Abstract
Background context
Studies have shown biomechanical superiority of cervical pedicle screw placement over other techniques. However, accurate placement is challenging due to the inherent risk of neurovascular complications. Navigation technology based on intraoperative 3D imaging allows highly accurate screw placement, yet studies specifically investigating screw placement in patients with traumatic atlantoaxial injuries are scarce. The aim of this study was to compare atlantoaxial screw placement as treatment of traumatic instabilities using iCT-based navigation or fluoroscopic-guidance with intraoperative 3D control scans.
Methods
This was a retrospective review of patients with traumatic atlantoaxial injuries treated operatively with dorsal stabilization of C1 and C2. Patients were either assigned to the intraoperative navigation or fluoroscopic-guidance group. Screw accuracy, procedure time, and revisions were compared.
Results
Seventy-eight patients were included in this study with 51 patients in the navigation group and 27 patients in the fluoroscopic-guidance group. In total, 312 screws were placed in C1 and C2. Screw accuracy was high in both groups; however, pedicle perforations > 1 mm occurred significantly more often in the fluoroscopic-guidance group (P = 0.02). Procedure time was on average 23 min shorter in the navigation group (P = 0.02).
Conclusions
This study contributes to the available data showing that navigated atlantoaxial screw placement proves to be feasible as well as highly accurate compared to the fluoroscopic-guidance technique without prolonging the time needed for surgery. When comparing these data with other studies, the application of different classification systems for assessment of screw accuracy should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compared to the thoracic and lumbar spine as well as the subaxial cervical spine, the upper cervical spine is characterized by its unique anatomy. Traumatic injuries in this anatomical region occur less frequently than in the thoracolumbar spine, yet the consequences may be more severe [1]. Because the injuries vary in morphology, different treatment options are viable [2,3,4]. In case of traumatic instabilities, studies have shown an advantage for surgical procedures in terms of higher fusion rates and pain reduction compared to conservative treatment [4, 5].
Depending on the morphology, surgical treatment can be performed via anterior, posterior, or combined anteroposterior fixation [3]. For posterior fixation with a screw and rod system, either lateral mass screws (LMS) or cervical pedicle screws (CPS) can be used for the axis. While LMS placement is less challenging, studies have shown biomechanical advantages for CPS regarding fixation stability [6].
Due to the spatial proximity of the screw corridor and neurovascular structures such as the vertebral artery, CPS must be placed with high accuracy to avoid neurovascular complications.
Intraoperative 3D imaging using cone beam CT (CBCT) or intraoperative computed tomography (iCT) has been recommended by different studies to detect potential screw misplacement intraoperatively and thus prevent secondary revision surgery [7,8,9,10,11]. Beyond intraoperative 3D imaging, other authors advocate the use of 3D image-guided navigation technology, potentially enhancing the accuracy of screw placement while reducing the need for fluoroscopic imaging during screw placement [12]. However, studies specifically comparing screw placement in the upper cervical spine for the treatment of traumatic injuries using either 3D-controlled fluoroscopic-guidance or 3D-based navigation are scarce and have included a very limited number of patients [13, 14].
Therefore, the aim of this study was to compare atlantoaxial screw placement for the treatment of traumatic instabilities using (1) fluoroscopic-guidance with additional intraoperative 3D control scans and (2) iCT-based navigation.
Material and methods
The authors hypothesize that iCT-based navigation can facilitate more accurate screw placement than 3D-assisted fluoroscopic-guidance techniques in cases of traumatic atlantoaxial injuries. In this single-center retrospective study, all patients with traumatic atlantoaxial injuries treated operatively with dorsal stabilization of C1 and C2 according to the techniques of Harms–Goel or Magerl between January 1, 2012, and December 31, 2022, were included. Atlantoaxial injuries included fractures of atlas and axis, as well as osteoligamentous and purely ligamentous instabilities. All included cases solely received dorsal fixation. No additional fusion was performed.
After retrospectively reviewing the digital patient charts, patients were assigned to two groups based on the use of iCT-based (Airo, Stryker GmbH & Co., KG, Kalamazoo, MI, USA) navigation (Curve, Brainlab Inc., Munich, Germany) (group 1) or the intraoperative use of fluoroscopic-guidance and additional 3D imaging using a C-arm CBCT (Arcadis Orbic or Cios Spin, both Siemens Healthineers, Erlangen, Germany) for implant control following screw placement (group 2).
