Abstract
Objective
The objective of this study was to explore the morphological characteristics of paraspinal muscles in young patients with unilateral neurological symptoms of lumbar disc herniation.
Methods
This study retrospectively analyzed young patients aged 18–40 years who were hospitalized for lumbar disc herniation in our hospital from June 2017 to June 2020. Data on sex, age, body mass index (BMI), subcutaneous fat tissue thickness (SFTT) at the L1-L2 level, duration of symptoms, degree of lumbar disc herniation, visual analog scale (VAS) for the lower back, Mo-fi-disc score, relative cross-sectional area (RCAS) of the paravertebral muscles (psoas major [PM], multifidus [MF], and erector spinae [ES]), and degree of fat infiltration (DFF) of the paravertebral muscles were collected. The VAS was used to evaluate the intensity of low back pain. Patients with VAS-back >4 points were defined as the low back pain group, and patients with ≤4 points were defined as the control group. The demographic characteristics, as well as the bilateral and ipsilateral paravertebral muscles, of the two groups were compared and analyzed.
Result
A total of 129 patients were included in this study (52 patients in the LBP group and 77 patients in the control group). There were no significant differences in sex, BMI, or Pfirrmann grade of lumbar disc herniation between the two groups (P > 0.05). The age of the LBP group (33.58 ± 2.98 years) was greater than that of the control group (24.13 ± 2.15 years) (P = 0.002), and the SFTT at the L1-L2 level (13.5 ± 7.14 mm) was higher than that of the control group (7.75 ± 6.31 mm) (P < 0.05). Moreover, the duration of symptoms (9.15 ± 0.31 months) was longer than that of the control group (3.72 ± 0.48 months) (P < 0.05), and the Mo-fi-disc score (8.41 ± 3.16) was higher than that of the control group (5.53 ± 2.85) (P < 0.05). At L3/4 and L5/S1, there was no significant difference in the RCSA and DFF of the bilateral and ipsilateral paraspinal muscles between the LBP group and the control group. At L4/5, there was no significant difference in the RCSA and DFF of the paraspinal muscles on either side in the LBP group (P > 0.05). In the control group, the RCSA of the MF muscle on the diseased side was smaller than that on the normal side (P < 0.05), and the DFF of the MF muscle on the diseased side was larger than that on the normal side (P < 0.05). In addition, there was no significant difference in the ES and PM muscles on both sides (P > 0.05). At L4/5, the RCSA of the MF muscle on the normal side was significantly smaller in the LBP group than in the control group (P < 0.05), and the DFF of the MF muscle on the normal side was significantly larger in the LBP group than in the control group (P < 0.05). There was no significant difference in the ES and PM muscles on the same side between the two groups (P > 0.05).
Conclusion
In young patients with unilateral neurological symptoms of lumbar disc herniation, symmetrical atrophy of the bilateral MF muscle is more prone to causing low back pain. Older age, higher SFTT at the L1-L2 levels, longer symptom duration, higher Mo-fi-di score, and greater muscle atrophy on the normal side of the MF increased the incidence of low back pain in young patients with unilateral lumbar disc herniation.
Similar content being viewed by others
Introduction
Low back pain (LBP) has gradually become an important health problem in present day society, and approximately 70–85% of adults have experienced low back pain at least once [1, 2]. LBP has become a leading cause of disability and loss of working time. In addition, LBP-related diseases cause a great economic burden to the patient’s family and to social medical care, and it can also reduce the patient’s quality of life. Early research on LBP mainly focused on the degeneration of the intervertebral disc and excessive loads, among other factors. It was initially believed that the degeneration of the intervertebral disc was the main cause of LBP [3, 4]. In recent years, with the further enrichment of the spinal stability theory and the development of imaging technology, the role of paraspinal muscles in the spinal system has attracted increasing attention from clinicians. The paraspinal muscles are an important part of the stability and movement of the spine, and the degeneration of the paraspinal muscles will accelerate the degeneration of the lumbar spine [5, 6]. It has been reported that patients with severe intervertebral disc degeneration are more likely to have increased fatty infiltration in the multifidus and ES muscles [7]. Panjabi proposed the concept of “three subseries” for maintaining the stability of the lumbar spine, which consists of passive substrains composed of the vertebral body, intervertebral disc, facet joints, and ligaments, among other factors, as well as the active subseries of muscles composed of muscles and tendons. The three subsystems are independent of each other, but they are related to each other. When a specific factor is damaged, it can be compensated for by other factors [8, 9]. LBP occurs when tissue damage exceeds the compensation range. Previous studies on the morphological changes of paraspinal muscles have mostly used imaging techniques for evaluation, such as ultrasound and CT [10, 11], which have a decreased ability to distinguish muscle tissue and large errors. MRI has the advantages of possessing low levels of radiation and a high degree of resolution, and the application of MRI to study muscle morphology improves the accuracy. The cross-sectional area of the paraspinal muscles and the degree of fatty infiltration are often used as indicators to evaluate muscle function [12, 13]. A literature report found that LBP in the population has been exhibiting a younger age trend [14]. At present, imaging studies on changes in paraspinal muscle groups are not uncommon, but no more detailed studies have been conducted on young patients with LBP caused by lumbar disc herniation, and no detailed distinction has been made on lumbar disc herniation patients with or without LBP. Thus, this study conducted a more detailed evaluation on the morphological changes of the paraspinal muscles in young patients with LBP.
