the clinical outcomes of this technique.
Methods
This retrospective study included 21 patients with atlantoaxial instability treated by posterior atlantoaxial internal fixation (Harms procedure) using the TiRobot surgical robot between March 2016 and June 2021. The precision of screw placement, perioperative parameters and clinical outcomes were recorded. Screw placement was assessed based on intraoperative guiding pin accuracy measurements on intraoperative C-arm cone-beam computed tomography (CT) images using overlay technology and the incidence of screw encroachment identified on CT images.
Results
Among the 21 patients, the mean age was 44.8 years, and the causes of atlantoaxial instability were os odontoideum (n = 11), rheumatoid arthritis (n = 2), unknown pathogenesis (n = 3), and type II odontoid fracture (n = 5). A total of 82 screws were inserted with robotic assistance. From intraoperative guiding pin accuracy measurements, the average translational and angular deviations were 1.52 ± 0.35 mm (range 1.14–2.25 mm) and 2.25° ± 0.45° (range 1.73°–3.20º), respectively. Screw placement was graded as A for 80.5% of screws, B for 15.9%, and C for 3.7%. No complications related to screw misplacement were observed. After the 1-year follow-up, all patients with a neurological deficit experienced neurological improvement based on Nurick Myelopathy Scale scores, and all patients with preoperative neck pain reported improvement based on Visual Analog Scale scores.
Conclusions
Posterior atlantoaxial internal fixation using the Harms technique assisted by a 3D-based navigation robot is safe, accurate, and effective for treating atlantoaxial instability.
Similar content being viewed by others
Background
Atlantoaxial disorders usually result in instability of the upper cervical spine and may cause spinal cord dysfunction, vascular impairment and cervical pain when left untreated for too long [1, 2]. Such disorders are mainly attributed to embryological, traumatic, or inflammatory factors. Currently, internal fixation is regarded as the main treatment for atlantoaxial instability. The Harms technique involves placement of C1 lateral mass screws in combination with C2 pedicle screws and was first described by Goel and Laheri in 1994 [3] and later by Harms and Melcher in 2001 [4]. This technique is now a popular method for atlantoaxial fixation that can achieve good pain relief and reliable biomechanical stability [5]. However, in clinical practice, screw placement has proven to be a high-risk procedure due to the complicated anatomical structures in the upper cervical region, and the possible adverse outcomes include vertebral artery and spinal cord injury. The risk is even greater when aberrant anatomy exists [6]. Relying on fluoroscopic images and surgeons’ experience alone, the conventional posterior screw insertion method is not sufficiently accurate and poses risks due to screw malposition [7].
With the rapid development of surgical robotics and its increased application in clinical practice, robotic assistance has been shown to improve the precision of screw placement in different spinal regions [8]. Compared with other surgically assisted techniques, robot-assisted spinal surgery demonstrates superior results in terms of reducing screw dislocation, shortening the duration of intraoperative radiation, and minimizing surgical bleeding [9]. Several orthopedic robotic systems, such as the TiRobot, SpineAssist, Renaissance, and Mazor, have been applied in spinal surgeries [10], and among them, the TiRobot system is the only one that can be used for posterior screw insertion in the craniocervical area [11].
In the present study, the TiRobot system was utilized to assist performance of the Harms procedure in patients with atlantoaxial instability. The resulting surgical outcomes as well as the accuracy rate of screw placement with the TiRobot surgical equipment were evaluated in the present study.
Methods
Patient enrollment
Patients with atlantoaxial instability due to embryological, traumatic, or inflammatory causes who underwent open or percutaneous Harms procedures in our institution between March 2016 and June 2021 were enrolled in this study. The exclusion criteria were atlantoaxial instability caused by neoplasm or infection, concurrent treatment with cervical procedures (such as anterior odontoid fixation, cervical laminoplasty or laminectomy, lower cervical pedicle screw placement, etc.), previous surgery in the upper cervical region, and failure to complete the 1-year follow-up or refusal to provide informed consent. This study was approved by the ethical board of our local institute (IRB: 201909-11).
Twenty-one patients who underwent robot-assisted Harms operation as treatment for atlantoaxial instability and met the inclusion and exclusion criteria for this study were enrolled. Among them, 11 patients were male and 10 were female. The mean patients age was 44.8 (range 19–63) years. The diagnosed causes of atlantoaxial instability in these patients included os odontoideum (n = 11, 52%), rheumatoid arthritis (n = 2, 10%), unknown pathogenesis (n = 3, 14%) and type II odontoid fracture (n = 5, 24%).
