Abstract
Purpose
High clinical success rates have been reported with the Masquelet technique in the treatment of traumatic bone loss. An increasing number of studies suggest that various factors can influence the properties of induced membranes. Goal of this systematic review is to answer the following questions: (1) which are the ideal spacer properties (material, surface topography, antibiotic supplementation) to booster the quality and osteogenic potential of induced membranes? (2) what is the ideal time to perform the second-stage operation?
Methods
A systematic search using the keywords “((Masquelet) OR (Induced Periosteum)) AND ((Spacer) OR (Time))” was performed in PubMed, Embase and Cochrane Library according to PRISMA guidelines. Studies published up to the 23rd of February 2022 were included and assessed independently by two reviewers.
Results
Thirteen animal and 1 clinical studies were identified to address the above questions. Spacer materials used were PMMA, silicone, titanium, polypropylene, PVA, PCL and calcium sulfate. With the exception of PVA sponges, all solid materials could induce membranes. Low union rates have been reported with titanium and rough surfaced spacers. Scraping of the inner surface of the IM also increased bony union rates. In terms of the ideal timing to perform the second-stage evidence suggests that membranes older than 8 weeks continue to have regenerative capacities similar to younger ones.
Conclusion
Membranes induced by smooth PMMA spacers loaded with low concentrations of antibiotics showed powerful osteogenic properties. Other materials such as Polypropylene or Calcium sulfate can also be used with good results. Despite current recommendation to perform the second stage operation in 4–8 weeks, membranes older than 8 weeks seem to have similar regenerative capacities to younger ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-bone defects resulting either from open fractures or from debridement for post-traumatic osteomyelitis represent still a major challenge for orthopaedic trauma surgeons. Various treatment options have been described to treat these defects including autologous bone grafting, vascularized fibular grafts, bone transport, diaphyseal replacement, allografts, titanium cages and the Masquelet technique [1]. Although several animal studies focus on the development of scaffolds/implants to allow guidance of bone formation [2,3,4], the two clinically most widely used techniques are bone transport described by Ilizarov [5] and the induced membrane (IM) technique described by Masquelet [6]. Bone transport has the advantage of no donor site morbidity, however, the treatment period is prolonged and complicated [7]. On the other hand, the IM technique is technically simple, since implants and approaches are familiar to most orthopaedic trauma surgeons and the union rates are high [8, 9].
The IM technique is performed as a 2-stage procedure. In the first stage, the bony defect is stabilized by an internal or external device and a spacer is placed into the defect to manage the dead space and to facilitate the formation of the membrane. In a second stage, 4–8 weeks later, the spacer is removed carefully, and the empty cavity surrounded by the IM (Fig. 1) is filled with bone graft [10]. Induced membranes are not simply a barrier, inhibiting soft tissue invasion into the bony defect, but have powerful osteogenic properties [11,12,13]. An increasing number of clinical and animal studies suggest that various factors (time to second operation, spacer characteristics, etc.) can influence these properties of induced membranes [13,14,15,16,17,18].
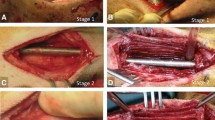
A Right distal femoral open fracture in a 18 year-old male patient sustained following a motorbike accident. B The fracture following debridement was stabilised with a locking plate and the bone defect was managed with a cement spacer (1st stage Masquelet technique). C Intraoperative picture during the second stage and prior to removal of cement spacer (green arrow) demonstrating the induction of the membrane (white arrows)
The goal of this systematic review is to answer the following questions: (1) Which are the ideal spacer properties (material, surface topography, porosity, antibiotic supplementation) to booster the formation and bioactivity of induced membranes? (2) What is the ideal time to perform the second-stage operation?
Materials and methods
Search strategy
A systematic search according to PRISMA [19] using the search terms “((Masquelet) OR (Induced Periosteum)) AND ((Spacer) OR (Time))” in PubMed, Embase and Cochrane Library was performed as of the earliest records till 23rd February 2022 by the first and second author. The search in PubMed and Embase was limited to the languages English and German.
Eligibility criteria
Studies were included if they met the following criteria: (1) studies investigated the Masquelet membrane in relation to the spacer or time; (2) the outcomes included histological and/or immunohistochemical and/or biomechanical and/or radiological data; (3) animal or clinical studies with the full-text paper published before the 23rd of February 2022.