Methods
This study retrospectively analyzed all young patients who were hospitalized for lumbar disc herniation at the authors’ institution from June 2017 to June 2020. The following inclusion criteria were used: (1) patients diagnosed with unilateral lumbar intervertebral disc herniation in the L4–5 segment via imaging examinations; obvious radiating pain in the lower limb on the diseased side, pain located on the side of the intervertebral disc herniation (with or without low back pain), and muscle strength of the lower limb on the diseased side being weakened to different degrees; the straight leg raising test on the diseased side being positive (> 30°, < 70°), and after a strict conservative treatment such as rest, nonsteroidal anti-inflammatory drugs, and epidural hormone administration for more than 3 months, the symptoms were not relieved, or the improvement effect was not obvious; and (2) the clinical data were complete. The following exclusion criteria were used: (1) patients with a history of previous spinal surgery, trauma, spinal infection, scoliosis, kyphosis, spinal canal stenosis, spondylolisthesis, spondylolysis, lumbarization, sacralization, metal located anywhere in the body, neurological or psychiatric disorders, rheumatic or endocrine diseases, malignancy, and pregnancy; (2) patients with bilateral lower extremity radiating pain; and (3) patients with LBP of unknown etiology. There were 129 patients who met the study criteria (aged 18–40-years-old), with an average age of 27.8-years-old, and a body mass index (BMI) of 18–30 kg/m2, with an average of 26.5 kg/m2 This study was conducted in accordance with the Declaration of Helsinki and received approval from the Ethics Committee of the Third Hospital of Hebei Medical University. Informed consent to participate in the study was obtained from all of the patients.
Data collection and image analysis
To reduce the influence of confounding factors, we selected patients with L4/5 disc herniation with typical unilateral symptoms as observation subjects. The visual analog scale (VAS) was used to assess low back pain intensity, with a score of 0 indicating no pain and a score of 10 indicating the most painful pain; in addition, patients were asked to choose one of 11 numbers to represent their pain level. The scale ranged from 0 to 10. In this study, the patients were divided into a LBP group (VAS score >4) and a control group (VAS score ≤ 4) [15, 16].
The MRI images of all of the patients were scanned by the radiologists, and the patients underwent conventional supine lumbar spine MRI scans. Each intervertebral space was parallel to the space for scanning 3 images. The axial and sagittal images of the T2-weighted images of the patients were collected, the MRI images of the three levels of L3/4, L4/5, and L5/S1 were axially collected, and the cross-sectional images of the center of the intervertebral disc were selected. The following MRI technical parameters were used: MRI was completed by using the 1.5 T MRI system (GE Company in the United States), and the axial T2 weighting parameters included a repetition time of 3000 ms/echo, an echo time of 100 ms, a field of view of 400 × 400 mm, and a thickness of 4 mm.
