Abstract
Background
In scoliosis corrective surgery, total blood loss is composed of visible blood loss, including intraoperative haemorrhage and drainage, and hidden blood loss in which blood extravasates into the tissues and accumulates in the surgical field. The purpose of this study was to investigate hidden blood loss (HBL) and its potential risk factors in adolescent idiopathic scoliosis patients undergoing posterior spinal fusion surgery and elucidate the influence of HBL on the necessity for postoperative blood transfusion.
Methods
We retrospectively studied adolescent idiopathic scoliosis patients undergoing posterior spine fusion for adolescent idiopathic scoliosis from January 2014 to December 2018 at our hospital. The patients’ demographics, blood loss-related parameters, surgeries and blood loss data were extracted. The association between patient characteristics and HBL was analyzed by Pearson or Spearman correlation analyses. Multivariate linear regression analysis was used to determine independent risk factors associated with HBL. Binary logistic regression analysis was used to analyze the influence of HBL on the necessity for postoperative blood transfusion.
Results
A total of 765 patients, of whom 128 were male and 637 were female (age range 10–18 years), were included in this study. The mean volume of HBL was 693.5 ± 473.4 ml, accounting for 53.9 % of the total blood loss. The multivariate linear regression analysis revealed that preoperative Hct (p = 0.003) and allogeneic blood transfusion (p < 0.001) were independent risk factors for HBL, while tranexamic acid (p = 0.003) was negatively correlated with HBL. Binary logistic regression analysis showed that HBL > 850 ml (P < 0.001, OR: 8.845, 95 % CI: 5.806–13.290) was an independent risk factor for the necessity for postoperative blood transfusion.
Conclusions
Substantial HBL occurred in adolescent idiopathic scoliosis patients undergoing posterior spinal fusion surgeries. Allogeneic blood transfusion and preoperative Hct were independent risk factors for HBL, while tranexamic acid was negatively related to HBL. HBL and its influencing factors should be considered when planning perioperative transfusion management. Patients with HBL greater than 850 ml should be closely monitored in cases of postoperative anaemia.
Level of evidence
Level III.
Similar content being viewed by others
Background
Blood loss remains a major focus in orthopaedic surgery for adolescent idiopathic scoliosis due to extensive and complex operating procedures. Anaesthesiologists and surgeons might primarily consider visible blood loss, including postoperative drainage, and ignore the component that penetrates the tissues, residual blood in the vertebral canal and blood loss due to haemolysis, which is known as hidden blood loss [1]. The concept of HBL was first proposed by Sehat [2] and has received increasing attention from clinicians in recent years. Xu et al. [3] showed that the HBL in posterior lumbar interbody fusion surgery accounted for approximately 61 % of the total blood loss in rheumatoid arthritis patients. Kai et al. [4] also reported that the volume of HBL reached 1084 ml, accounting for 82 % of total blood loss, after hip hemiarthroplasty. HBL could exacerbate postoperative haemoglobin level reductions, resulting in increased blood transfusion requirements. Clarifying HBL will be helpful for clinicians to improve clinical assessment capabilities and manage postoperative blood transfusion. However, to date, few published studies have examined HBL in posterior spinal fusion surgeries for adolescent idiopathic scoliosis. In this study, we retrospectively explored the volume of HBL in adolescent idiopathic scoliosis surgery patients and analyzed the influencing factors of HBL.
A previous study reported that 23.4 % of patients required postoperative transfusion in posterior spinal fusion surgeries for adolescent idiopathic scoliosis [5]. Furthermore, we also found that a considerable number of patients still experienced anaemia after surgery at our medical centre, which required transfusion. Postoperative anaemia has a negative effect on illness recovery and leads to an extended hospital stay. This phenomenon may be associated with hidden blood loss. Therefore, clarifying the relationship between hidden blood loss and postoperative transfusion will guide perioperative blood transfusion practices.
