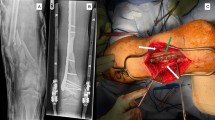
Abstract
Background
The induced membrane technique (IMT) is an effective strategy to repair bone defects and involves a two-stage set of surgical procedures. Although the IM has osteogenic activity, bone grafting is necessary in standard IMT. Bone defects repaired completely by osteogenic activity of the IM alone without bone grafts are rare.
Case presentation
We present a case of infected fractures and bone defects of the ulna and radius treated with IMT. After the first stage using polymethylmethacrylate (PMMA) beads, X-rays showed that new callus developed after 2 to 4 months, and the defects were repaired completely by 5 months. We also present a literature review on spontaneous osteogenesis of the IM in patients.
Conclusions
We present a case of infected ulnar and radial bone defects that healed by 5 months after the first stage of the IMT using a PMMA spacer. This finding suggests that local associated inflammatory reactions and bone tissue might enhance the osteogenic activity of the IM, causing spontaneous healing of bone defects. This appears to be the first such case reported in the literature.
Similar content being viewed by others
Background
Bone defects in the extremities caused by severe trauma, infection and tumor resection are common in clinical practice. Bone defects greater than two cm cannot heal spontaneously, and reconstruction surgery is necessary [1, 2]. The induced membrane technique (IMT) is a recently developed effective strategy to repair bone defects [3,4,5]. Standard IMT includes a two-stage set of surgical procedures. The first stage involves insertion of a polymethylmethacrylate (PMMA) cement spacer after radical debridement of bone and soft tissue. A membrane around the spacer formed as a foreign body reaction is known as an induced membrane (IM). The second stage is bone grafting within the membrane after removal of the cement spacer [4, 5]. The mechanisms of IMT mainly include the following: (i) the IM acts as a barrier to prevent the soft tissue from growing into the fracture site, and plays a role as a wrapping device to prevent loosening and absorption of the bone graft; (ii) the IM secretes osteogenic factors and contains mesenchymal stem cells (MSCs), which express bone morphogenetic protein-2 (BMP-2), vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β); (iii) the surface of the IM has rich micro-vessels to increase the blood supply [1, 3]. Therefore, the IM has intrinsic osteogenic activity [6]. However, bone defects repaired completely by intrinsic osteogenic activity of the IM alone are rare in clinical practice. We report a case of infected fractures with ulnar and radial bone defects that were repaired completely by osteogenic activity of the IM and review the literature on spontaneous osteogenesis of the IM.
Case presentation
A previously fit and healthy 44-year-old female with no history of hypertrophic ossifications was admitted with a complaint of pain and limited movement of the left upper limb following an accident in which her arm was entangled and twisted injury in a machine. Physical examinations and X-rays demonstrated that the patient had fractures of the left humerus, ulna and radius (AO classification: 12-A3, 13-B1, 22-A3) with a forearm skin contusion (Fig. 1). Open reduction and definitive stabilization using plates and screws of the fractures of the left humerus, ulna and radius were performed immediately on admission (Fig. 2). She was discharged after the incisions healed. Six weeks following internal fixation, her left forearm became red, swollen and painful. A small hole was made, and pus began to exude. Pseudomonas aeruginosa was cultured from the secretion. X-rays showed obvious bone resorption and slight periosteal reactions on the left ulna and radius (Fig. 3). A deep incision infection after internal fixation of the ulna and radius fractures was confirmed. Because conservative treatment is invalid, surgical intervention was required. Following radical debridement including removal of the hardware, 0.7 cm segmental defects of the fracture ends, and 4–5 cm partial defects of the ulna and radius were noted. External fixators were used for fixation and maintaining the length of the ulna and radius, then PMMA bone cement beads loaded with vancomycin (40:4) were inserted into and around the defects (Fig. 4). Cefmetazole was administered intravenously for 2 weeks, followed by oral rifampin for 4 weeks. The wound healed uneventfully, and she was encouraged to perform early postoperative rehabilitation activities. Follow-up X-rays two and 4 months after the first stage displayed a continuing process of new bone regeneration in the bone defects (Figs. 5 and 6). The ulnar and radial fractures and defects were healed by clinical evaluation by 4.5 months, and the external fixators were removed in the clinic at 5.5 months. The second stage of the IMT (bone grafting) was not necessary. The bone cement spacers were removed 6 months after filling with PMMA (Fig. 7). Intraoperative exploration revealed that parts of the spacers were wrapped by new callus. No additional surgeries were between the two surgeries. The humeral fracture healed by 3 months postoperatively. The patient recovered the normal length of her humerus, ulna and radius, as well as most of the function of her left upper limb. One year postoperatively, there was no recurrence of infection, her hand grip strength recovered to 80% of the contralateral side, her range of motion: wrist flexion recovered to 56°, wrist extension recovered to 53°, pronation of the forearm recovered to 69°, supination recovered to 73°, elbow flexion recovered to 95°, and elbow extension recovered to 0°.