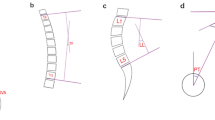
The degree of fatty infiltration of the paraspinal muscles (psoas major [PM], multifidus [MF], and erector spinae [ES]) was measured by using ImageJ software via the same method [17,18,19] (Fig.1). ImageJ software was also used to measure subcutaneous fat thickness at the L1-L2 level of the lumbar MRI. Özcan-Ekşi et al. recently showed that SFTT at the L1-L2 level on MRI is superior to BMI in predicting low back pain [20]. The imaging data of the relative cross-sectional area of the paraspinal muscles were processed by using the Picture Archiving and Communication Systems (PACS) system; in addition, the cross-sectional sizes of the paraspinal muscles and the vertebral body were measured. To eliminate individual differences in physique, the paraspinal muscle cross-sectional area/corresponding vertebral body area was used to obtain relative cross-sectional area (RCSA) = paraspinal muscle cross-sectional area/vertebral body cross-sectional area× 100%. The degree of disc herniation was graded by using the Pfirrmann criteria. Pfirrmann grading is an intuitive grading standard for assessing the relationship between herniated intervertebral discs and nerve roots during lumbar disc herniation under MRI images and is divided into four grades [21]. The Mo-Fi-Disc score of all of the patients was calculated; specifically, the Mo-Fi-Disc score is a simple and objective method for evaluating spinal degeneration [22]. The image measurement and evaluation of all of the patients’ imaging data were completed by two senior deputy chief physicians, and the inter- and intraobserver reliabilities of every parameter were calculated by using the interclass correlation coefficient for continuous variables. The interobserver reliability of all of the paraspinal muscles was good or excellent (0.75–0.88). Moreover,the intraobserver reliability of all of the variables was excellent (0.85–0.91), and the final data were recorded. During the reading process, the relevant data of the two groups of patients were concealed and blindly processed to reduce the potential influence on the reading.
Statistical analysis
Statistical analysis was performed by using SPSS 26.0 statistical software (IBM SPSS Statistics 26.0, IBM Corporation, Armonk, NY). The Shapiro–Wilk method was used for normality testing (P > 0.05). All of the data were in accordance with a normal distribution; thus, the measured data were described by using the mean ± standard deviation (M ± SD). Continuous variables within groups were analyzed by using a paired samples T test, and the continuous variables between groups were analyzed by using an independent samples t test or chi-square test. P < 0.05 indicates a statistically significant difference.
Results
A total of 129 patients were included in this study, including 52 patients in the LBP group and 77 patients in the control group. There was no significant difference in sex (P > 0.05), BMI (P > 0.05), or Pfirrmann grade (P > 0.05) between the two groups, whereas the average age of the LBP group (33.58 ± 2.98 years) was higher than that of the control group (24.13 ± 2.15 years) (P = 0.002), the SFTT at the L1-L2 level (13.5 ± 7.14 mm) was higher than that of the control group (7.75 ± 6.31 mm) (P < 0.05), the duration of symptoms (9.15 ± 0.31 months) was longer than that of the control group (3.72 ± 0.48 months) (P < 0.05), and the Mo-fi-disc score (8.41 ± 3.16) was higher than that of the control group (5.53 ± 2.85) (P < 0.05) (Table 1).
At L3/4 and L5/S1, the paraspinal muscles on both sides of the LBP group and the control group were not significantly different (P > 0.05). At L4/5, there was no significant difference in the RCSA and DFF of the paraspinal muscles on either side in the LBP group (P > 0.05) (Table 2) (Fig. 2). In the control group, the RCSA of the MF muscle on the diseased side (35.39 ± 7.15) was smaller than that on the normal side (46.76 ± 6.91) (P < 0.001), and the DFF of the MF muscle on the diseased side (22.51 ± 3.58) was larger than that on the normal side (14.10 ± 3.12) (P < 0.001) (Table 3) (Fig. 3). Furthermore, there was no significant difference in the RCSA and DFF of the ES and PM muscles on either side in the NBLP group (P > 0.05).
At L3/4 and L5/S1, there was no significant difference in the RCSA and DFF of the ipsilateral paraspinal muscle between the LBP and control groups (P > 0.05). At L4/5, the RCSA of the MF muscle on the normal side was significantly smaller in the LBP group (36.44 ± 6.59) than in the control group (46.76 ± 6.91) (P < 0.001), and the DFF of the MF muscle on the normal side was significantly larger in the LBP group (21.96 ± 4.78) than in the control group (14.10 ± 3.12) (P < 0.001). Additionally, there was no significant difference in the RCSA and FF of the paraspinal muscles on the diseased side between the LBP and control groups (P > 0.05). Moreover, there was no significant difference in the ES and PM muscles on the same side between the two groups (P > 0.05) (Table 4).
