Abstract
Background
Studies have shown that the combined application of hyaluronic acid (HA) and platelet-rich plasma (PRP) can repair degenerated cartilage and delay the progression of knee osteoarthritis (KOA). The purpose of this study was to explore the efficacy and safety of the intra-articular injection of PRP combined with HA compared with the intra-articular injection of PRP or HA alone in the treatment of KOA.
Methods
The PubMed, Cochrane Library, EMBASE and China National Knowledge Infrastructure (CNKI) databases were searched from inception to December 2019. Randomized controlled trials and cohort studies of PRP combined with HA for KOA were included. Two orthopaedic surgeons conducted the literature retrieval and extracted the data. Outcome indicators included the Western Ontario and McMaster Universities Arthritis Index (WOMAC), the Lequesne Index, the visual analogue scale (VAS) for pain, and adverse events (AEs). Review Manager 5.3 was used to calculate the relative risk (RR) or standardized mean difference (SMD) of the pooled data. STATA 14.0 was used for quantitative publication bias evaluation.
Results
Seven studies (5 randomized controlled trials, 2 cohort studies) with a total of 941 patients were included. In the VAS comparison after 6 months of follow-up, PRP combined with HA was more likely to reduce knee pain than PRP alone (SMD: − 0.31; 95% confidence interval (CI): − 0.55 to − 0.06; P = 0.01 < 0.05). PRP combined with HA for KOA achieved better improvements in the WOMAC Function Score (SMD: -0.32; 95% CI: − 0.54 to − 0.10; P < 0.05) and WOMAC Total Score (SMD: -0.42; 95% CI: − 0.67 to − 0.17; P < 0.05) at the 12-month follow-up than did the application of PRP alone. In a comparison of Lequesne Index scores at the 6-month follow-up, PRP combined with HA improved knee pain scores more than PRP alone (SMD: -0.42; 95% CI: − 0.67 to − 0.17; P < 0.05). In terms of AEs, PRP combined with HA was not significantly different from PRP or HA alone (P > 0.05).
Conclusions
Compared with intra-articular injection of PRP alone, that of PRP combined with HA can improve the WOMAC Function Scores, WOMAC Total Score, 6-month follow-up VAS ratings, and Lequesne Index scores. However, in terms of the incidence of AEs, PRP combined with HA is not significantly different from PRP or HA alone.
Similar content being viewed by others
Background
Knee osteoarthritis (KOA) is a common knee degenerative disease characterized by cartilage degeneration, cartilage exfoliation, and subchondral bone hyperplasia, leading to knee pain, joint instability and functional limitations [1]. KOA severely affects patients’ quality of life and is a major public health issue [2]. An epidemiological survey published in Proceedings of the National Academy of Sciences (PNAS) showed that the incidence of KOA in the U.S. population has doubled since the mid-twentieth century [3]. KOA has become a high-incidence human disease and has caused a great negative impact on people’s lives and work.
The Osteoarthritis Society International (OARSI) recommends conservative treatment rather than surgery as the first-line management solution for KOA, which emphasizes the importance of conservative treatment in the treatment of KOA [4]. The American College of Rheumatology (ACR) has proposed a classification in which conservative treatment includes drug treatment and non-drug treatment [5]. Non-drug treatment includes general exercise and muscle exercise, but non-drug methods often depend heavily on patient compliance and are difficult to control [5]. The main drug therapies include analgesics, non-steroidal anti-inflammatory drugs and corticosteroid injections [6]. Although the above drug therapies are effective to a certain degree, there are also major side effects [6, 7]. In recent years, there have been an increasing number of studies on the application of intra-articular injection of platelet-rich plasma (PRP) or hyaluronic acid (HA) in the treatment of KOA. Many systematic reviews suggest that intra-articular injection of PRP, compared to HA, can alleviate pain symptoms and improve knee function in patients with KOA [6, 8, 9]. However, a double-blind randomized controlled trial with a 5-year follow-up showed that the combination of HA and PRP improved pain and function in patients with a history of chronic symptomatic knee degenerative changes and osteoarthritis [10]. An RCT showed that PRP is an effective treatment for mild to moderate KOA and that the combined use of HA and PRP is better than the use of HA (1 year) and PRP (3 months) alone [11]. The RCT also revealed that PRP does not provide better overall clinical improvement than HA in terms of symptom-function improvement at different follow-up points or in terms of duration of effect [10]. In recent years, an increasing number of studies have focused on the rationality of PRP combined with HA for KOA, and their mechanisms have been discussed in depth. Experimental studies comparing the migration capabilities of tendon cells and synovial fibroblasts in pure PRP solution and PRP plus HA solution have shown that mixing PRP with HA can significantly improve cell mobility [12]. Marmotti found that the addition of HA to PRP can effectively promote the proliferation of chondrocytes and improve the ability of cartilage repair [13]. Studies have shown that the combination of PRP and HA may benefit from its different biological mechanisms and facilitate the activity of signal molecules such as inflammatory molecules, catabolic enzymes, cytokines and growth factors, thereby playing a positive role in the treatment of KOA [11, 14].