A X-ray images preoperative, intraoperative, and postoperative of a C2 Anderson II fracture stabilized according to Harms–Goel. B CT images preoperative, intraoperative, and postoperative of a C2 Anderson II fracture stabilized according to Harms–Goel. C Images preoperative, intraoperative, and postoperative of a C2 Effendi II fracture stabilized according to Magerl
In both groups, patients were positioned on a radiolucent carbon-fiber surgical table in prone position with the head placed on an adjustable carbon headrest (Doro, Getinge, Maquet, Rastatt, Germany). After skin incision, soft tissue preparation, and electric drilling (drill diameter: VuePoint, NuVasive, San Diego, USA, Symphony, DePuy Synthes, Raynham, USA: 2.4 mm (3.5-mm screws); Mountaineer, DePuy Synthes, Raynham, USA: 2.7 mm (3.5-mm screws); Ennovate, Aesculap, Tuttlingen, Germany: 2.3 mm (3.5-mm screws) and 2.8 mm (4.0-mm screws); and Neon, Ulrich Medical, Ulm, Germany: 2.6 mm) of the preferred screw trajectory, guide wires were inserted. In the fluoroscopic-guidance group, lateral fluoroscopic images were obtained during the drilling process, while a navigated drill guide allowed live navigation of the drilling trajectory in the navigation group. Fluoroscopic imaging as well as 3D imaging was performed after guide wire placement to confirm the correct wire position, followed by screw placement with long shank screws for C1 and C2 screws in the technique described by Harms–Goel (Fig. 1A, B). In some cases, transarticular C2 screws were placed in the Magerl technique (Fig. 1C). After placement of all screws, intraoperative 3D imaging was performed to control screw position.
The following implants were used: VuePoint (NuVasive, San Diego, USA; group 1: 33 (64.7%); group 2: 6 (22.2%)), Symphony (DePuy Synthes, Raynham, USA; group 1: 16 (31.4%); group 2: 1 (3.7%)), Ennovate (Aesculap, Tuttlingen, Germany; group 1: 1 (2%); group 2: 0 (0%)), Mountaineer (DePuy Synthes, Raynham, USA; group 1: 1 (2%); group 2: 18 (66.7%)), and Neon (Ulrich Medical, Ulm, Germany; group 1: 0 (0%); group 2: 2 (7.4%)).
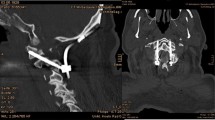
The operating room setup for navigated screw placement is depicted in Fig. 2A. In this group, the reference array was mounted on the spinous process of C2. After obtaining an iCT scan, the surgical instruments were calibrated, and screw trajectory planning was performed using the navigation system. The screws were inserted utilizing a navigated screwdriver, with the surgeons continuously monitoring the trajectory of the instruments and screws on the navigation displays in real time (Fig. 3). After screw placement, radiographic 3D control of implant position is performed. In both groups, postoperative CT scans were performed to confirm and document implant position with high-quality imaging (Fig. 1).
Radiological evaluation of screw placement accuracy was performed by two blinded observers using the postoperative CT scans to avoid a potential bias as a result of differences in image quality between CBCT and iCT as well as to ensure blinding of the observers. If a breach of the cortex was detected, the distance of the exterior cortex to the furthest screw rim was measured. Screw size measured in the scans was compared to the actual screw size to account for potential metal artifacts.
Screw placement was graded according to two different classification systems: the Neo classification with grades 0–3 as well as the Bredow classification with five grades (grade 1 to grade 5) at intervals of 1 mm (Table 1). Perforations of ≥ 2 mm and for Bredow classification the perforation of the transverse foramen by more than half a screw diameter (Neo grades 2 and 3, Bredow grades 3–5) were considered critical. Kappa was calculated and interpreted according to Landis and Koch to evaluate interobserver reliability.
The statistical analysis was performed using Prism 9 (GraphPad, San Diego, California, USA). Kolmogorov–Smirnov test was used to test for normal distribution of the data. Depending on the distribution, either a non-paired t test or the Mann–Whitney U test was performed. Binary data were analyzed using Fisher’s exact test. The significance level was set at P < 0.05.