Discussion
The paraspinal muscles (especially the MF and ES muscles) have important functions in maintaining spinal stability and controlling lumbar motion. The cross-sectional area of the MF muscle gradually increases from the upper lumbar vertebrae in a downward direction, whereas the cross-sectional area of the ES muscle gradually decreases in this direction. Due to the fact that the MF muscle has the characteristics of short fibers, a large cross-section, and large mobility, it is the most important muscle in the paraspinal muscles for stabilizing the spine and for controlling the movement of the spine [23]. Paraspinal muscle atrophy mainly manifests as a reduction in the cross-sectional area of the paraspinal muscle and an increase in fat content. In the earlier stages of research, the understanding of the stability of the lumbar spine focused on the intervertebral disc, vertebral body, and other structures. With the progress of research, researchers have gradually demonstrated the importance of muscles in maintaining the stability of the spine, especially regarding the “neutral zone” and the stabilization of the lumbar spine. Wilke has proposed the concept of the “neutral zone” of the low back muscles and believed that the stability of the MF muscle in the “neutral zone” accounted for at least 2/3 [24]. The MF muscle is more sensitive to local lumbar degeneration (such as nerve compression, as well as intervertebral disc and facet joint degeneration), which may be related to its single innervation by the posterior rami of the spinal nerve, whereas the ES and PM muscles are multisegment spinal nerve innervations [25, 26]. This may be the reason why the morphological changes in the MF muscle are more obvious than those in the ES and PM muscles.
In this study, it was found that the patients in the LBP group had a longer disease duration than the control group. We believe that the degree of MF muscle atrophy may be related to the duration of symptoms. Specifically, a longer disease course corresponds to a longer duration of symptoms and a more severe atrophy of the MF muscle. This is consistent with the findings of Barker [27], who believed that the degree of MF muscle atrophy is clearly related to the duration of symptoms and that MF muscle atrophy can lead to the occurrence of LBP. Similarly, the patients in the LBP group were older than those in the control group. It is possible that with increasing age, various factors (such as degeneration of the lumbar vertebral body, lumbar intervertebral disc, and facet joints) can lead to the degeneration of the spine becoming gradually increased, as well as the flexibility and mobility of the waist decreasing and the MF muscle appearing to exhibit disuse atrophy. Shahidi and Kim also believed that age was an independent factor for RCSA and FF of the MF muscle [28, 29]. In addition, the Mo-fi-disc score in the low back pain group was higher than that in the control group. The Mo-fi-disc scoring system is a useful tool for predicting the degree of severe LBP and spinal degeneration. In this study, patients with more intense LBP had higher “Mo-fi-disc” scores. It has also been proven that the degree of spinal degeneration in patients with low back pain is greater than that in the control group. This study also compared the genders of the two groups of patients and found that there was no significant difference in gender between the two groups. Of course, this may be related to the age of the female patients in this study. In this study, the female cases were young patients because we excluded postmenopausal estrogen cases; therefore, there may be no significant difference between the sexes. Moreover, there were no statistically significant differences in BMI between the two groups. However, due to some limitations of BMI, we added L1-L2 level SFTT to predict LBP and spinal degeneration at the lower lumbar spine level. The SFTT of L1-L2 levels in the LBP group was higher than that in the control group. The appropriate cutoff values for SFTT in women and men have been reported to be 8.45 mm and 9.4 mm, respectively. When this critical value is exceeded, the rate of spinal degeneration in patients significantly increases, and low back pain is more likely to occur [30]. We compared the Pfirrmann grading of the degree of intervertebral disc herniation between the two groups and found that there was no significant difference in the Pfirrmann grading between the LBP group and the control group. We believe that as long as the nerve root is compressed (regardless of the degree of compression), the MF muscle will undergo significant pathological changes. Therefore, atrophy of the lumbar paraspinal muscles may not be solely caused by denervation. Farshad also studied the relationship between the degree of nerve root compression and the changes in the MF muscle in patients with lumbar disc herniation and believed that the changes in the MF muscle had nothing to do with the degree of nerve root compression [31].