Method of literature review
Studies were retrieved using the terms “bone defect”, “IMT” or “Masquelet technique”, “osteogenesis” or “bone formation” and “bone healing” from PubMed and Web of Science databases between 2000 and 2020 to collect cases of bone defects repaired using the IMT or Masquelet technique using PMMA as a spacer, in which new bone formed without bone graft.
Retrieval results
There were only two case reports of bone defects repaired by IMT, in which new bone was found with detailed clinical and follow-up data. One of these patients was a 42-year-old male with severe femoral fractures and defects accompanied by traumatic brain injury (TBI). The interval from filling with PMMA to clinical healing was 48 days [7]. The other was a 7-year-old male boy with mandibular Ewing’s sarcoma treated by a combination of neoadjuvant chemotherapy, resection surgery, and adjuvant radiochemotherapy. A macroplate and a PMMA cement spacer were used for the reconstruction. The spacer was removed after 17 days. X-ray films 2 months postoperatively showed that a small amount of new bone had formed along the macroplate; however, bone grafting was performed in a second stage of surgery [8].
Discussion
In 2009, Klaue et al. [6] first found in an animal model that the IM is spontaneously osteogenic. They designed a reproducible animal model producing a stable 3 cm mid-diaphyseal bone resection and periosteal defects on sheep femurs and created a foreign-body membrane. After removal of the spacer, radiographs in the group without bone grafts at 16–18 weeks demonstrated that a small amount of woven bone formed at the proximal junction of the bone end and the IM. However, the spontaneous osteogenesis of the IM was weak. No constant osteogenesis was observed in other studies, so it did not attract attention.
The rapid callus formation in the case of femoral fractures and defects accompanying TBI reported by Hotchen et al. [7] was attributed to the dual effects of the TBI and the IM, especially the TBI. The exact mechanisms of fracture that accompany TBI to increase callus formation are not yet completely understood. Potential mechanisms may include: (i) Bone fracture triggers the release of cytokines including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and interleukin-6 (IL-6) in both the serum and the cerebrospinal fluid that attract MSCs to the fracture site and generate an osteogenic effect [7, 8] . (ii) The enhanced inflammatory response caused by TBI creates a receptive environment for the occurrence of heterotopic ossification (HO), and the upregulation of some inflammatory factors in the body fluids accelerates bone formation. (iii) Leptin is increased in the circulation following TBI and is also released in the stress response to cytokines and hormonal factors. Leptin is positively correlated with the amount of callus formation at a fracture site [7, 9]. (iv) Patients who suffer from TBI are typically in a coma and require mechanical ventilation. The combination of these factors promotes the incidence of HO and rapid bone formation in TBI patients.
A 7-year-old boy with sarcoma resection of the mandibular branch was reported by Sorin et al. [8]. Only a small amount of new bone was found in the boy and this was attributed to the osteogenic activity of the IM and younger age.