Surgical techniques
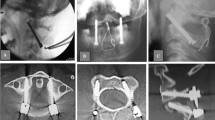
The procedures were conducted with the TiRobot system (TINAVI Medical Technologies, Beijing, China). After general anesthesia, the patient was placed in a prone position, and the head was fixed onto the operating table with the Mayfield frame. In open surgery, the midline approach was routinely performed to expose the C1 posterior arch and C2 lamina. Then the patient tracker was anchored onto the Mayfield frame. For the percutaneous minimally invasive approach, the patient tracker was anchored first and then two para-median incision and intermuscular approaches were created under robotic guidance. The C-arm (ARCADIS Orbic 3D, Siemens Medical Solutions, Erlangen, Germany) was employed to take X-ray images, which were then transferred to the robotic workstation. The senior surgeon planned the optimal trajectory for the C1 lateral screws and C2 pedicle screws on the workstation. Then, the robotic arm was moved to the target position guided by an NDI stereo camera (Northern Digital Inc., Ontario, Canada). The guiding cannula was placed onto the end of the robotic arm. Once the deviation of guidance was within 0.5 mm and became steady, a K-wire was drilled as a guiding pin into the vertebrae along the guiding cannula to the optimal depth (Fig. 1). The position of the guiding pin was verified by re-scanning of the C-arm. Once satisfactory positioning of the guiding pin was achieved, the pilot hole was tapped, followed by insertion of 3.5-mm diameter polyaxial screws (Mountaineer, Medtronic Sofamor Danek, Memphis, TN or Summit, Depuy Spine, Raynham, MA). The cartilage of the C1–C2 articular joint was drilled out, and an autograft harvested from the iliac crest was placed into that space.
We performed the reduction maneuver by adjusting the position of the Mayfield frame and monitoring it on lateral fluoroscopy. After the reduction was satisfactory on the fluoroscopy, we acquired the 3D images using the C-arm to ensure that C1–C2 was in the anatomical reduction position or the space available for the spinal cord (distance from the posterior margin of dens to the laminar line) was more than 13 mm on the sagittal image. If the reduction procedure was satisfactory, the connecting rod and pre-tightened screw heads were installed. When needed, continuous efforts were made to adjust the atlantoaxial alignment until the reduction was satisfactory.
Evaluation of screw placement accuracy
Intraoperative guiding pin accuracy
Guiding pin accuracy was measured on the basis of the X-ray images obtained by the C-arm intraoperatively. The steps of the workflow were as follows:
-
Step 1: Image fusion. Based on the manual fusion method through overlay technology, the screw planning C-arm cone-beam computed tomography (CBCT) image was matched with the guiding pin placement CBCT image on the TiRobot Spine Software platform (Fig. 2).
-
Step 2: Entry and target point identification. The spatial coordinate values (X, Y, Z) of the entry point and target point were determined on the screw planning CBCT image as well as guiding pin placement CBCT image, separately.
-
Step 3: Accuracy calculation. The accuracy of the entry point and target point were calculated separately based on Euclidean distance. Then the translational and angular deviations in the axial and sagittal planes were calculated using the following formulae:
$$Entry\; error = \sqrt {\left( {EX - EX^\prime } \right)^{2} + \left( {EY - EY^\prime } \right)^{2} + \left( {EZ - EZ^\prime } \right)^{2} }$$$${\text{Target}}\; error = \sqrt {\left( {TX - TX^\prime } \right)^{2} + \left( {TY - TY^\prime } \right)^{2} + \left( {TZ - TZ^\prime } \right)^{2} }$$$$Total\; positioning\; error = \frac{Entry\; error + Target\; error}{2}$$
Screw encroachment grading
The accuracy of the screw placement also was evaluated on postoperative CT images. The precision of screw placement was classified according to three grades of the scale introduced by Gertzbein and Robbins [12]. A screw inserted completely within the pedicle was regarded as grade A; a screw inserted with a pedicle cortical breach less than 2 mm was regarded as grade B; and a screw inserted with a pedicle cortical breach exceeding 2 mm was classified as grade C.