In this study, there was no significant difference in the RCSA and DFF of the bilateral MF muscles in the LBP group, which exhibited symmetrical atrophy. In the control group, the MF muscle on both sides was asymmetrically atrophied, and the MF muscle on the diseased side was significantly atrophied (compared with the normal side). This may be related to muscle compensatory function. After the atrophic changes of the MF muscle on the diseased side of the body in the early stage of lumbar degeneration, with the decline of muscle function and to stabilize the stability of the lumbar spine, compensatory hypertrophy of the MF muscle on the normal side may occur to replace part of the lost function on the diseased side. However, with the passage of time, the normal-side MF muscle cannot compensate for the lost function of the diseased side and will gradually exhibit changes in atrophy and degeneration. By comparing the symmetry of the lumbar multifidus muscle and the cross-sectional size of the multifidus muscle between patients with chronic low back pain and healthy asymptomatic volunteers, Hides found that patients with low back pain had significantly more lumbar multifidus atrophy than healthy asymptomatic healthy volunteers. In that study, it was found that the atrophy of the multifidus muscle was most pronounced in patients with low back pain at the level of the L5 vertebral body [32]. Additionally, Hyun et al. examined the CSA of the MF muscle in patients with lumbar disc herniation and found that in the specific scenario when the course of disease was greater than 30 days, the CSA of the MF muscle was healthy, and the diseased side of the body exhibited obvious asymmetric atrophy [33]. Kamath et al. observed imaging changes after muscle denervation by applying MRI and found that tissue edema quickly occurred several days after muscle denervation and lasted for several weeks. During this period of time, even muscle atrophy may not be observed. Specifically, and with the extension of the course of the disease, muscle atrophy will begin to slowly appear [34]. This may be the reason why patients with LBP have a longer course of disease and symmetrical atrophy of the bilateral MF muscle than patients without LBP.
In the ipsilateral comparison between the low back pain group and the control group, it was found that the atrophy of the multifidus muscle on the normal side of the body in the low back pain group was more obvious than that in the control group. Kjaer et al. demonstrated MF muscle atrophy by examining changes in the cross-sectional area of the MF muscle on lumbar MRI in adults with LBP. They correlated the observations with the general condition of the patients. They eventually demonstrated a strongly significant link between MF muscle atrophy and LBP in adults (independent of BMI) [35]. Furthermore, wan. Q et al. found that paraspinal muscle atrophy was more pronounced in patients with LBP than in patients without LBP [36]. MF muscle atrophy in the diseased segment may lead to local muscle weakness and spinal instability that exacerbates muscle atrophy. During the process of muscle atrophy, adipose tissue gradually replaces normal muscle fibers, thus resulting in decreased spinal stability, which may be one of the important reasons for LBP [37]. In this study, it was also believed that atrophy of the MF muscle may be the cause of LBP. Due to the decline in MF muscle function, muscle glycogen cannot be fully utilized, and a large amount of lactic acid and various metabolites accumulate in the tissue, which correspondingly leads to muscle edema and pain. Of course, the causal relationship between pain and paraspinal muscle atrophy still requires further research.
The average infiltration rate of lumbar paraspinal muscles in normal adults is not greater than 9% [38]. Due to the sensitivity of the multifidus, pathological changes in the multifidus mostly occur in the early stage. Early training that only targets the lumbar multifidus muscle can typically achieve better clinical results [39]. Franc, Koppenhaver believed that a training program specifically aimed at patients with low back pain with multifidus atrophy can restore the innervation of the multifidus muscle [40, 41]. At present, many studies have confirmed that [42] by strengthening paraspinal muscle group exercise to reduce atrophy and fat infiltration of the paraspinal muscle, one can significantly improve the pain and dysfunction of the lumbar spine and legs caused by lumbar disc herniation, as well as enhance its function and maintain the lumbar spine. Thus, biomechanical balance can be achieved.
There were still some limitations of this study. First, two physicians completed all of the measurements. When measuring the paraspinal muscle CSA and FF, the subjective factors were manually delineated. Second, the determination of the course of the patient’s disease can only rely on the patient’s chief complaint, and there is no objective evidence. In addition, the sample size was small, and there was no normal control group. In the future, the sample size should be expanded in prospective studies and multicenter studies. Finally, when evaluating the relationship between paraspinal muscles and low back pain, only patients with unilateral neurological symptoms of intervertebral disc herniation in the L4/5 segment were selected as the research subjects.