In recent years, clinical workers have begun treating KOA with intra-articular injections of HA combined with PRP to take advantage of their synergistic therapeutic effects. The purpose of this study was to explore the efficacy and safety of intra-articular injection of PRP combined with HA compared with PRP or HA alone, providing an evidence-based strategy for the treatment of KOA.
Methods
This meta-analysis was performed strictly in accordance with the relevant requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.
Inclusion and exclusion criteria
Inclusion criteria. (1) Type of study: Published randomized controlled trial (RCT) or cohort study. (2) Research subjects: Individuals with a clear diagnosis of KOA, regardless of age, gender or nationality. (3) Intervention: Administration of intra-articular injection of PRP combined with Ha to the test group and intra-articular injection of PRP or HA to the control group. Two- or three-arm studies were eligible. (4) At least one of the following outcome indicators: Western Ontario and McMaster Universities Arthritis Index (WOMAC), Lequesne Index, visual analogue scale (VAS), and adverse events (AEs). (5) No application of language exclusions.
Exclusion criteria. (1) Reviews, meeting abstracts, case reports. (2) Subjects with both KOA and hip osteoarthritis. (3) Studies in which the intervention did not include intra-articular injection of PRP combined with HA. (4) Duplicate publications or studies with similar data. (5) Incomplete, unclear, or obviously erroneous data that could not be resolved by contacting the authors.
Literature retrieval strategy
The PubMed, Cochrane Library, EMBASE and China National Knowledge Infrastructure (CNKI) databases were searched, and RCTs and cohort studies that met the inclusion criteria were included. The retrieval period was from the establishment of each database to December 2019. Two researchers also performed cross-referencing to reduce retrieval errors. The search terms included “platelet-rich plasma”, “PRP”, “hyaluronic acid”, “HA”, “knee osteoarthritis” and “KOA”. See Supplement 1 for the database retrieval strategies.
Literature screening and data extraction
Two orthopaedic surgeons conducted the literature retrieval; the preliminary and secondary screenings of the literature were performed strictly in accordance with the pre-established inclusion and exclusion criteria. The two researchers extracted the data independently, and a third researcher compared their outputs. In the event of an error or difference, the third researcher and corresponding author assisted in the judgement.
The main data extracted in this study included first author, publication year, sample size, intervention measures, ethical approval, gender, age, BMI, follow-up periods, radiographic classification, relevant items for literature quality evaluation and relevant outcome indicators of clinical efficacy and safety.
Risk of bias assessment of the included studies
Regarding RCTs, the Cochrane risk of bias tool was used for quality evaluation [15]. The tool includes evaluation in seven areas: random sequence generation, allocation blinding, blinding of participants, blinding of outcome measures, incomplete outcome data, selective reporting, and other biases. The risk of bias in each area was judged to be low, high or unclear [16]. For cohort studies, the Newcastle-Ottawa Scale (NOS) was used for quality assessment. This scale includes three aspects: (1) selection of study groups; (2) ascertainment of exposure and outcome; and (3) group comparability. Studies with scores greater than or equal to 7 were considered to have a low risk of bias, scores of 4 to 6 indicated a moderate risk of bias, and scores less than 4 indicated a high risk of bias.