Clinical assessment
The operative time, estimated volume of blood loss and perioperative hospitalization duration were recorded and analyzed. Clinical examinations were performed preoperatively and 1 year after surgery and included the acquisition of Nurick Myelopathy Scale (NMS) and Visual Analog Scale (VAS) scores. All intraoperative and postoperative complications were recorded. The fusion status was assessed by CT scanning 1 year after surgery.
Statistical analysis
All statistical analyses were performed using SPSS 26.0 (SPSS Inc, Chicago, IL). For quantitative data, differences were evaluated using the t-test if the data conformed to a normal distribution. P < 0.05 was regarded as the threshold for statistical significance.
Results
Operative information
Twenty of the twenty-one patients received bilateral C1 lateral screw placement together with C2 pedicle screw fixation. The only exception was a patient who had an anatomic variation that allowed for only one C1 lateral screw to be placed with one C2 pedicle screw on the left side. Thus, 82 screws were inserted in total with the aid of the robotic system. These procedures were performed by three senior spine surgeons though an open approach in 17 patients and a minimally invasive approach in 4 patients.
Overall, the mean operative time was 266.9 ± 64.7 min, and the mean blood loss volume was 348.6 ± 250.9 mL. The mean operative time for the open procedure was 265.3 ± 63.5 min, and that for the minimally invasive procedure was 273.8 ± 79.7 min (t = − 0.0230, P = 0.821). The estimated blood loss volume was 394.1 ± 253.6 mL for the open procedure and 155.0 ± 121.5 mL for the minimally invasive procedure (t = 1.810, P = 0.086). The mean postoperative hospital stay was 6.8 ± 5.1 days for all patients, 7.5 ± 5.5 days after the open procedure and 4.0 ± 1.4 days after the minimally invasive procedure (t = 1.232, P = 0.233; Fig. 3).
Screw placement accuracy
The average translational and angular deviations of the 82 screw guiding pins were 1.52 ± 0.35 mm (range 1.14–2.25 mm) and 2.25° ± 0.45° (range 1.73–3.20º), respectively. Sixty-six out of 82 screws (80.5%) were inserted with perfect precision (grade A); 13 screw placements were classified as grade B (15.9%, 13/82); and 3 were classified as grade C (3.7%, 3/82). No complications were found to be related to unsatisfactory screw placement.
Clinical outcomes
No complications were detected after 1 year of follow-up. Additionally, no additional complications were observed in percutaneous surgery, especially with regard to greater occipital neuralgia. All patients with a neurological deficit experienced neurological improvement throughout the follow-up period. The preoperative NMS scores were 0 for 5 patients, 1 for 4 patient, 2 for 6 patients, 3 for 4 patients, and 4 for 2 patients, whereas at the 1-year follow-up, 11 patients had no neurological deficits, 7 cases had a score of 1, and 3 cases had a score of 2 (Fig. 4). Symptoms were relieved or improved in all patients with preoperative neck pain, with VAS scores showing improvement by more than 70% in all of these patients (Fig. 4). All patients achieved solid fusion of the C1–C2 articular joint at 1 year postoperatively.
Discussion
The TiRobot system is a multi-functional orthopedic surgical robotic system that can be utilized for thoracolumbar pedicle screw insertion, percutaneous vertebroplasty and lumbar-pelvic fixation [13,14,15,16].
For spinal surgery, the precision of screw placement is very important at the craniocervical junction, where the surgical window is very narrow for superior cervical screw placement, as many vital structures traverse adjacent to bony structures, making screw placement in this area dangerous. Anatomical variations make this situation even more difficult [17]. Since the Harms technique was first described, the construct has been considered safer than transarticular screws, because it is less-dependent on vertebral artery alignment due to the flexibility in screw trajectory [18, 19]. However, the risk of vertebral artery injury for C2 pars or pedicle screw placement cannot be completely avoided [20], and such injury can lead to severe blood loss, neurological impairment, stroke, and even death [21, 22].