Materials and methods
Patients
Institutional review board approval was waived as patients’ private information was not retrieved. A total of 765 patients undergoing posterior spinal fusion surgeries for adolescent idiopathic scoliosis from January 2014 to December 2018 were included in current study. All surgeries were performed by highly experienced surgeons using a uniform approach. The inclusion criteria were as follows: patients (1) younger than 18 years and diagnosed adolescent idiopathic scoliosis; (2) who underwent posterior spinal fusion surgery; and (3) obtained stable perioperative fluid balance and haemodynamics. The exclusion criteria were as follows: patients (1) with coagulation disorders or perioperative infection; (2) who were treated with anticoagulant drugs; (3) who experienced intraoperative blood loss greater than 2.5 L on account of larger bias with excessive blood loss [6]; and (4) who suffered cerebrospinal fluid leakage.
Date extraction
Demographic information was collected from electronic medical records, including age, sex, height, weight, body mass index (BMI), preoperative and postoperative haematocrit (Hct) and haemoglobin (Hb), American Society of Anaesthesiologists (ASA) classification, preoperative Cobb angle, prothrombin time and activated partial thromboplatin time. Surgery information included operation time, pedicle screw number, bone mineral density, the number of surgical segments, whether osteotomy was performed, the use of tranexamic acid, intraoperative blood loss, postoperative drainage volume, and allogeneic and autologous blood transfusion volume.
Blood loss management and calculation of HBL
Intraoperative blood loss was recorded by the anaesthesiologist and was mainly determined from the volume of blood in the suction apparatus and in the soaked gauzes that were used during the entire operation. Postoperative blood loss was calculated by measuring the blood volume in the haemovac. Most of the haemovac was removed on the third postoperative day. If the drainage tube could not be removed when calculating the blood loss, we measured the blood in the drainage tube on the day when the blood was taken. The total visible blood loss was calculated as the sum of the volume of intraoperative blood loss and the volume of postoperative drainage. Blood lost during surgery could be collected by the blood salvage technique and reinfused into the patient as autologous blood; whether to perform this procedure was decided by the anaesthesiologist. Patients were given a blood transfusion when the haemoglobin level was below 70 g/dL or below 80 g/dL with significant symptoms of anaemia, such as increased heart rate hypotension. We calculated the patient blood volume (PBV) according to the formula described by Nadler et al. [7]: PBV (L) = k1 × height (m)3 + k2 × weight (kg) + k3, (k1 = 0.3669, k2 = 0.03219, k3 = 0.6041 for males, and k1 = 0.3561, k2 = 0.03308, k3 = 0.1833 for females). The total red cell volume can be calculated by multiplying the PBV and the patient Hct together. Therefore, a reduction in Hct reflects a change in red cell volume [8]. As the haemorrhage continues, the transfusion is used to sustain the patient’s circulating volume. The linear formula proposed by Gross was found to intimately follow a logarithmic formula [9]. According to the Gross formula [9], total blood loss = PBV (Hctpre - Hctpost)/Hctave, where Hctpre is the initial preoperative Hct, Hctpost was Hct on the second or the third day postoperatively, and Hctave is the average of the Hctpre and the Hctpost. Hidden blood loss was calculated by subtracting the visible blood loss from the total blood loss according to the formula of Sehat et al. [10] as follows:
When perioperative blood transfusion is performed, hidden blood loss = total blood loss + allogeneic blood transfusion + autologous blood transfusion - visible blood loss.