Statistical analysis
The relative risk (RR) was used to evaluate the effects of binary variables, the standardized mean difference (SMD) was used to evaluate the effects of continuous variables, and 95% confidence intervals (CIs) of the RR and SMD were calculated. Review Manager 5.3.5 software (Cochrane Collaboration, Oxford, UK) was used to calculate the efficacy and safety indicators and their 95% CIs. In addition, for homogeneous data sets, P > 0.1 and I2 < 50% were used as the test standards. When the above two statistical conditions were met, a fixed-effects model was used for the meta-analysis because the pooled effect sizes were relatively homogenous. If one of the above standards did not conform, the homogeneity of the pooled effect size was not ideal, and a random-effects model was applied.
To quantitatively assess whether there are publication biases in different outcome indicators, this study used Stata 14.0 (STATA Corporation, Lakeway, Texas, USA) software to perform Egger’s and Begg’s linear regression tests on the outcome indicators included in the combined analysis of three or more studies.
Results
Literature screening process and results
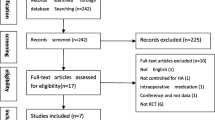
A total of 653 related studies were obtained in the preliminary inspection, including n = 170 from PubMed, n = 218 from Embase, n = 128 from the Cochrane Library, n = 37 from CNKI, and n = 0 from other manual searches. After reading the titles and abstracts and excluding irrelevant documents, a total of 27 articles remained. After excluding duplicate studies, following the inclusion criteria and exclusion criteria, this study eventually included 5 RCTs and 2 cohort studies; all 7 studies clearly stated that they had received ethical approval. The included literature included two three-arm trials, and the rest were two-arm trials, for a total of 941 patients. The literature screening process and results are shown in Fig. 1. Analysing the basic characteristics of the included cases, it was found that the age of the included cases was mainly concentrated in the 40–60 year range, the Kellgren and Lawrence grading scale was I to IV, and the follow-up time was 6–12 months. The basic information of the included literature is shown in Table 1. Baseline materials such as age, BMI, and sample size of the patients included in the 7 studies were comparable, all with p > 0.05. The preparation of PRP combined with HA and the dosage, frequency, and duration of treatment of the 7 studies included were systematically summarized. Of the 7 studies included in this study, the PRP used by patients was derived from the patient’s own whole blood. PRP is a blood product containing high concentrations of platelets, white blood cells and a large number of growth factors produced by whole blood centrifugation. The amount of PRP per serving was approximately 2–8 ml. The frequency of injection of PRP and HA therapy was once a week for 3–9 weeks, which has certain theoretical significance for clinical application. There were some differences in PRP concentrations in the literature included in this study, which may have had an impact on the efficacy of treating KOA. The results are shown in Supplement 2.
Quality evaluation of the included literature
Quality evaluation of the 5 RCTs
There were 3 studies that explicitly reported the specific method of using random allocation, such as the random number table method, and 2 studies merely mentioned randomness and did not explain the specific method. Three papers did not explain the allocation and concealment, and 1 paper did not perform allocation and concealment. The blinding risk of the participants in the blind method and the outcome index measurement process was mainly unclear risk and low risk, and no high risk was found in the literature. None of the five RCTs had missing data, selective reporting, or other risks (Fig. 2 and Fig. 3).
Quality evaluation of the 2 cohort studies
The NOS scores of both cohort studies were 9, and both were low risk (Table 2). In general, the 7 articles included were of good quality, with a standardized research design and good research value.
Meta-analysis
Vas
A total of 3 studies reported VAS scores at 1 month after treatment. The heterogeneity test indicated that the homogeneity was not ideal (I2 = 97%, P < 0.00001). A random-effects model was used for the meta-analysis. The results showed that PRP combined with HA was not significantly different from PRP alone (SMD: -1.13, 95% CI: − 2.84 to 0.13, P = 0.19 > 0.05) (Fig. 4).
A total of 2 articles reported VAS scores at 3 months after treatment. The heterogeneity test indicated heterogeneity (I2 = 57%, P = 0.13), and a random-effects model was used for meta-analysis. The results showed that PRP combined with HA was not significantly different from PRP alone (SMD: -0.36, 95% CI: − 0.92 to 0.20, P = 0.20 > 0.05) (Fig. 4).