Results
Overall, in our institution, 78 patients with traumatic atlantoaxial instability were treated surgically with posterior stabilization of C1/C2 in the study period. While navigated screw placement was performed in 51 patients (group 1), fluoroscopic-guidance with additional intraoperative 3D imaging was used in 27 patients (group 2). Accordingly, 312 screws were placed in total (204 in group 1 and 108 in group 2) with 156 screws placed in C1 and C2 each. In C2, 138 screws were placed in the Harms–Goel technique (96 in group 1 and 42 in group 2) and 18 screws in the Magerl technique (6 in group 1 and 12 in group 2).
The comparison of demographic data of the patients included showed no statistically significant differences between the groups (Table 2).
Accuracy of screw placement
In C1, 97.1% of the screws placed in group 1 showed either no perforation or a perforation of < 1 mm. In the fluoroscopic-guidance group, this was true for 88.9% of the screws placed in C1.
According to both Neo and Bredow, 100% of the screws placed in C1 using navigation were accurately placed. In the fluoroscopic-guidance group, 100% (Neo) and 99.0% (Bredow) of the screws were accurately placed, respectively (Table 3).
In C2, 88.2% of the screws placed using navigation showed either no perforation or a perforation of < 1 mm, while this applied to 75.9% of the screws placed with fluoroscopic-guidance.
Considering the Neo classification, C2 pedicle screw placement accuracy was 99.5% in group 1 and 100.0% in group 2. However, according to Bredow, the C2 pedicle screw accuracy rates were 99.5% (group 1) and 95.2% (group 2). According to Neo, C2 Magerl screw placement was 100.0% accurate in both groups, while according to Bredow, Magerl screws were placed with an accuracy of 100.0% (group 1) and 91.7% (group 2) (Table 3). The direction of the pedicle perforations is shown in Table 4.
Overall, statistical analysis revealed a significant difference regarding pedicle perforations of ≥ 1 mm between the groups (P = 0.02*). Revision surgery due to secondary atlantoaxial dislocation had to be performed in one case of the fluoroscopic-guidance group (3.7%). There were no intraoperative or postoperative neurovascular complications in any of the patients included.
Screw diameter/pedicle width ratio (SPR) was analyzed. The median SPR was 0.50 in the navigation group and 0.47 in the fluoroscopic-guidance group, with a statistically significant difference (P = 0.02*). Further examination revealed that this significance was driven by the disparity in SPRs at C2, where they were 0.82 (group 1) and 0.60 (group 2) (P < 0.001**), while SPR of C1 showed no significant difference (P = 0.56).
Table 5 shows that significantly more scans were performed in the fluoroscopic-guidance group and that significantly more K-wires had to be repositioned in this group (P < 0.001**).
Interobserver reliability
Overall interobserver reliability showed moderate agreement (ICC = 0.675) for all screws assessed.
The isolated analysis of interobserver reliability for group 1 (ICC = 0.641) and group 2 (ICC = 0.691) also showed moderate agreement.
Operating time
The mean operating time was 135 ± 40 min in group 1 and 158 ± 44 min in group 2. This difference of 23 min on average proved to be statistically significant (P = 0.02*).
Discussion
The aim of this study was to compare atlantoaxial screw placement using either fluoroscopic-guidance with additional intraoperative 3D imaging control or iCT-based navigation in patients with traumatic injuries. The results of this study show that the use of navigation technology facilitates atlantoaxial screw placement, leading to higher screw placement accuracy while reducing the operating time by 23 min on average.
While there are multiple studies reporting on the advantages of navigated screw placement over fluoroscopic-guidance for the thoracic and lumbar spine [15, 16], the literature regarding the application of navigation in the upper cervical spine and the number of cases included in these studies are very limited [17]. In 2021, Bertram et al. [14] published a study on iCT-based navigation versus fluoroscopic-guidance screw placement in all levels of the cervical spine. A higher accuracy was found for the navigated technique, yet in contrast to the present study, the majority of the cases included in their study were patients with degenerative pathologies, and most screws were placed in the subaxial spine. The focus of the present study was traumatic injuries of the upper cervical spine and their surgical treatment. This must be considered when interpreting accuracy rates since the presence of fractures entails a higher mobility of the spine and vertebrae which is further enhanced by general anesthesia and muscle relaxation. This may result in discrepancies between the real anatomy and the displayed anatomy according to the navigation system, presenting a challenging situation for the surgeon and potentially reducing the accuracy of screw placement [13, 14, 17, 18]. In addition, inaccuracies may also occur as the result of unintentional manipulation of the reference array positioned inside or close to the surgical field. Accordingly, special consideration must be given to the positioning of the reference array. Furthermore, during surgery, navigation accuracy should be verified regularly on different anatomical landmarks using the navigated instruments. To prevent being misled by a miss-match of screwdriver trajectory and screw trajectory, pressure should be removed from the screwdriver every now and again to confirm the actual screw trajectory.