Studies were excluded if they met the following criteria: (1) reviews, conference abstracts, case reports, letters, or comments; (2) full-text paper written in English or German was not available.
Results
The flowchart of the literature search is displayed in Fig. 2. Thirteen animal studies [11, 14,15,16,17,18, 20,21,22,23,24,25,26] and 1 clinical study [13] have been identified by both reviewers as appropriate to answer our research questions. The most important results of these studies are summarized in Table 1.
Animal models
Rat animal models are the most commonly used in relation to the Masquelet technique. Overall, 10 of 13 animal studies included in this review used rats [11, 14,15,16,17,18, 20,21,22,23, 26]. In one study, New Zealand’s white rabbits [24] and in another study, skeletally mature female goats [25] have been used. According to definition of critical size defect (length of defect exceeds the diameter of the affected bone by a factor of 2–2.5) [27], the defect size depended on the size of the animal used. In 9 out of 11 rat models, large femoral defects that ranged from 6 to 10 mm length were created. In one study, no bone defect was created and the spacer was placed in a 15 mm long subcutaneous pocket [20] and in another, a 6 mm long and 1 mm wide slot defect was created in the femur that required no osteosynthesis [26]. In four out of nine rat studies with critical size defects, plates have been used [18, 21, 22], in three studies, an external fixator [14,15,16], in one study, an internal fixator [17] and in one study, no specification was made [23].
As far as larger animal studies are concerned, rabbits had a 10 mm defect in the radius that was intramedullary stabilized with a K wire [24] and goats had a 50 mm defect at the tibia that was stabilized with an intramedullary nail [25].
Materials
PMMA has been used in all studies included in this review and acted as the control group in all spacer material comparing studies [14,15,16,17, 20,21,22, 26]. Other materials tested were: Silicone [20, 22], Titanium [14,15,16], Polypropylene [17], Calcium Sulfate [21], Polycaprolactone (PCL) [26] and Polyvinyl alcohol sponge (PVA) [15]. All solid materials could induce biologically active membranes with similar histological structures to that of PMMA. This was not the case for PVA sponges, that did not induce a membrane [15].
Comparing Titanium spacers with PMMA spacers, PMMA spacers showed better union rates in all studies [15, 16]. According to these results, we conclude that titanium spacers are not a viable option for clinical application.
Polypropylene spacers manufactured from disposable syringes induced bioactive membranes and showed no difference in bone formation at the 10 week microCT analysis [17].
Calcium sulfate spacers did not just induce highly bioactive membranes (VEGF and BMP-2 levels slightly higher than PMMA), but the IM was also thicker than PMMA. Additionally, the calcium sulfate IM showed signs of endochondral ossification, which is unique for this material and makes one-staged procedures conceivable.
Silicone IM showed no significant difference to PMMA as far as thickness of the membrane, vascular density and BMP2 expression are concerned [20, 22]. However, bony union has not been evaluated by these studies. In addition to that De Mones et al. did not create bone defects but placed the spacers in subcutaneous pocket [20]. The results of this study should be looked critically, since it is known that membranes formed in subcutaneous pockets have different bioactivity compared to membranes induced around bone defects [11].
Potential toxic effects of MMA (the major monomeric component of PMMA) have been investigated in a recent study from Stahl et al. [26]. They compared membrane formation around polycaprolactone (PCL) with high-dose MMA eluting PCL, low-dose MMA eluting PCL and PMMA to find robust IM around all groups. No statistically significant effect in regard to levels of VEGF and BMP2 has been detected. From these results, we can conclude (1) that PCL is a material capable of producing a bioactive IM and (2) that the monomer MMA cannot be used to boost the biologic potency of the membrane.
Spacer topography and porosity
Except for the material itself, each spacer is characterized by its surface roughness, porosity and overall external shape. These properties can have an influence on the effective surface area of the spacer (contact area between spacer and soft tissue) [25].
Roughness of the spacer has been thoroughly investigated by two animal studies [14, 16]. Toth et al. showed that roughening the spacer surface led to increased overall IL6 levels and that higher union rates could be achieved with smooth surfaced spacers 10 weeks post-engraftment in the microCT analysis [16]. Gaio et al. showed that roughening had also an effect on IM biomechanics, creating membranes that are more elastically compliant and failed at significantly higher strains in both directions [14].