The case presented in the current study involved a situation in which the infected bone defects healed spontaneously by osteogenic activity of the IM alone. This case differed from that of Hotchen et al. [7] which was based on the presence of TBI, and from that of Sorin et al. [8] based on the amount of osteogenesis. The possible reasons for spontaneous healing of the bone defects are: (i) the local inflammatory responses increase the release of inflammatory factors, which increase the thickness of the IM and the level of vascularization as well as recruitment of pro-osteogenic cytokines [10,11,12] and (ii) some fracture fragments or periosteum are present near the cement spacer, which can provide seed cells and enhance the osteogenic activity of the IM [13,14,15].
Catros et al. [14] conducted an in vivo experiment to investigate the osteoinductive properties of the IM on a macroporous hydroxyapatite-tricalcium phosphate (HA-TCP) scaffold in a non-osseous subcutaneous site in rabbits. The results showed that the IM placed in a non-osseous site had no osteoinductive properties. In this current case, callus growth was observed only on the side near the bone defects, but not on the side near the subcutaneous tissue, consistent with the results of Catros et al. [14] and Klaue et al. [6]. In view of the local associated inflammatory responses, fracture fragments or periosteum around the cement spacer may improve the osteogenic activity of the IM, causing spontaneous healing of the bone defects. Therefore, we assume that filling with PMMA beads as a spacer for partial bone defects or adding a small amount of bone graft for segmental bone defects around the beads may stimulate spontaneous osteogenesis. Of course, this hypothesis needs to be tested experimentally.
It is a slow process to completely repair bone defects by the osteogenic activity of IM alone. This is especially true in the long bones of the lower limb because it may lead to stiffness of adjacent joints and other sequelae; it is not a preferred method at present. The infection of the incision after internal fixation of the ulnar and radial fractures was related to improper surgery for a complex severe injury of the upper extremity (floating elbow) combined with a forearm skin contusion, or to improper aseptic conditions. The lesson of this case is that for a complex severe injury with a skin contusion, multiple site incisions, especially large incisions, for open reduction and internal fixation should not be performed emergently at the same time.
Conclusions
We present a case of infected bone defects of the ulnar and radial bone. After the first stage of the IMT using PMMA beads, X-rays showed that new callus developed and the defects healed by 5 months. This finding most likely can be attributed to local associated inflammatory reactions and bone tissue, which enhance the osteogenic activity of the IM, causing spontaneous healing of the bone defects. This case differed from those reported in the literature. It appears to be the first such case reported in the literature.
Availability of data and materials
This is a case report of a single patien, the imaging data are presented within the manuscript. The original reports, laboratory studies, imaging studies and outpatient clinic records are retained as per normal procedure within the medical records of our institution.
Abbreviations
- IMT:
-
Induced membrane technique
- IM:
-
Induced membrane
- PMMA:
-
Polymethylmethacrylate
- MSC:
-
Mesenchymal stem cell
- TBI:
-
Traumatic brain injury
- HO:
-
Heterotopic ossification
References
Masquelet AC, Fitoussi F, Begue T, et al. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45(3):346–53.
Yee MA, Mead MP, Alford AI, Hak DJ, Mauffrey C, Hake ME. Scientific understanding of the induced membrane technique: current status and future directions. J Orthop Trauma. 2017;31(Suppl 5):S3–8. https://doi.org/10.1097/BOT.0000000000000981.
Bezstarosti H, Metsemakers WJ, Lieshout EMMV, et al. Management of critical-sized bone defects in the treatment of fracture-related infection: a systematic review and pooled analysis. Arch Orthop Trauma Surg. 2020;1(Suppl 4):3.
Dhar SA, Dar TA, Mir NA. The management of infected nonunion of the forearm by the Masquelet technique. Strat Trauma Limb Reconstruct. 2019;14(1):1–5. https://doi.org/10.5005/jp-journals-10080-1411.