The present study demonstrated that use of the TiRobot system could ensure the accuracy of Harms construct placement, helping to avoid complications. The high accuracy achieved with usage of the robotic system is consistent with the findings of previous reports for other types of surgeries. One meta-analysis demonstrated that the rate of acceptable pedicle screw placement, as categorized by Gerztbein-Robbin Grade A + B, was approximately 95% with the aid of the robot, which was significantly superior to the free-hand technique (odds ratio = 1.54) [23]. Compared with our present robot-assisted study, conventional methods offered much lower precision of screw placement according to the literature for Harms surgery. Zhan et al. reported that using conventional fluoroscopy guidance for Harms procedure, the proportion of “clinically acceptable” screws (graded A and B) was only 87.5% [24]. Similar results were seen in the studies conducted by Li et al. with safe screws accounting for 81.7% of 120 screws [25] and by Sancipriano et al. in which the incidence of screw malpositioning was 13% [26]. Previous studies also concluded that the application of robotics in spinal surgery greatly reduces the intraoperative time and radiation dosage in comparison to conventional procedures [23, 27]. The magnitude of screw deviations observed in the present study demonstrated good consistency with those reported previously. Van Dijk et al. used the Mazor robot to place percutaneous lumbar pedicle screws and reported a mean deviation at the entry point of 2.0 ± 1.2 mm and mean differences in the angle of insertion of 2.2° ± 1.7° on the axial plane and 2.9° ± 2.4° on the sagittal plane [28]. Devito et al. compared the planned screw insertion angles with the actual insertion of screws with the assistance of the Mazor robot and reported mean deviations of 1.2 ± 1.5 mm on the axial plane and 1.1 ± 1.2 mm on the sagittal plane [29]. A previous study by Jiang et al. showed that use of the ExcelsiusGPS surgical robot afforded a deviation of the screw tip of 2.1 mm (range 0.8–5.2 mm), a mean deviation of the screw caudal of 3.2 mm (range 0.9–5.4 mm) and a mean angular deviation of 2.4° (range 0.7–3.8°) [30]. These findings demonstrate good consistency with results in the present study, in which the TiRobot system facilitated good precision with about 1.5 mm deviation and 2° offset with an acceptable accuracy of 96.4% in the Gertzbein-Robbins evaluation. The source of this deviation is multifactorial [30, 31]. Entering the pedicle with no flat drilling surface might predispose the guiding pin to slip off the exact entry point, causing lower accuracy and higher deviation [32]. This slipping can be minimized by choosing an entry point that is not located on the steep slope of the bony surface, by pre-preparing the surface using a highspeed drill or an ultrasonic osteotome, or by using a sharp tip pin driven by a highspeed drill.
In the present study, we used two approaches, open and minimally invasive, to perform the Harms procedure. The estimated blood loss tended to be less in the minimally invasive procedure compared with the open approach, although the observed difference was not significant. Because minimally invasive screw fixation is performed through a muscle-expanding approach that significantly reduces the number of iatrogenic soft tissue injuries, it has been found to offer several potential advantages over open techniques, including reductions in blood loss, postoperative pain, recovery time, and the emotional impact on the patient [33]. Multiples studies have described minimally invasive Harms procedures [34,35,36]. However, a major problem of this technique is the absence of anatomical landmarks, which makes screw fixation technically challenging. The application of a navigational surgical robot is an optimal method to solve this problem. We did not compare the accuracy of screw placement between the open and minimally invasive approaches directly in the present study due to our small sample size. However, the screw accuracy overall was high with the assistance of the TiRobot system. An additional advantage of the robotic platform is that it allows the surgeon to locate the entry point at the skin level, thereby reducing the required incision size. Further studies are needed to clarify the benefit of a minimally invasive Harms technique. In the present study, the operative time and postoperative hospital stay achieved with minimally invasive surgery were comparable with those associated with the open surgery, implying the minimally invasive approach is likely as feasible as open surgery in clinical application.
The main limitation of this study was that comparison was only possible between the preoperative planning images and the observation of guiding pins on intraoperative CBCT. Although the pilot hole was tapped following placement of the guiding pin, the screw was inserted without the aid of the robot, which could lead to errors. However, because of the impact of artifacts, it is impossible to compare the preoperatively planed locations to the final screw placement on postoperative CT. This study is also limited in that it was a case series study without a control group. Thus, the benefit and utility of the robot-assisted Harms procedure remain to be elucidated in future randomized control trials. Still, the present study provides preliminarily evidence of the significance of the investigated procedure and demonstrates that promising outcomes were achieved.
Conclusions
Posterior atlantoaxial internal fixation using the Harms technique assisted by 3D-based navigational robot is safe, accurate, and effective for the treatment of atlantoaxial instability.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CBCT:
-
C-arm cone-beam computed tomography
- NMS:
-
Nurick Myelopathy Scale
- VAS:
-
Visual Analog Scale
References
Panda S, Ravishankar S, Nagaraja D. Bilateral vertebral artery dissection caused by atlantoaxial dislocation. J Assoc Physicians India. 2010;58:187–9.