Luangphakdy et al. used microCT to compare bone formation between smooth PMMA spacers and textured surface PMMA spacers with 2-mm thick and 2-mm deep linear grooves [25]. Micro-CT analysis found no difference between the smooth and ribbed PMMA spacers as far as total bone volume in the central region of the defect is concerned. Histologic analysis confirmed these results showing that a comparable range of new bone area was measured in both groups. Finally, the authors showed that scraping the inner surface of the IM, increased significantly bone formation.
As far as spacer porosity is concerned, to the best of our knowledge, there are no studies comparing spacers of different porosities to evaluate the quality of IM and bone formation. Shah et al. used high-porosity PMMA spacers [23], reporting induction highly bioactive membranes. The authors used PMMA powder and methylmethacrylate liquid with a carboxymethylcellulose (CMC) hydrogel to impart porosity and PLGA microspheres loaded with or without clindamycin to control drug release [28]. However, the focus of the study was not the porosity itself but the effect of clindamycin on infected bone defects.
Antibiotics
Three animal studies evaluated the influence of antibiotics and its dosage on the quality of IM [18, 23, 24]. Gentamicin, Clindamycin, and Vancomycin have been analyzed since these antibiotics can be easily mixed with cement powder and reliably eluted from these spacers.
Nau et al. compared commercially available antibiotic supplemented spacers and reported that IM thickness varied significantly in dependency from the antibiotics used [18]. IM formed by a gentamicin (Palacos R + G: 0,25 g gentamicin in 20 g cement powder) and gentamicin-vancomycin spacers (Copal G + V: 0,5 g gentamicin + 2 g vancomycin in 43 g cement powder) were thicker than IM formed around gentamicin-clindamycin supplemented spacers (Copal G + C: 1 g gentamicin + 1 g clindamycin in 42,7 g cement powder) at 6 weeks after surgery. The Copal and Palacos cements used in this are based on the same PMMA formula and were hand mixed in all cases.
Xie et al. explored the effects of different vancomycin concentrations (0, 1, 2, 4, 6, 8, and 10 g per 40 g cement powder) on IM formation in a rabbit radius bone defect model [24]. An obvious decrease in osteogenic and angiogenic capacity measured by immunohistochemical markers (CD31, STRO-1, Ki67) was observed when the concentration of vancomycin was more than 6 g per cement dose. In contrast low concentrations of vancomycin (1–4 g per cement dose) did not interfere with the proliferative capacity of IM, and even promoted their capacity. Even with the highest concentrations, no toxic effects could be observed on the animals.
Shah et al. compared membranes evoked by PMMA spacers with or without clindamycin in contaminated (Staph aureus) and not contaminated bone defects and found the expression of BMP-2 und VEGF to be high in all IM [23]. However, clindamycin treatment of the contaminated defects restored inflammatory cytokine and BMP-5 to the same levels as in the non-contaminated group.
Time
Plethora of knowledge is available regarding IM changes between the 2nd and 8th week [11, 17, 18, 21]. Animal studies suggest that the thickness of IM is increasing in the first 4 weeks and that the peak of osteogenic and angiogenic activity (measured by Ki67, STRO1 and VEGF) is reached between 2 and 4 weeks. After the 6th week the bioactivity of the IM is subsiding [11].
Little is known about histological and biological changes after this time period. We could identify only one clinical study analyzing IM created after significant longer time periods (up to 113 days) [13]. The authors of this study harvested 60 IM after spacer removal at the second stage of revision total knee arthroplasty. All patients had large femoral defects. The defects were stabilized with intramedullary static spacers. The samples were taken far from the joint structures. ELISA and protein microarray were used to quantify osteogenesis related factors for the different time points. Osteocalcin and osteopontin were found over all time periods without significant differences. VEGF levels seemed to be fluctuating over time but did not show a decreasing tendency. All membranes enhanced proliferation of cultured mesenchymal stem cells (MSC). Thus, the authors concluded that membranes older than 8 weeks retain their biological potency.
Tests used to evaluate biological potency of the membrane
Although most studies used similar methods to evaluate the quality of the IM, often different outcome parameters were chosen. This makes comparisons and correlations of the results problematic. Here, we summarize the most commonly performed tests in Masquelet-related studies.