Statistical analysis
All data analyses were performed using IBM SPSS 22.0 software. Categorical variables are presented as frequencies, and the chi-squared test was used to compare dichotomous variables. Normally distributed continuous variables are presented as the mean ± SD, and an independent samples t test was used to compare intergroup differences. Pearson’s correlation (used for normally distributed data), Spearman’s correlation (used for the non-normally distributed data) and multivariate linear regression analyses were performed to identify risk factors for HBL, using 13 quantitative (age, ASA classification, BMI, preoperative Hct, preoperative Cobb angle, prothrombin time, activated partial thromboplatin time, bone mineral density, operation time, segment number, pedicle screw number, autologous blood transfusion, allogeneic blood transfusion, and postoperative drainage) and qualitative variables (osteotomy, tranexamic acid and sex). For the qualitative variables, osteotomy and tranexamic acid were set as “1”. Non-osteotomy and non-tranexamic acid were set as “0”. A binary logistic regression model was established to identify the relationship between hidden blood loss and postoperative transfusion. Statistically significant continuous variables were converted to categorical variables through their cut-off points, which were determined by receiver operating characteristic curve (ROC) analysis. The level of statistical significance was set at P < 0.05.
Results
Demographic and clinical characteristics
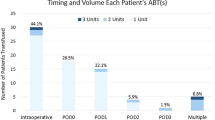
A total of 765 patients undergoing posterior spinal fusion corrective surgeries for adolescent idiopathic scoliosis, 128 of whom were male and 637 of whom were female, were analyzed. The mean hidden blood loss volume was 693.5 ± 473.4 ml, accounting for 53.9 % of the total blood loss volume. The demographic and clinical characteristics are summarized in Table 1. The mean total blood loss volume was 1285.7 ± 437.5 ml. The mean change in Hct level was 11.0 ± 10.9 %, and the mean Hb loss was 36.8 ± 13.9 g/L. Approximately 690 (90.2 %) patients required intraoperative blood transfusions, and the mean transfusion volume was 672.4 ± 657.1 ml. A total of 205 (26.8 %) patients required transfusions of suspended red blood cells after the operation, and the mean transfusion volume was 126.5 ± 242.9 ml.
Analysis of risk factors for HBL in patients undergoing scoliosis surgery
Pearson or Spearman correlation analyses for hidden blood loss showed the following parameters with p < 0.05 (Table 2): sex (p = 0.000), operation time (p = 0.002), preoperative Hct (p = 0.000), preoperative Cobb angle (p = 0.024), tranexamic acid (p = 0.000), allogeneic blood transfusion (p = 0.000) and segment number (p = 0.040). To further explore the association between HBL and the risk factors mentioned above, we performed a multivariate linear regression analysis. The results indicated that preoperative Hct (p = 0.003) and allogeneic blood transfusion (p < 0.0001) were independent risk factors for HBL, while tranexamic acid (p = 0.003) was negatively correlated with HBL (Table 3).
HBL was an independent risk factor for the requirement for postoperative transfusion in scoliosis corrective surgery patients
As illustrated in Table 4, the univariate analysis showed that variables sex, BMI, preoperative Hb, segment number, pedicle screw number, HBL and postoperative drainage were significantly different between the postoperative transfusion and postoperative nontransfusion groups. Statistically significant continuous variables were transformed into categorical variables by their cut-off points. These variables were further analyzed by binary logistic regression, and the results revealed that HBL > 850 ml (P < 0.001, OR: 8.845, 95 % CI: 5.806–13.290) was an independent risk factor for postoperative blood transfusion (Table 5).
Discussion
Posterior spinal fusion corrective surgeries for adolescent idiopathic scoliosis are often associated with significant intraoperative blood loss [11,12,13]. The volume of visible blood loss can be easily measured in the perioperative period, which can attract the attention of clinicians. In clinical practice, we always observe a degree of postoperative haemoglobin decline that is inconsistent with the volume of intraoperative blood loss. Thus, there may be other sources of blood loss. In 2000, the concept of HBL was first proposed by Sehat et al. [2], which can explain this phenomenon. However, the bleeding defined HBL which was caused by the leaking of blood into tissue spaces, blood accumulated in the surgical field and blood haemolysis is always ignored although reported with a large volume. Fortunately, an increasing number of studies on HBL after spinal surgery have been performed in recent years. Xu et al. [3] reported that the average volume of HBL after posterior lumbar interbody fusion surgery for lumbar spinal stenosis was 423 ml, accounting for 61 % of total blood loss; Derong et al. determined that HBL accounted for 47 % of the total blood loss [14]. These numbers were higher than expected. Our study showed that the mean HBL volume during scoliosis surgery was 693.5 ml, accounting for 53.9 % of the total blood loss. These results indicate that HBL accounts for a considerable proportion of perioperative blood loss; therefore, surgeons and anaesthesiologists should pay more attention to physiological derangement related to HBL.