Yin Q, Ai F, Zhang K, Chang Y, Xia H, Wu Z, et al. Irreducible anterior atlantoaxial dislocation: one-stage treatment with a transoral atlantoaxial reduction plate fixation and fusion. Report of 5 cases and review of the literature. Spine. 2005;30:E375–81.
Goel A, Laheri V. Plate and screw fixation for atlanto-axial subluxation. Acta Neurochir (Wien). 1994;129:47–53.
Harms J, Melcher RP. Posterior C1–C2 fusion with polyaxial screw and rod fixation. Spine. 2001;26:2467–71.
Du JY, Aichmair A, Kueper J, Wright T, Lebl DR. Biomechanical analysis of screw constructs for atlantoaxial fixation in cadavers: a systematic review and meta-analysis. J Neurosurg Spine. 2015;22:151–61.
Derman PB, Lampe LP, Lyman S, Kueper J, Pan TJ, Girardi FP, et al. Atlantoaxial fusion: sixteen years of epidemiology, indications, and complications in New York State. Spine. 2016;41:1586–92.
Yang YL, Zhou DS, He JL. Comparison of isocentric C-arm 3-dimensional navigation and conventional fluoroscopy for C1 lateral mass and C2 pedicle screw placement for atlantoaxial instability. J Spinal Disord Tech. 2013;26:127–34.
Peng YN, Tsai LC, Hsu HC, Kao CH. Accuracy of robot-assisted versus conventional freehand pedicle screw placement in spine surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Transl Med. 2020;8:824.
Fan Y, Du J, Zhang J, Liu S, Xue X, Huang Y, et al. Comparison of accuracy of pedicle screw insertion among 4 guided technologies in spine surgery. Med Sci Monit. 2017;23:5960–8.
Zhang Q, Han XG, Xu YF, Fan MX, Zhao JW, Liu YJ, et al. Robotic navigation during spine surgery. Expert Rev Med Devices. 2020;17:27–32.
Tian W. Robot-assisted posterior C1–2 transarticular screw fixation for atlantoaxial instability: a case report. Spine. 2016;41(Suppl 19):B2-b5.
Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine. 1990;15:11–4.
Tian W, Liu YJ, Liu B, He D, Wu JY, Han XG, et al. Guideline for thoracolumbar pedicle screw placement assisted by orthopaedic surgical robot. Orthop Surg. 2019;11:153–9.
Wang B, Cao J, Chang J, Yin G, Cai W, Li Q, et al. Effectiveness of Tirobot-assisted vertebroplasty in treating thoracolumbar osteoporotic compression fracture. J Orthop Surg Res. 2021;16:65.
Liu ZJ, Hu YC, Tian W, Jin X, Qi HT, Sun YX, et al. Robot-aided minimally invasive lumbopelvic fixation in treatment of traumatic spinopelvic dissociation. Orthop Surg. 2021;13:563–72.
Tian W, Wang H, Liu YJ. Robot-assisted anterior odontoid screw fixation: a case report. Orthop Surg. 2016;8:400–4.
Murphy TM, McEvoy L, Bolger C. Surgical considerations of rheumatoid disease involving the craniocervical junction and atlantoaxial vertebrae. Rheumatoid Arthritis-Etiology, Consequences and Co-Morbidities. 2012:275–82.
Heuer GG, Hardesty DA, Bhowmick DA, Bailey R, Magge SN, Storm PB. Treatment of pediatric atlantoaxial instability with traditional and modified Goel-Harms fusion constructs. Eur Spine J. 2009;18:884–92.
Brockmeyer DL, York JE, Apfelbaum RI. Anatomical suitability of C1–2 transarticular screw placement in pediatric patients. J Neurosurg. 2000;92:7–11.
Hong JT, Lee SW, Son BC, Sung JH, Yang SH, Kim IS, et al. Analysis of anatomical variations of bone and vascular structures around the posterior atlantal arch using three-dimensional computed tomography angiography. J Neurosurg Spine. 2008;8:230–6.
Yeom JS, Buchowski JM, Park KW, Chang BS, Lee CK, Riew KD. Undetected vertebral artery groove and foramen violations during C1 lateral mass and C2 pedicle screw placement. Spine. 2008;33:E942–9.