Histological analysis was used by almost all studies included in this review article [11, 13, 16,17,18, 20, 21, 23, 24]. Hematoxylin/Eosin and Elastica van Gieson were the most used stainings (5 μm sections). Next to the histological structure, the thickness of the membrane was measured. IM consisted mostly of two-layers, a thin inner cellular layer and a larger external collagen rich layer [13, 20, 22]. The membrane thickness generally increases in the first 4 weeks, but this can be influenced by various factors (e.g. clindamycin supplemented spacer, radiation) [18, 20]. Mathieu et al. used histologic images to assess cell density, expressed by the number of cell nuclei per mm2 [17].
Immunochemistry was also used by most studies to quantify factor expression in the IM. Antibodies specific to growth factors (BMP-2, TGFβ, VEGF) and cytokines (IL6) were commonly used [11, 15,16,17, 21]. To reveal endothelial cells (mature vessels) immunohistochemical analysis was performed with anti-CD31 antibodies [18, 22, 24]. Immature blood vessels could be identified by von Willebrand Factor antibodies [11, 18]. Cells containing the nuclear protein Ki-67 were also identified with immunohistochemical methods to assess cellular proliferation [11, 18, 24]. Stro-1 antibodies were used to identify MSC [11, 18, 24]. Monocytes that play a major role in the early phases of the induced membrane formation were identified by the expression of the CD14 antigen [18].
The ELISA method has been used by some studies to quantify various bioactive factors such as BMP2 and VEGF [13, 22, 26]. Interestingly, Gessmann et al. co-cultured the IM in contact with growing MSC for 2 weeks before performing ELISA to analyze for supernatants like osteoprotegerin, osteocalcin and bone specific alkaline phosphatase [13]. Western Blot was used by two studies al to quantify the expression of BMP2, TGF-β1 and VEGF in IM extracts [20, 21].
Two studies used cell cultures of Human Bone Marrow Stromal cells (HBMSCs) to assess cell differentiation by measuring alkaline phosphatase (ALP) activity with a colorimetric assay [20, 22]. To achieve that, cell cultures were treated every day with 100 μg of IM lysates. A culture of bone marrow stromal cells in induction medium served as an internal control. Flow cytometry was used by Mathieu et al. to assess the presence of MSCs in IM with various antibodies (Immunophenotyping) [17].
Another two studies used the Real-time PCR to measure the level of expression of m-RNA for inflammatory cytokines (IL-6, IL-10, TNFa) and/or growth factors (VEGF, TGF-β1, BMP-5 and BMP-2) [21, 23].
Micro-CT was used to quantify bone regeneration, bone volume and bone mineral density after Stage 2 of the Masquelet technique [16, 17, 21, 25].
A summary of all the findings related to IM morphological and biological characteristics in relation to the length of time of maintaining the spacer prior to proceeding to the second stage is shown in Table 2.
Discussion
The success of Masquelet technique is based on creating a vascularized soft tissue envelope with similar—but not identical—properties to that of periosteum (“induced periosteum”) [29, 30]. This membrane is not just a barrier that impedes graft resorption but a biologic chamber that secretes various growth factors critical for bone regeneration [31, 32]. To further improve the biologic properties of the membrane, scientific efforts are made to optimize modifiable variables (spacer characteristics, timepoint of second operation) that could influence membrane formation.
Spacer material
Traditionally, the spacer used during the IM technique is made of polymethylmethacrylate (PMMA), a material well known to most orthopaedic surgeons [33]. PMMA can be moulded in the operating room to suit all defect sizes and morphologies. This gives the operating surgeon more flexibility compared to prefabricated spacers, an important feature, since defect size can change after debridement. In addition, PMMA provides mechanical stability and can be easily supplemented with various antibiotics in various dosages to treat deep infections and prevent biofilm formation [18]. On the other hand, concerns have been raised regarding potential toxicity of PMMA adjuvants and detrimental heat because of the exothermic reaction during cement polymerization [17, 20, 22].
The current literature does not support these concerns since no differences have been detected neither histologically in IM structure nor in cell density between PMMA and other solid materials such as titanium, polypropylene, silicone and calcium sulfate [14, 17, 21]. In addition to that, no significant differences have been recorded in BMP-2 levels and mesenchymal stem cells (MSCs) between PMMA and other solid materials [16, 17, 22]. Stahl et al. also showed that even high concentrations of MMA (the major monomeric component of PMMA) exerted no statistically significant effect on hMSC proliferation in vitro, nor on the levels of VEGF and BMP2 in vivo [26].