Wang J, Yin Q, Gu S, Wu Y, Rui Y. Induced membrane technique in the treatment of infectious bone defect: a clinical analysis. Orthop Traumatol Surg Res. 2019;105(3):535–9. https://doi.org/10.1016/j.otsr.2019.01.007.
Klaue K, Knothe U, Anton C, et al. Bone regeneration in long-bone defects: tissue compartmentalisation? In vivo study on bone defects in sheep. Injury. 2009;40(Supp-S4):95–S102.
Hotchen AJ, Barr LV, Krkovic M. Bridging hard callus at 48 days in an open femoral shaft fracture with segmental defect treated with a first-stage Masquelet technique: I wasn’t expecting that. Strat Trauma Limb Reconstr. 2018;13(1):57–60. https://doi.org/10.1007/s11751-017-0300-z.
Sorin T, Mansuy L, Colson T, Minetti C, Brix M, Simon E. Ewing's sarcoma of the mandible in children: reconstruction by induced membrane. Revue de stomatologie, de chirurgie maxillo-faciale et de chirurgie orale. 2014;115(5):318–22. https://doi.org/10.1016/j.revsto.2014.05.001.
Wu Y, Yin Q, Zhou Z, Gu S, Rui Y, Li F. Similarities and differences of induced membrane technique and wrap bone graft technique for repair of segmental bone defects. J Indian Ortho. 2020;54(2):156–63. https://doi.org/10.1007/s43465-019-00006-4.
Tsitsilonis S, Seemann R, Misch M, Wichlas F, Haas NP, Schmidt-Bleek K, et al. The effect of traumatic brain injury on bone healing: an experimental study in a novel in vivo animal model. Injury. 2015;46(4):661–5. https://doi.org/10.1016/j.injury.2015.01.044.
Huang H, Cheng WX, Hu YP, Chen JH, Zheng ZT, Zhang P. Relationship between heterotopic ossification and traumatic brain injury. J Orthop Transl. 2018;12:16–25. https://doi.org/10.1016/j.jot.2017.10.002.
Wang L, Tang X, Zhang H, Yuan J, Ding H, Wei Y. Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture. J Spinal Cord Med. 2011;34(5):501–9. https://doi.org/10.1179/2045772311Y.0000000034.
Liu H, Hu G, Shang P, Shen Y, Nie P, Peng L, et al. Histological characteristics of induced membranes in subcutaneousintramuscular sites and bone defect. Orthop Traumatol Surg Res. 2013;99(8):959–64. https://doi.org/10.1016/j.otsr.2013.08.009.
Catros S, Zwetyenga N, Bareille R, et al. Subcutaneous-induced membranes have no osteoinductive effect on macroporous HA-TCP in vivo. J Orthop Res. 2010;27(2):155–61.
Pélissier P, Lefevre Y, Delmond S, et al. Influences of induced membranes on heterotopic bone formation within an osteo-inductive complex. Experimental study in rabbits. Ann Chir Plast Esthet. 2009;54(1):16.
Acknowledgements
None.
Funding
Funding for this project was provided by the top medical expert team of “Taihu Talent Program” in Wuxi (2020).
Author information
Authors and Affiliations
Contributions
YL and JW wrote the initial draft of this manuscript and subsequent revisions. J W and QY are the senior authors who is treating the patient. YY collected the data and provided the Figs. YY and QY is responsible for the oversight of the report and editing the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics and consent to participate
The study was approved by ethics committee of Wuxi No.9 People’ s Hospital Affiliated to Suzhou University (No:LY202020–0115). Written informed consent for participation was obtained from the patient.
Consent for publication
Written informed consent for print and electronic publication of this case report was obtained from the patient.
Competing interests
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, Y., Wang, J., Yang, Y. et al. Bone defects are repaired by enhanced osteogenic activity of the induced membrane: a case report and literature review. BMC Musculoskelet Disord 22, 447 (2021). https://doi.org/10.1186/s12891-021-04317-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04317-2