Another study performed by Carl et al. [13] analyzed differences between navigation based on a preoperative CT scan with subsequent intraoperative surface matching and iCT-based navigation. Again, with 16 patients included, the study group is rather small. With the application of mobile C-arm CBCT-based intraoperative navigation, Bredow et al. [19] reported accuracy rates of 90.5% for C1 and 95.4% for C2 according to the same classification system as used in the present study. A similar accuracy rate of 92% has been published by Ling et al. [20] in a case series with a total of 37 screws placed in the atlantoaxial cervical spine using iCT-based navigation. In contrast, in their systematic review and meta-analysis, Azimi et al. [21] reported a higher pooled accuracy rate for freehand C2 pedicle screw placement (93.8%) compared to navigated C2 pedicle screw placement (92.2%). A possible reason for the contrasting results could be the heterogeneity of the studies included in their work.
The present study also analyzed the SPR in both groups. Particularly for the C2 level, SPR was significantly greater in the navigation group. Despite these findings, screw placement accuracy was higher in the navigation group. This should also find consideration when interpreting the results presented.
Investigating complications of dorsal stabilization in the upper cervical spine, Buchmann et al. [22] reported vertebral artery occlusion in four of 127 patients and a revision surgery rate of 6% in fluoroscopic-guidance procedures. Shuman et al. [23] compared complications and revision surgery rates for navigated and non-navigated cervical fusion with navigated procedures having decreased odds of complications overall, with no significant difference regarding revision surgery. In the present study, none of the patients suffered from vertebral artery occlusion. In one case of the fluoroscopic-guidance group, revision surgery (3.7%) had to be performed.
Comparison of different studies and their screw accuracy rates reported proved to be difficult due to use of different classification systems. In their meta-analysis, Azimi et al. [21] listed 12 different grading systems for screw placement in the cervical spine. Both systems for screw grading used in this study, the classifications introduced by Neo and Bredow, consider medial and lateral perforations only. According to the Neo classification with its 2-mm intervals, a lateral breach of an entire 3.5-mm screw would result in the screw being classified as grade B. The exact same screw may be graded as Bredow grade E if it crosses the transverse foramen. This example illustrates why the results presented above differ considerably depending on the grading system used. The results according to the Neo classification suggest comparable accuracy in both groups, while the Bredow classification indicates more severe perforations in the fluoroscopic-guidance group. Still, in contrast to the grading system introduced by Laine et al. [24], neither of the two classifications reflect the direction of the perforation although it may play a crucial role in predicting the clinical significance of a pedicle breach.
In summary, as long as sophisticated grading systems are missing, authors should use the existing classifications for screw accuracy with discretion to their weaknesses. Furthermore, when reviewing screw placement accuracy, the grading system used should be considered.
While other authors have reported an increased total procedure time for navigated cervical screw placement, we found a significant decrease in operating time for navigated procedures [13, 25]. This may be due to the fact that significantly more scans were performed in the fluoroscopic-guidance group and significantly more K-wires were repositioned. As elucidated above, despite these measures, navigated accuracy rates were not reached. Accordingly, in our setup, navigated atlantoaxial screw placement has proven to be very feasible, highly accurate and, anecdotally, ensuring minimal radiation exposure to the surgical team present as they leave the control zone during scan acquisition.
As with every study, the results should be interpreted considering its limitations. First, due to the study being a retrospective single-center investigation, the generalizability of the results is limited. However, we found no evidence that the results of any of the groups might be subject to bias. The initial decision on which intraoperative imaging would be used was solely dependent on availability of the systems. The respective surgeon would use intraoperative 3D navigation on any case if possible since it was introduced in 2017; therefore, we see no indication of a possible selection bias. While two different screw fixation techniques (88.5% Harms–Goel and 11.5% Magerl) were included, this does not diminish the fact that screw placement tended to be more accurate using navigation, especially because the accuracy rates for the few Magerl screws do not differ substantially from the accuracy rates found for the Harms–Goel instrumentation in either group. Second, the number of patients included in the study, especially in the group with fluoroscopic-guidance, remains limited. Nevertheless, we have included the largest study population for traumatic instabilities published so far. Third, as discussed, the grading of screw accuracy is highly dependent on the classification system used. Due to the lack of better alternatives and in favor of higher transparency, we decided to report two different frequently used classifications.