Although titanium spacers can induce bioactive membranes the bony union rates are significantly lower compared to that of PMMA, making this material not a viable option currently for clinical use [15, 16].
Polypropylene, on the other hand, evoked bioactive membranes and showed no difference in bone formation at the 10 week microCT analysis [17]. Use of Polypropylene spacers, manufactured from disposable syringes has also been described in a clinical case series study to reconstruct metacarpal bone defects with very good results [34]. Polypropylene, however, cannot be used in clinic as a local antibiotic carrier for treatment of infections.
Calcium sulfate is complete biodegradable and has some osteogenic properties [35]. In addition, it can be used as a vehicle to deliver antibiotics. Ma et al. reported that the calcium sulfate-induced membranes were generally thicker than the PMMA membranes. More interestingly at 8 weeks, some endochondral ossification in calcium sulfate-induced membranes has been observed. No significant differences have been reported as expression levels of VEGF and BMP-2 are concerned. Jiang et al. [36] used calcium sulfate to reconstruct a calcaneal defect in a case report. Eight weeks after introduction of the spacer, a membrane was visible around the spacer and autologous bone graft was used. Although a one-stage strategy was not performed, the authors postulate that for smaller defects a one-stage procedure without use of bone grafts would be possible.
Silicone membranes produce in the animal model membranes with similar characteristics to that of PMMA. However, bone healing has not been evaluated and to the best of our knowledge this material has never been used in clinical practice for a Masquelet procedure.
PVA sponge, on the other hand had a very different response. There was little to no tissue between the spacer material and muscle. Furthermore, the PVA spacer has been infiltrated by fibrous tissues [15].
Spacer topography and porosity
In a recent animal study, smooth PMMA spacers have been compared with rough PMMA spacers, showing significantly higher union rates for the smooth spacers [16]. Smooth titanium spacers showed also better union rates compared to rough surfaced titanium spacers. Interestingly, roughening has also an effect on IM mechanical properties, creating membranes that are more compliant [14]. IM created by rough surfaces are more likely to be deformed in situations such as graft overfilling or weight bearing, which in turn could affect bone regeneration.
A large animal study on goats compared PMMA spacers with a smooth or textured surface, showing no difference in bone formation [25]. Here, it is important to emphasize that a textured surface is not a rough surface. Scraping of the IM before grafting improved significantly bone healing.
The influence of the porosity of the spacer on IM and bone formation has not been explicitly evaluated via comparative animal or clinical studies. However, we know that a highly porous implants have usually a rough surface and a higher surface area. This could be beneficial for antibiotic eluting spacers since antibiotics and/or other drugs are eluted from the surface and pores of the implant as well as from microcracks within it [37]. Porosity of PMMA can be increased by avoiding vacuum-type mixing devices, since hand mixing introduces air into the cement mixture.
Supplementation with antibiotics
Bone defects in orthopaedic trauma patients are usually the result of open fractures or deep infections. Therefore, control of infection plays a central role in the treatment of these patients. Ilizarov claimed, “infection burns in the fire of regeneration” and suggested callus distraction to treat bone defects [38]. The IM technique allows surgeons to use the spacer as a tool to introduce antibiotics at the very center of infection. Antibiotics, most commonly supplementing bone cements are clindamycin, gentamicin and vancomycin. In high concentrations, all the above antibiotics can have cytotoxic effects with clindamycin being the most toxic, followed by vancomycin and gentamicin [18, 39,40,41].
Current literature suggests that local delivery of antibiotics is effective in mitigating deep infections and promotes the expression of growth factors at the IM in relatively low concentrations [23, 24]. However, differences have been reported between commercially available PMMA cements with antibiotic release from Copal bone cement being more extensive than from Palacos [42]. The concomitant use of more than one local antibiotics in combination with Copal cement seems to have a negative effect on the maturation of the IM [18].
Ideal time point to perform second step procedure
Current clinical recommendation is to perform the second surgery between 4 and 8 weeks post-spacer implantation, assuming the highest IM bioactivity [11, 43]. This narrow time frame has been questioned only by a few studies, that show membranes older than 8 weeks (even years after the 1st stage) to have regenerative capacities similar to younger ones [13, 44, 45]. Highly controlled animal studies are needed to evaluate IM bioactivity beyond the frame of 8 weeks.