Conclusion
In young patients with unilateral neurological symptoms of lumbar disc herniation, symmetrical atrophy of the bilateral MF muscle is more prone to causing low back pain. Older age, higher SFTT at the L1-L2 levels, longer symptom duration, higher Mo-fi-di score, and greater muscle atrophy on the normal side of the MF increased the incidence of low back pain in young patients with unilateral lumbar disc herniation. Therefore, young patients with lumbar intervertebral disc herniation with or without LBP should perform standardized low back muscle rehabilitation exercises at early stages.
Availability of data and materials
The datasets generated during and analyzed during the current study are.
not publicly available due to hospital regulations but are available from the.
corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- VAS:
-
Visual Analogue Scale
- PM:
-
Psoas major
- MF:
-
Multifidus
- ES:
-
Erector spinae
- RCSA:
-
Relative cross-sectional area
- LBP:
-
Low back pain
- DFF:
-
Degree of fatty infiltration
- PACS:
-
Picture archiving and com-munication systems
- SFTT:
-
Subcutaneous fat tissue thickness
- Mo-fi-disc:
-
Modic changes (MO)-fatty infiltration (fi) -intervertebral disc degeneration (disc)
References
Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(6):968–74. https://doi.org/10.1136/annrheumdis-2013-204428.
March L, Smith EU, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F, et al. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol. 2014;28(3):353–66. https://doi.org/10.1016/j.berh.2014.08.002.
Ogon I, Takebayashi T, Takashima H, Tanimoto K, Ida K, Yoshimoto M, et al. Analysis of chronic low back pain with magnetic resonance imaging T2 mapping of lumbar intervertebral disc. J Orthop Sci. 2015;20(2):295–301. https://doi.org/10.1007/s00776-014-0686-0.
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873–8. https://doi.org/10.1097/00007632-200109010-00011.
Kim JC, Lee SU, Jung SH, Lim JY, Kim DH, Lee SY. Natural aging course of paraspinal muscle and back extensor strength in community-dwelling older adults (sarcopenia of spine, SarcoSpine): a prospective cohort study protocol. BMJ Open. 2019;9(9):e032443. https://doi.org/10.1136/bmjopen-2019-032443.
Lee JC, Cha JG, Kim Y, Kim YI, Shin BJ. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine (Phila Pa 1976). 2008;33(3):318–25. https://doi.org/10.1097/BRS.0b013e318162458f.
Özcan-Ekşi EE, Ekşi MŞ, Akçal MA. Severe lumbar intervertebral disc degeneration is associated with Modic changes and fatty infiltration in the Paraspinal muscles at all lumbar levels, except for L1-L2: a Cross-sectional analysis of 50 symptomatic women and 50 age-matched symptomatic men. World Neurosurg 2019 ;122:e1069-e1077. doi: https://doi.org/10.1016/j.wneu.2018.10.229. Epub 2018 Nov 9. PMID: 30415054.
Panjabi MM. The stabilizing system of the spine. Part I. function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5(4):383–9; discussion 397. https://doi.org/10.1097/00002517-199212000-00001.
Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5(4):390–6; discussion 397. https://doi.org/10.1097/00002517-199212000-00002.
Keller A, Gunderson R, Reikerås O, Brox JI. Reliability of computed tomography measurements of paraspinal muscle cross-sectional area and density in patients with chronic low back pain. Spine (Phila Pa 1976). 2003;28(13):1455–60. 12838105. https://doi.org/10.1097/01.BRS.0000067094.55003.AD.
Hides JA, Richardson CA, Jull GA. Magnetic resonance imaging and ultrasonography of the lumbar multifidus muscle. Comparison of two different modalities. Spine (Phila Pa 1976). 1995 Jan 1;20(1):54–8. doi: https://doi.org/10.1097/00007632-199501000-00010. PMID: 7709280.
Kalichman L, Carmeli E, Been E. The association between imaging parameters of the Paraspinal muscles, spinal degeneration, and low Back pain. Biomed Res Int. 2017;2017:2562957. Doi: https://doi.org/10.1155/2017/2562957. Epub 2017 mar 20. .
Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9(4):266–72. https://doi.org/10.1007/s005860000190 PMID: 11261613; PMCID: PMC3611341.