Conclusions
In conclusion, this study contributes to the data available showing that navigated atlantoaxial screw placement proves to be feasible as well as highly accurate compared to the fluoroscopic-guidance technique without prolonging the time needed for surgery. Using intraoperative navigation concurrently leads to a significant decrease in the need to reposition K-wires. When comparing these data with other studies, the availability of different classification systems for assessment of screw accuracy should be considered. Prospective randomized studies are needed to increase the quality of evidence available in the literature.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V (2015) Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J 24(5):905–918
Elliott RE, Tanweer O, Boah A, Morsi A, Ma T, Frempong-Boadu A, Smith ML (2014) Outcome comparison of atlantoaxial fusion with transarticular screws and screw-rod constructs: meta-analysis and review of literature. J Spinal Disord Tech 27(1):11–28
Nourbakhsh A, Hanson ZC (2022) Odontoid fractures: a standard review of current concepts and treatment recommendations. J Am Acad Orthop Surg 30(6):e561–e572
Kleinstück FS, Fekete TF, Loibl M, Jeszenszky D, Haschtmann D, Porchet F, Mannion AF (2021) Patient-rated outcome after atlantoaxial (C1–C2) fusion: more than a decade of evaluation of 2-year outcomes in 126 patients. Eur Spine J 30(12):3620–3630
Wagner SC, Schroeder GD, Kepler CK, Schupper AJ, Kandziora F, Vialle EN, Oner C, Fehlings MG, Vaccaro AR (2017) Controversies in the management of geriatric odontoid fractures. J Orthop Trauma 31(Suppl 4):S44-s48
Duff J, Hussain MM, Klocke N, Harris JA, Yandamuri SS, Bobinski L, Daniel RT, Bucklen BS (2018) Does pedicle screw fixation of the subaxial cervical spine provide adequate stabilization in a multilevel vertebral body fracture model? An in vitro biomechanical study. Clin Biomech (Bristol, Avon) 53:72–78
Tonetti J, Boudissa M, Kerschbaumer G, Seurat O (2020) Role of 3D intraoperative imaging in orthopedic and trauma surgery. Orthop Traumatol Surg Res 106(1s):S19-s25
Keil H, Luxenhofer M, Vetter SY, Beisemann N, Grützner PA, Franke J (2021) Evaluation of image quality and assessability of a new flat-panel 3D C-arm compared to mobile and fixed computed tomography in posterior spinal fixation. Int J Med Robot 17(2):e2181
Chachan S, Bin Abd Razak HR, Loo WL, Allen JC, Shree Kumar D (2018) Cervical pedicle screw instrumentation is more reliable with O-arm-based 3D navigation: analysis of cervical pedicle screw placement accuracy with O-arm-based 3D navigation. Eur Spine J 27(11):2729–2736
Uehara M, Takahashi J, Ikegami S, Kuraishi S, Futatsugi T, Kato H (2017) Screw perforation rates in 359 consecutive patients receiving computer-guided pedicle screw insertion along the cervical to lumbar spine. Eur Spine J 26(11):2858–2864
Hecht N, Yassin H, Czabanka M, Föhre B, Arden K, Liebig T, Vajkoczy P (2018) Intraoperative computed tomography versus 3D C-arm imaging for navigated spinal instrumentation. Spine (Phila Pa 1976) 43(5):370–377
Hecht N, Kamphuis M, Czabanka M, Hamm B, König S, Woitzik J, Synowitz M, Vajkoczy P (2016) Accuracy and workflow of navigated spinal instrumentation with the mobile AIRO(®) CT scanner. Eur Spine J 25(3):716–723
Carl B, Bopp M, Pojskic M, Voellger B, Nimsky C (2019) Standard navigation versus intraoperative computed tomography navigation in upper cervical spine trauma. Int J Comput Assist Radiol Surg 14(1):169–182
Bertram U, Schmidt TP, Clusmann H, Albanna W, Herren C, Riabikin A, Mueller CA, Blume C (2021) Intraoperative computed tomography-assisted spinal navigation in dorsal cervical instrumentation: a prospective study on accuracy regarding different pathologies and screw types. World Neurosurg 149:e378–e385
Baldwin KD, Kadiyala M, Talwar D, Sankar WN, Flynn JJM, Anari JB (2022) Does intraoperative CT navigation increase the accuracy of pedicle screw placement in pediatric spinal deformity surgery? A systematic review and meta-analysis. Spine Deform 10(1):19–29