Conclusions
Spacers made of materials offering a rigid barrier with a smooth surface, produce membranes with comparable characteristics in terms of histology, growth factors, and stem cell contents. PMMA is the golden standard for IM technique. Other materials such as Polypropylene or Calcium sulfate can be used.
Supplementing the PMMA spacer with relatively low concentrations of antibiotics (gentamicin, vancomycin, clindamycin) is an effective tool for controlling local infection and can even promote the osteogenic capacity of IM.
Despite current recommendation to perform the second stage procedure in 4–8 weeks, IM older than 8 weeks seems to have regenerative capacities similar to younger ones.
References
Liodakis E, Kenawey M, Krettek C, Wiebking U, Hankemeier S. Comparison of 39 post-traumatic tibia bone transports performed with and without the use of an intramedullary rod: the long-term outcomes. Int Orthop. 2011;35(9):1397–402. https://doi.org/10.1007/s00264-010-1094-5.
Pobloth AM, Schell H, Petersen A, Beierlein K, Kleber C, Schmidt-Bleek K, Duda GN. Tubular open-porous beta-tricalcium phosphate polycaprolactone scaffolds as guiding structure for segmental bone defect regeneration in a novel sheep model. J Tissue Eng Regen Med. 2018;12(4):897–911. https://doi.org/10.1002/term.2446.
Tarchala M, Harvey EJ, Barralet J. Biomaterial-stabilized soft tissue healing for healing of critical-sized bone defects: the Masquelet technique. Adv Healthc Mater. 2016;5(6):630–40. https://doi.org/10.1002/adhm.201500793.
Torbjorn M, Amela T, Andreas T, Stina E, Cecilia L, Caroline OM, Petra HJ, Marianne JW, Patricia H. Guided bone tissue regeneration using a hollow calcium phosphate based implant in a critical size rabbit radius defect. Biomed Mater. 2021;16(3):035018. https://doi.org/10.1088/1748-605X/abde6f.
Ilizarov GA, Lediaev VI. Replacement of defects of long tubular bones by means of one of their fragments. Vestn Khir Im I I Grek. 1969;102(6):77–84.
Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45(3):346–53.
Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990;250:81–104.
Morwood MP, Streufert BD, Bauer A, Olinger C, Tobey D, Beebe M, Avilucea F, Buitrago AR, Collinge C, Sanders R, Mir H. Intramedullary nails yield superior results compared with plate fixation when using the Masquelet technique in the Femur and Tibia. J Orthop Trauma. 2019;33(11):547–52. https://doi.org/10.1097/BOT.0000000000001579.
Stafford PR, Norris BL. Reamer-irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: a review of 25 cases. Injury. 2010;41(Suppl 2):S72–7. https://doi.org/10.1016/S0020-1383(10)70014-0.
Mauffrey C, Hake ME, Chadayammuri V, Masquelet AC. Reconstruction of long bone infections using the induced membrane technique: tips and tricks. J Orthop Trauma. 2016;30(6):e188-193. https://doi.org/10.1097/BOT.0000000000000500.
Henrich D, Seebach C, Nau C, Basan S, Relja B, Wilhelm K, Schaible A, Frank J, Barker J, Marzi I. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J Tissue Eng Regen Med. 2016;10(10):E382–96. https://doi.org/10.1002/term.1826.
Gindraux F, Rondot T, de Billy B, Zwetyenga N, Fricain JC, Pagnon A, Obert L. Similarities between induced membrane and amniotic membrane: novelty for bone repair. Placenta. 2017;59:116–23. https://doi.org/10.1016/j.placenta.2017.06.340.
Gessmann J, Rosteius T, Baecker H, Sivalingam K, Peter E, Schildhauer TA, Koller M. Is the bioactivity of induced membranes time dependent? Eur J Trauma Emerg Surg. 2021. https://doi.org/10.1007/s00068-021-01844-4.
Gaio N, Martino A, Toth Z, Watson JT, Nicolaou D, McBride-Gagyi S. Masquelet technique: the effect of altering implant material and topography on membrane matrix composition, mechanical and barrier properties in a rat defect model. J Biomech. 2018;72:53–62. https://doi.org/10.1016/j.jbiomech.2018.02.026.