Mather L, Ropponen A, Mittendorfer-Rutz E, Narusyte J, Svedberg P. Health, work and demographic factors associated with a lower risk of work disability and unemployment in employees with lower back, neck and shoulder pain. BMC Musculoskelet Disord. 2019;20(1):622. https://doi.org/10.1186/s12891-019-2999-9.
Shafshak TS, Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low Back pain. J Clin Rheumatol2021 ;27(7):282–285. doi: https://doi.org/10.1097/RHU.0000000000001320. PMID: 31985722.
Boonstra AM, Schiphorst Preuper HR, Balk GA, Stewart RE. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain. 2014;155(12):2545–2550. doi: https://doi.org/10.1016/j.pain.2014.09.014. Epub 2014 Sep 17. PMID: 25239073.
Niemeläinen R, Briand MM, Battié MC. Substantial asymmetry in paraspinal muscle cross-sectional area in healthy adults questions its value as a marker of low back pain and pathology. Spine (Phila Pa 1976). 2011;36(25):2152–7. https://doi.org/10.1097/BRS.0b013e318204b05a.
Fortin M, Omidyeganeh M, Battié MC, Ahmad O, Rivaz H. Evaluation of an automated thresholding algorithm for the quantification of paraspinal muscle composition from MRI images. Biomed Eng Online. 2017;16(1):61. https://doi.org/10.1186/s12938-017-0350-y.
Valentin S, Licka T, Elliott J. Age and side-related morphometric MRI evaluation of trunk muscles in people without back pain. Man Ther. 2015 Feb;20(1):90–5. doi: https://doi.org/10.1016/j.math.2014.07.007. Epub 2014 Jul 17. Erratum in: Man Ther. 2015 Jun;20(3):513.
Özcan-Ekşi EE, Kara M, Berikol G, Orhun Ö, Turgut VU, Ekşi MŞ. A new radiological index for the assessment of higher body fat status and lumbar spine degeneration. Skelet Radiol 2022;51(6):1261–1271. doi: https://doi.org/10.1007/s00256-021-03957-8. Epub 2021 Nov 18. PMID: 34792625.
Pfirrmann CW, Dora C, Schmid MR, Zanetti M, Hodler J, Boos N. MR image-based grading of lumbar nerve root compromise due to disk herniation: reliability study with surgical correlation. Radiology. 2004;230(2):583–8. https://doi.org/10.1148/radiol.2302021289.
Ekşi MŞ, Özcan-Ekşi EE, Orhun Ö, Turgut VU, Pamir MN. Proposal for a new scoring system for spinal degeneration: Mo-fi-disc. Clin Neurol Neurosurg 2020 ;198:106120. doi: https://doi.org/10.1016/j.clineuro.2020.106120. Epub 2020 Jul 31. PMID: 32889115.
Macintosh JE, Bogduk N. 1987 Volvo award in basic science. The morphology of the lumbar erector spinae. Spine (Phila pa 1976). 1987 Sep;12(7):658-68. doi: https://doi.org/10.1097/00007632-198709000-00004.
Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine (Phila Pa 1976). 1995 Jan 15;20(2):192–8. doi: https://doi.org/10.1097/00007632-199501150-00011.
Rageot E. Le syndrome des branches postérieures des nerfs rachidiens. Bases anatomiques, sémiologiques et thérapeutiques [Syndrome of the posterior branches of spinal nerves. Anatomic, symptomatologic and therapeutic basis]. J Chir (Paris). 1982 Aug-Sep;119(8–9):517–22. French.
Mahan MA, Sanders LE, Guan J, Dailey AT, Taylor W, Morton DA. Anatomy of psoas muscle innervation: cadaveric study. Clin Anat. 2017;30(4):479–86. https://doi.org/10.1002/ca.22879.
Barker KL, Shamley DR, Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine (Phila Pa 1976). 2004;29(22):E515–9. https://doi.org/10.1097/01.brs.0000144405.11661.eb.
Shahidi B, Parra CL, Berry DB, Hubbard JC, Gombatto S, Zlomislic V, et al. Contribution of lumbar spine pathology and age to Paraspinal muscle size and fatty infiltration. Spine (Phila Pa 1976). 2017;42(8):616–23. https://doi.org/10.1097/BRS.0000000000001848.