Elgafy H, Pompo F, Vela R, Elsamaloty HM. Ipsilateral arcuate foramen and high-riding vertebral artery: implication on C1–C2 instrumentation. Spine J. 2014;14:1351–5.
Fatima N, Massaad E, Hadzipasic M, Shankar GM, Shin JH. Safety and accuracy of robot-assisted placement of pedicle screws compared to conventional free-hand technique: a systematic review and meta-analysis. Spine J. 2021;21:181–92.
Zhan J, Xu W, Lin J, Luan J, Hou Y, Wang Y, et al. Accuracy and safety of robot-assisted versus fluoroscopy-guided posterior C1 lateral mass and C2 pedicle screw internal fixation for atlantoaxial dislocation: a preliminary study. Biomed Res Int. 2022;2022:8508113.
Li Y, Lin J, Wang Y, Luo H, Wang J, Lu S, et al. Comparative study of 3D printed navigation template-assisted atlantoaxial pedicle screws versus free-hand screws for type II odontoid fractures. Eur Spine J. 2021;30:498–506.
Sancipriano V, Penner F, Cofano F, Ajello M, Marengo N, Petrone S, et al. Intraoperative computed tomography for C1–C2 stabilization by goel-harms: analysis of clinical efficacy and a novel classification of screw placement accuracy. World Neurosurg. 2022;158:e19–37.
Li HM, Zhang RJ, Shen CL. Accuracy of pedicle screw placement and clinical outcomes of robot-assisted technique versus conventional freehand technique in spine surgery from nine randomized controlled trials: a meta-analysis. Spine. 2020;45:E111–9.
van Dijk JD, van den Ende RP, Stramigioli S, Köchling M, Höss N. Clinical pedicle screw accuracy and deviation from planning in robot-guided spine surgery: robot-guided pedicle screw accuracy. Spine. 2015;40:E986–91.
Devito DP, Kaplan L, Dietl R, Pfeiffer M, Horne D, Silberstein B, et al. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine. 2010;35:2109–15.
Jiang B, Karim Ahmed A, Zygourakis CC, Kalb S, Zhu AM, Godzik J, et al. Pedicle screw accuracy assessment in ExcelsiusGPS® robotic spine surgery: evaluation of deviation from pre-planned trajectory. Chin Neurosurg J. 2018;4:23.
Zhang Q, Fan MX, Han XG, Liu YJ, He D, Liu B, et al. Risk factors of unsatisfactory robot-assisted pedicle screw placement: a case-control study. Neurospine. 2021;18:839–44.
Tian W, Han X, Liu B, Liu Y, Hu Y, Han X, et al. A robot-assisted surgical system using a force-image control method for pedicle screw insertion. PLoS ONE. 2014;9: e86346.
Holly LT, Schwender JD, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus. 2006;20:E6.
Meyer M, Farah K, Graillon T, Dufour H, Blondel B, Fuentes S. Minimally invasive percutaneous C1–C2 fixation using an intraoperative three-dimensional imaging-based navigation system for management of odontoid fractures. World Neurosurg. 2020;137:266–71.
Bodon G, Patonay L, Baksa G, Olerud C. Applied anatomy of a minimally invasive muscle-splitting approach to posterior C1–C2 fusion: an anatomical feasibility study. Surg Radiol Anat. 2014;36:1063–9.
Holly LT, Isaacs RE, Frempong-Boadu AK. Minimally invasive atlantoaxial fusion. Neurosurgery. 2010;66:193–7.
Acknowledgements
None.
Funding
This work was supported by the Beijing Natural Science Foundation [Grant Number L192048] and Beijing Jishuitan Hospital Elite Young Scholar Programme [Grant Number XKGG202104].
Author information
Authors and Affiliations
Contributions
ZL: Data curation, Writing-original draft. XH: Data curation. MF: Methodology. YL: Resources. DH: Supervision. WT: Conceptualization, Methodology, Writing-review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study were planned and conduced in accordance with the ethical standards of the Institutional and National Research Committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, and approved by the medical ethics committee of Beijing Jishuitan Hospital [ethical approval number: 201909-11]. Informed consent was obtained from all individual participants involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lang, Z., Han, X., Fan, M. et al. Posterior atlantoaxial internal fixation using Harms technique assisted by 3D-based navigation robot for treatment of atlantoaxial instability. BMC Surg 22, 378 (2022). https://doi.org/10.1186/s12893-022-01826-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01826-2