McBride-Gagyi S, Toth Z, Kim D, Ip V, Evans E, Watson JT, Nicolaou D. Altering spacer material affects bone regeneration in the Masquelet technique in a rat femoral defect. J Orthop Res. 2018. https://doi.org/10.1002/jor.23866.
Toth Z, Roi M, Evans E, Watson JT, Nicolaou D, McBride-Gagyi S. Masquelet technique: effects of spacer material and micro-topography on factor expression and bone regeneration. Ann Biomed Eng. 2019;47(1):174–89. https://doi.org/10.1007/s10439-018-02137-5.
Mathieu L, Murison JC, de Rousiers A, de l’Escalopier N, Lutomski D, Collombet JM, Durand M,. The Masquelet technique: can disposable polypropylene syringes be an alternative to standard PMMA spacers? A rat bone defect model. Clin Orthop Relat Res. 2021;479(12):2737–51. https://doi.org/10.1097/CORR.0000000000001939.
Nau C, Seebach C, Trumm A, Schaible A, Kontradowitz K, Meier S, Buechner H, Marzi I, Henrich D. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury. 2016;47(2):325–34. https://doi.org/10.1016/j.injury.2015.10.079.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. https://doi.org/10.1016/j.ijsu.2010.02.007.
de Mones E, Schlaubitz S, Oliveira H, d’Elbee JM, Bareille R, Bourget C, Couraud L, Fricain JC. Comparative study of membranes induced by PMMA or silicone in rats, and influence of external radiotherapy. Acta Biomater. 2015;19:119–27. https://doi.org/10.1016/j.actbio.2015.03.005.
Ma YF, Jiang N, Zhang X, Qin CH, Wang L, Hu YJ, Lin QR, Yu B, Wang BW. Calcium sulfate induced versus PMMA-induced membrane in a critical-sized femoral defect in a rat model. Sci Rep. 2018;8(1):637. https://doi.org/10.1038/s41598-017-17430-x.
Sagardoy T, Ehret C, Bareille R, Benoit J, Amedee J, De Mones E. Influence of external beam radiotherapy on the properties of polymethyl methacrylate-versus silicone-induced membranes in a bilateral segmental Bone defect in rats. Tissue Eng Part A. 2018;24(9–10):703–10. https://doi.org/10.1089/ten.TEA.2017.0095.
Shah SR, Smith BT, Tatara AM, Molina ER, Lee EJ, Piepergerdes TC, Uhrig BA, Guldberg RE, Bennett GN, Wenke JC, Mikos AG. Effects of local antibiotic delivery from porous space maintainers on infection clearance and induction of an osteogenic membrane in an infected bone defect. Tissue Eng Part A. 2017;23(3–4):91–100. https://doi.org/10.1089/ten.TEA.2016.0389.
Xie J, Wang W, Fan X, Li H, Wang H, Liao R, Hu Y, Zeng M. Masquelet technique: effects of vancomycin concentration on quality of the induced membrane. Injury. 2021. https://doi.org/10.1016/j.injury.2021.11.003.
Luangphakdy V, Elizabeth Pluhar G, Piuzzi NS, D’Alleyrand JC, Carlson CS, Bechtold JE, Forsberg J, Muschler GF. The effect of surgical technique and spacer texture on bone regeneration: a caprine study using the Masquelet technique. Clin Orthop Relat Res. 2017;475(10):2575–85. https://doi.org/10.1007/s11999-017-5420-8.
Stahl A, Park YB, Park SH, Lin S, Pan CC, Kim S, Yang YP. Probing the role of methyl methacrylate release from spacer materials in induced membrane bone healing. J Orthop Res. 2021. https://doi.org/10.1002/jor.25147.
Wiese A, Pape HC. Bone defects caused by high-energy injuries, bone loss, infected nonunions, and nonunions. Orthop Clin North Am. 2010;41(1):1–4. https://doi.org/10.1016/j.ocl.2009.07.003.
Shi M, Kretlow JD, Nguyen A, Young S, Scott Baggett L, Wong ME, Kasper FK, Mikos AG. Antibiotic-releasing porous polymethylmethacrylate constructs for osseous space maintenance and infection control. Biomaterials. 2010;31(14):4146–56. https://doi.org/10.1016/j.biomaterials.2010.01.112.