The underlying mechanism of HBL has not been well clarified. The mainstream view is that HBL primarily occurs as extravasation of the blood into the surgical-site surrounding tissue, blood haemolysis and blood accumulated in the surgical field. Research has shown that main source of HBL is the extravasation of blood into tissues by using labelled red blood cells [15,16,17]. In our study, the extensive surgical area required for scoliosis surgery provided a wide area for bleeding, which consequently led to more hidden blood loss than transforaminal lumbar interbody fusion surgery [18, 19]. Therefore, paravertebral tissue incisions and wound sutures need to be more rigorously applied in corrective scoliosis surgery. Pattison et al. [20] showed that haemolysis was another possible cause of HBL. Faris et al. [21] reported that the employment of unwashed, filtered and reinfused blood increased haemolysis. Furthermore, in this study, intraoperative allogeneic blood transfusion was an independent risk factor for HBL in the multivariate linear regression analysis. Therefore, we speculated that haemolysis of erythrocytes induced by allogeneic blood transfusion might be, at least in part, responsible for HBL in posterior spinal fusion surgeries. Further prospective studies are needed to elucidate the underlying mechanism of HBL.
In this study, we investigated and examined the potential risk factors for HBL in scoliosis corrective surgery by multivariate linear regression analysis. The results showed that preoperative Hct was another independent risk factor for HBL. That is, patients with higher preoperative Hct appear to be in a state of haemoconcentration. More red blood cells are lost during intraoperative bleeding, which results in a lower Hct when combined with infusion dilution. We speculated that this may be one of the reasons for the higher HBL. Our study revealed that the use of tranexamic acid was negatively correlated with HBL. Tranexamic acid is a synthetic lysine analogue that is often used in orthopaedic surgery as an antifibrinolytic drug. Previous studies have shown that tranexamic acid can effectively reduce the total volume of intraoperative bleeding and the need for blood transfusion in scoliosis corrective surgery [22, 23], but there are few reports on whether tranexamic acid can reduce HBL in scoliosis corrective surgery. In transforaminal thoracic interbody fusion, Wang et al. found that the application of tranexamic acid significantly reduced HBL [24]. This group recommended that tranexamic acid be used as a standard drug to reduce HBL during the perioperative period. In the current study, tranexamic acid was used at a loading dose of 1 g before skin incision, and HBL was significantly reduced compared to that in the control group of posterior spinal fusion surgery patients. Therefore, intraoperative use of tranexamic acid may contribute to reducing HBL in scoliosis corrective surgery patients. However, the sample size of these studies was relatively small, and the effect of tranexamic acid on HBL, including drug dosage, still needs to be confirmed in more rigorous prospective studies with a larger sample size.
Excessive blood loss can lead to relevant postoperative complications [25], especially for minors, because their compensatory ability is less sound than that of adults. Wen et al. suggested a positive association between HBL and operative complications, although postoperative complications were not an independent risk factor for HBL [16]. The volume of HBL should be of sufficient concern to clinicians. In this research, the incidence of postoperative blood transfusion was as high as 26.8 %. Compared with intraoperative blood transfusion, postoperative anaemia is not easily detected in a timely manner. Therefore, we explored whether the volume of HBL was related to the necessity for postoperative transfusion. Binary logistic regression showed that HBL was an independent risk factor for the necessity for postoperative transfusion for scoliosis corrective surgery patients. In scoliosis corrective surgery, patients with HBL volumes greater than 850 ml, especially those with anaemia, should be closely monitored postoperatively in case of potential complications. As opposed to visible blood loss, HBL is more likely to be overlooked, especially in the postoperative period. The clinical application of hidden blood loss determination will provide clinicians with a new indicator for the management of postoperative blood transfusion, in addition to postoperative drainage volumes and haemoglobin levels, which will facilitate more accurate postoperative management strategies and ensure patients’ safety in perioperative period.