Kim WH, Lee SH, Lee DY. Changes in the cross-sectional area of multifidus and psoas in unilateral sciatica caused by lumbar disc herniation. J Korean Neurosurg Soc. 2011;50(3):201–4. https://doi.org/10.3340/jkns.2011.50.3.201.
Berikol G, Ekşi MŞ, Aydın L, Börekci A, Özcan-Ekşi EE. Subcutaneous fat index: a reliable tool for lumbar spine studies. Eur Radiol 2022;32(9):6504–6513. doi: https://doi.org/10.1007/s00330-022-08775-7. Epub 2022 Apr 5. PMID: 35380225.
Farshad M, Gerber C, Farshad-Amacker NA, Dietrich TJ, Laufer-Molnar V, Min K. Asymmetry of the multifidus muscle in lumbar radicular nerve compression. Skelet Radiol. 2014;43(1):49–53. https://doi.org/10.1007/s00256-013-1748-7.
Hides J, Gilmore C, Stanton W, Bohlscheid E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther. 2008;13(1):43–9. https://doi.org/10.1016/j.math.2006.07.017.
Hyun JK, Lee JY, Lee SJ, Jeon JY. Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine (Phila Pa 1976). 2007;32(21):E598–602. https://doi.org/10.1097/BRS.0b013e318155837b.
Kamath S, Venkatanarasimha N, Walsh MA, Hughes PM. MRI appearance of muscle denervation. Skelet Radiol. 2008;37(5):397–404. https://doi.org/10.1007/s00256-007-0409-0.
Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007;5:2. https://doi.org/10.1186/1741-7015-5-2.
Wan Q, Lin C, Li X, Zeng W, Ma C. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol. 2015;88(1053):20140546. https://doi.org/10.1259/bjr.20140546.
D'hooge R, Cagnie B, Crombez G, Vanderstraeten G, Dolphens M, Danneels L. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man Ther. 2012;17(6):584–8. https://doi.org/10.1016/j.math.2012.06.007.
Parkkola R, Rytökoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine (Phila Pa 1976). 1993;18(7):830–6. https://doi.org/10.1097/00007632-199306000-00004.
Willemink MJ, van Es HW, Helmhout PH, Diederik AL, Kelder JC, van Heesewijk JP. The effects of dynamic isolated lumbar extensor training on lumbar multifidus functional cross-sectional area and functional status of patients with chronic nonspecific low back pain. Spine (Phila Pa 1976). 2012;37(26):E1651–8. https://doi.org/10.1097/BRS.0b013e318274fb2f.
França FR, Burke TN, Caffaro RR, Ramos LA, Marques AP. Effects of muscular stretching and segmental stabilization on functional disability and pain in patients with chronic low back pain: a randomized, controlled trial. J Manip Physiol Ther. 2012;35(4):279–85. https://doi.org/10.1016/j.jmpt.2012.04.012.
Koppenhaver SL, Fritz JM, Hebert JJ, Kawchuk GN, Parent EC, Gill NW, et al. Association between history and physical examination factors and change in lumbar multifidus muscle thickness after spinal manipulation in patients with low back pain. J Electromyogr Kinesiol. 2012;22(5):724–31. https://doi.org/10.1016/j.jelekin.2012.03.004.
Akuthota V, Nadler SF. Core strengthening. Arch Phys Med Rehabil. 2004;85(3 Suppl 1):S86–92. https://doi.org/10.1053/j.apmr.2003.12.005.
Acknowledgements
Not applicable.
Funding
There was no direct funding source aligned to this study.
Author information
Authors and Affiliations
Contributions
XZ conceptualized and designed the study, carried out the initial analyses, composed the initial manuscript, and reviewed and revised the manuscript. HQL,ZJH,WSL,and LFW designed the data collection instruments, collected data, and reviewed and revised the manuscript. YS,JL coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and received approval from the Ethics Committee of the Third Hospital of Hebei Medical University. Informed Consent to participate were obtained from all the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, X., Liang, H., Hua, Z. et al. The morphological characteristics of paraspinal muscles in young patients with unilateral neurological symptoms of lumbar disc herniation. BMC Musculoskelet Disord 23, 994 (2022). https://doi.org/10.1186/s12891-022-05968-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-022-05968-5