Cuthbert RJ, Churchman SM, Tan HB, McGonagle D, Jones E, Giannoudis PV. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone. 2013;57(2):484–92. https://doi.org/10.1016/j.bone.2013.08.009.
Dai K, Deng S, Yu Y, Zhu F, Wang J, Liu C. Construction of developmentally inspired periosteum-like tissue for bone regeneration. Bone Res. 2022;10(1):1. https://doi.org/10.1038/s41413-021-00166-w.
Niikura T, Oda T, Jimbo N, Komatsu M, Oe K, Fukui T, Matsumoto T, Hayashi S, Matsushita T, Itoh T, Kuroda R. Immunohistochemical analysis revealed the expression of bone morphogenetic proteins-4, 6, 7, and 9 in human induced membrane samples treated with the Masquelet technique. J Orthop Surg Res. 2022;17(1):29. https://doi.org/10.1186/s13018-022-02922-y.
Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury. 2011;42(6):591–8. https://doi.org/10.1016/j.injury.2011.03.036.
Masquelet AC. Induced Membrane Technique: Pearls and Pitfalls. J Orthop Trauma. 2017;31(Suppl 5):S36–8. https://doi.org/10.1097/BOT.0000000000000979.
Murison JC, Pfister G, Amar S, Rigal S, Mathieu L. Metacarpal bone reconstruction by a cementless induced membrane technique. Hand Surg Rehabil. 2019;38(2):83–6. https://doi.org/10.1016/j.hansur.2019.01.002.
Thomas MV, Puleo DA. Calcium sulfate: properties and clinical applications. J Biomed Mater Res B Appl Biomater. 2009;88(2):597–610. https://doi.org/10.1002/jbm.b.31269.
Jiang N, Qin CH, Ma YF, Wang L, Yu B. Possibility of one-stage surgery to reconstruct bone defects using the modified Masquelet technique with degradable calcium sulfate as a cement spacer: a case report and hypothesis. Biomed Rep. 2016;4(3):374–8. https://doi.org/10.3892/br.2016.584.
Jaeblon T. Polymethylmethacrylate: properties and contemporary uses in orthopaedics. J Am Acad Orthop Surg. 2010;18(5):297–305. https://doi.org/10.5435/00124635-201005000-00006.
Kanellopoulos AD, Soucacos PN. Management of nonunion with distraction osteogenesis. Injury. 2006;37(Suppl 1):S51–5. https://doi.org/10.1016/j.injury.2006.02.041.
Naal FD, Salzmann GM, von Knoch F, Tuebel J, Diehl P, Gradinger R, Schauwecker J. The effects of clindamycin on human osteoblasts in vitro. Arch Orthop Trauma Surg. 2008;128(3):317–23. https://doi.org/10.1007/s00402-007-0561-y.
Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 2011;29(7):1070–4. https://doi.org/10.1002/jor.21343.
Antoci V Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin Orthop Relat Res. 2007;462:200–6. https://doi.org/10.1097/BLO.0b013e31811ff866.
Ensing GT, van Horn JR, van der Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin Orthop Relat Res. 2008;466(6):1492–8. https://doi.org/10.1007/s11999-008-0203-x.
Taylor BC, French BG, Fowler TT, Russell J, Poka A. Induced membrane technique for reconstruction to manage bone loss. J Am Acad Orthop Surg. 2012;20(3):142–50. https://doi.org/10.5435/JAAOS-20-03-142.
Taylor BC, Hancock J, Zitzke R, Castaneda J. Treatment of bone loss with the induced membrane technique: techniques and outcomes. J Orthop Trauma. 2015;29(12):554–7. https://doi.org/10.1097/BOT.0000000000000338.
Assal M, Stern R. The Masquelet procedure gone awry. Orthopedics. 2014;37(11):e1045–8. https://doi.org/10.3928/01477447-20141023-93.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical approval
Each author certifies that all investigations were conducted in conformity with ethical principles of research.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liodakis, E., Giannoudis, V.P., Sehmisch, S. et al. Bone defect treatment: does the type and properties of the spacer affect the induction of Masquelet membrane? Evidence today. Eur J Trauma Emerg Surg 48, 4403–4424 (2022). https://doi.org/10.1007/s00068-022-02005-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-022-02005-x