Chan A, Parent E, Narvacan K, San C, Lou E (2017) Intraoperative image guidance compared with free-hand methods in adolescent idiopathic scoliosis posterior spinal surgery: a systematic review on screw-related complications and breach rates. Spine J 17(9):1215–1229
Sancipriano V, Penner F, Cofano F, Ajello M, Marengo N, Petrone S, Zenga F, Crobeddu M, Bianco A, Cossandi C et al (2022) Intraoperative computed tomography for C1–C2 stabilization by Goel–Harms: analysis of clinical efficacy and a novel classification of screw placement accuracy. World Neurosurg 158:e19–e37
Zhang HL, Zhou DS, Jiang ZS (2011) Analysis of accuracy of computer-assisted navigation in cervical pedicle screw installation. Orthop Surg 3(1):52–56
Bredow J, Oppermann J, Kraus B, Schiller P, Schiffer G, Sobottke R, Eysel P, Koy T (2015) The accuracy of 3D fluoroscopy-navigated screw insertion in the upper and subaxial cervical spine. Eur Spine J 24(12):2967–2976
Ling JM, Tiruchelvarayan R, Seow WT, Ng HB (2013) Surgical treatment of adult and pediatric C1/C2 subluxation with intraoperative computed tomography guidance. Surg Neurol Int 4(Suppl 2):S109-117
Azimi P, Yazdanian T, Benzel EC, Aghaei HN, Azhari S, Sadeghi S, Montazeri A (2020) Accuracy and safety of C2 pedicle or pars screw placement: a systematic review and meta-analysis. J Orthop Surg Res 15(1):272
Buchmann N, Schweizer C, Kirschke JS, Rienmüller A, Gempt J, Ringel F, Meyer B, Ryang Y-M (2020) C1–C2 posterior screw fixation in atlantoaxial fractures revisited: technical update based on 127 cases. Eur Spine J 29(5):1036–1042
Shuman WH, Valliani AA, Chapman EK, Martini ML, Neifert SN, Baron RB, Schupper AJ, Steinberger JM, Caridi JM (2022) Intraoperative navigation in spine surgery: effects on complications and reoperations. World Neurosurg 160:e404–e411
Laine T, Lund T, Ylikoski M, Lohikoski J, Schlenzka D (2000) Accuracy of pedicle screw insertion with and without computer assistance: a randomised controlled clinical study in 100 consecutive patients. Eur Spine J 9(3):235–240
Czabanka M, Haemmerli J, Hecht N, Foehre B, Arden K, Liebig T, Woitzik J, Vajkoczy P (2017) Spinal navigation for posterior instrumentation of C1–2 instability using a mobile intraoperative CT scanner. J Neurosurg Spine 27(3):268–275
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PAG, JF, and SYV were involved in conception and design of the study. JG, EM, AM, and BB helped with data acquisition. JG, BB, and SYV participated in analysis and interpretation of data. JG and EM were involved in writing of the manuscript. AM, PAG, JF, and SYV were involved in revision of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The research group MINTOS had grants/grants pending and technical support from Siemens Healthineers (Erlangen, Germany) and NuVasive Inc. (San Diego, USA). The funders had no involvement in the study conceptualization, design, data collection, analysis, nor the decision to publish or the preparation of the manuscript. JF and PAG serve as unpaid members of a consulting/advisory board for Siemens Healthineers. The other authors declare that they have no financial or non-financial interests to disclose.
Ethical approval
The study was reviewed and approved by the responsible Ethics Committee (application number 2022–16388). All patients provided verbal and written consent. All procedures were performed in accordance with the ethical standards of the Institutional and/or National Research Committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gierse, J., Mandelka, E., Medrow, A. et al. Comparison of iCT-based navigation and fluoroscopic-guidance for atlantoaxial screw placement in 78 patients with traumatic cervical spine injuries. Eur Spine J 33, 2304–2313 (2024). https://doi.org/10.1007/s00586-024-08232-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-024-08232-7