There were several limitations in this study. Since this was a descriptive study, bias may have been present. Further prospective studies are required to confirm the risk factors for HBL in scoliosis corrective surgery patients. In addition, Hct was measured twice before surgery in some patients, and the latest Hct was extracted to calculate HBL. Whether the latest measurements reflect preoperative Hct levels is still controversial. Finally, we could not rule out the effect of racial differences on HBL because most patients included in this study were native residents.
Conclusions
In conclusion, a large volume of HBL occurred in adolescent idiopathic scoliosis patients undergoing posterior spinal fusion surgeries, and this level of HBL was higher than previously expected in posterior spinal fusion surgeries. Allogeneic blood transfusion and preoperative Hct were independent risk factors for HBL, while tranexamic acid was negatively correlated with HBL. HBL and its influencing factors should be considered when determining perioperative transfusion management strategies for scoliosis corrective surgery patients. In addition, HBL volumes greater than 850 ml were an independent risk factor for the necessity for postoperative transfusion in scoliosis corrective surgery patients. Physicians should closely monitor patients with hidden blood loss volumes greater than 850 ml in cases of postoperative anaemia.
Availability of data and materials
The datasets generated during and analyzed during the current study are not publicly available due to hospital regulations but are available from the corresponding author on reasonable request.
Abbreviations
- HBL:
-
Hidden blood loss
- Hct:
-
Hematocrit
- Hb:
-
Hemoglobin
- BMI:
-
Body mass index
- ASA:
-
American society of Anesthesiologists
- PLT:
-
Platelet
- INR:
-
International Normalized Ratio
- APTT:
-
Activated partial thromboplastin time
- PT:
-
Prothrombin time
- BUN:
-
Blood urea nitrogen
- SCR:
-
Serum creatinine
References
Elgafy H, Bransford RJ, McGuire RA, Dettori JR, Fischer D. Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine (Phila Pa 1976). 2010;35(9 Suppl):S47-56.
Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–5.
Xu S, Liang Y, Wang J, Yu G, Guo C, Zhu Z, Liu H. Blood Loss of posterior lumbar interbody fusion on lumbar stenosis in patients with rheumatoid arthritis: a case-control study. Spine (Phila Pa 1976). 2019;44(17):E1045–52.
Xu K, Anwaier D, He R, Zhang X, Qin S, Wang G, Duan X, Tong D, Ji F. Hidden blood loss after hip hemiarthroplasty using the superPATH approach: a retrospective study. Injury. 2019;50(12):2282–6.
Soliman HAG, Beausejour M, Joncas J, Roy-Beaudry M, Barchi S, Mac-Thiong JM, Labelle H, Grimard G, Parent S. Predicting lowest hemoglobin level and risk of blood transfusion in spinal fusion surgery for adolescent idiopathic scoliosis. Eur Spine J. 2019;28(6):1342–8.
Smorgick Y, Baker KC, Bachison CC, Herkowitz HN, Montgomery DM, Fischgrund JS. Hidden blood loss during posterior spine fusion surgery. Spine J. 2013;13(8):877–81.
Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32.
Bourke DL, Smith TC. Estimating allowable hemodilution. Anesthesiology. 1974;41(6):609–12.
Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58(3):277–80.
Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561–5.
Koerner JD, Patel A, Zhao C, Schoenberg C, Mishra A, Vives MJ, Sabharwal S. Blood loss during posterior spinal fusion for adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2014;39(18):1479–87.
Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion for pediatric patients with idiopathic scoliosis in the United States, 2004–2009. Spine (Phila Pa 1976). 2014;39(22):1860–7.
Ialenti MN, Lonner BS, Verma K, Dean L, Valdevit A, Errico T. Predicting operative blood loss during spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(4):372–6.
Xu D, Ren Z, Chen X, Zhuang Q, Hui S, Sheng L, Li S. The further exploration of hidden blood loss in posterior lumbar fusion surgery. Orthop Traumatol Surg Res. 2017;103(4):527–30.
Ren Z, Li S, Sheng L, Zhuang Q, Li Z, Xu D, Chen X, Jiang P, Zhang X. Efficacy and safety of topical use of tranexamic acid in reducing blood loss during primary lumbar spinal surgery: a retrospective case control study. Spine (Phila Pa 1976). 2017;42(23):1779–84.
Wen L, Jin D, Xie W, Li Y, Chen W, Ding J, Xu J, Ren D. Hidden blood loss in posterior lumbar fusion surgery: an analysis of risk factors. Clin Spine Surg. 2018;31(4):180–4.
Nagabhushan RM, Shetty AP, Dumpa SR, Subramanian B, Kanna RM, Shanmuganathan R. Effectiveness and safety of batroxobin, tranexamic acid and a combination in reduction of blood loss in lumbar spinal fusion surgery. Spine (Phila Pa 1976). 2018;43(5):E267–73.
Yang Y, Zhang L, Liu B, Pang M, Xie P, Chen Z, Wu W, Feng F, Rong L. Hidden and overall haemorrhage following minimally invasive and open transforaminal lumbar interbody fusion. J Orthop Traumatol. 2017;18(4):395–400.
Zhang H, Chen ZX, Sun ZM, Jiang C, Ni WF, Lin Y, Wu YS. Comparison of the total and hidden blood loss in patients undergoing open and minimally invasive transforaminal lumbar interbody fusion. World Neurosurg. 2017;107:739–43.
Pattison E, Protheroe K, Pringle RM, et al. Reduction in haemoglobin after knee joint surgery. Ann Rheum Dis. 1973;32:582–4.
Faris PM, Ritter MA, Keating EM, et al. Unwashed filtered shed blood collected after knee and hip arthroplasties. A source of autologous red blood cells. J Bone Joint Surg Am. 1991;73(8):1169–78.
Yuan QM, Zhao ZH, Xu BS. Efficacy and safety of tranexamic acid in reducing blood loss in scoliosis surgery: a systematic review and meta-analysis. Eur Spine J. 2017;26(1):131–9.
Yagi M, Hasegawa J, Nagoshi N, Iizuka S, Kaneko S, Fukuda K, Takemitsu M, Shioda M, Machida M. Does the intraoperative tranexamic acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine (Phila Pa 1976). 2012;37(21):E1336-1342.
Wentao W, Duan K, Minjie M, Jiang Y. Tranexamic acid decreases visible and hidden blood loss without affecting prethrombotic state molecular markers in transforaminal thoracic interbody fusion for treatment of thoracolumbar fracture-dislocation. Spine (Phila Pa 1976). 2018;43(13):E734–9.
Huang YH, Ou CY. Significant blood loss in lumbar fusion surgery for degenerative spine. World Neurosurg. 2015;84(3):780–5.
Acknowledgements
None.
Funding
NO.
Author information
Authors and Affiliations
Contributions
LPW participated in the design of the study, wrote the manuscript and performed the study, XXS and MHL collected and analyzed the data. JLL and YQC designed and supervised the entire study. LPW was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No patients’ private information is involved. The retrospective study was waived for the ethical approval and informed consent by the ethic committee of Yijishan Hospital of Wannan Medical College. We confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests from a commercial party related directly or indirectly to the subject of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, L., Liu, J., Song, X. et al. Hidden blood loss in adolescent idiopathic scoliosis patients undergoing posterior spinal fusion surgery: a retrospective study of 765 cases at a single centre. BMC Musculoskelet Disord 22, 794 (2021). https://doi.org/10.1186/s12891-021-04681-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04681-z