A total of 4 studies reported VAS scores at 6 months after treatment. The heterogeneity test suggested a high degree of homogeneity (I2 = 0%, P = 1.00), and a fixed-effects model was used for meta-analysis. The results showed that the difference between PRP combined with HA compared with PRP alone was statistically significant (SMD: -0.31, 95% CI: − 0.55 to − 0.06, P = 0.01 < 0.05) (Fig. 5). The results showed that at 6 months after treatment, the group receiving PRP combined with HA had an average VAS score that was 0.31 points lower than that of the group receiving PRP alone.
WOMAC function score
A total of 2 studies reported the WOMAC Function Score at 12 months after treatment. The heterogeneity test showed good homogeneity (I2 = 40%, P = 0.20), and a fixed-effects model was used for the meta-analysis. The results showed that the difference between PRP and HA compared with PRP alone was statistically significant (SMD: -0.32, 95% CI: − 0.54 to − 0.10, P = 0.004 < 0.05) (Fig. 6). The results showed that at 12 months after treatment, the WOMAC Function Score of the group receiving PRP combined with HA was 0.32 points lower than that of the group receiving PRP alone.
WOMAC Total score
A total of 2 studies reported comparisons of the WOMAC Total Score at 12 months after treatment. The heterogeneity test indicated that the homogeneity was good (I2 = 0%, P = 0.90), and a fixed-effects model was used for meta-analysis. The results showed that the difference between PRP combined with HA compared with PRP alone was statistically significant (SMD: -0.42, 95% CI: − 0.67 to − 0.17, P = 0.001 < 0.05) (Fig. 7). The results showed that at 12 months after treatment, the WOMAC Total Score of the group receiving PRP combined with HA was 0.42 points lower than that of the group receiving PRP alone.
Lequesne index
A total of 2 studies reported Lequesne Index scores 6 months after treatment. The heterogeneity test indicated that the homogeneity was good (I2 = 15%, P = 0.28), and a fixed-effects model was used for meta-analysis. The results showed that PRP combined with HA had significant differences compared with PRP alone (SMD: -0.42, 95% CI: − 0.67 to − 0.17, P < 0.00001) (Fig. 8). The results showed that at 6 months after treatment, the Lequesne Index of the group receiving PRP combined with HA was reduced by 0.42 points compared with that of the group receiving PRP alone.
AEs
A total of 5 studies reported the comparison of AEs of PRP combined with HA and PRP alone on KOA. The heterogeneity test showed that the homogeneity was good (I2 = 13%, P = 0.33), and a fixed-effects model was used for meta-analysis. The results showed no significant difference between PRP combined with HA compared with PRP alone (RR: 0.92, 95% CI: 0.54 to 1.58, P = 0.77) (Fig. 9). The main types of adverse reactions were pain, proteinuria, redness, peripheral oedema, constipation and worsening of pain, without serious adverse reactions.
A total of 2 studies reported the comparison of AEs of PRP combined with HA and the application of HA alone in the treatment of KOA. The heterogeneity test suggested a high degree of homogeneity (I2 = 0%, P = 0.50), and a fixed-effects model was used for meta-analysis. The results showed that PRP combined with HA had no significant difference compared with the application of HA alone (RR: 0.92, 95% CI: 0.49 to 1.75, P = 0.81) (Fig. 10).
Evaluation of publication bias
To quantitatively analyse whether there are publication biases in the relevant outcome indicators of this study, Egger’s and Begg’s tests were conducted on the outcome indicators combined with 3 or more studies. The results showed that there was no publication bias in the results of VAS after 1 month of treatment (Begg’s test: Pr>|z| = 1.000>0.05; Egger’s test: P = 0.857>0.05), VAS after 6 months of treatment (Begg’s test: Pr>|z| = 0.734>0.05; Egger’s test: P = 0.619>0.05), and AEs (Begg’s test: Pr>|z| = 0.221>0.05; Egger’s test: P = 0.269>0.05). The data analysis process and statistical results of publication bias are shown in Supplementary 3.
Discussion
KOA is a disease that can cause lower extremity disability, reduce the quality of life of patients, and seriously affect the physical and mental health of middle-aged and elderly people [24]. With the ageing of the population, KOA will gradually become a common and frequently occurring disease, which is a major challenge that health systems in various countries need to meet [25]. The pathogenesis of KOA is still unclear, and there is still no continuous and effective conservative treatment [10]. For patients with KOA who are successfully treated with conservative treatment, surgical treatment is mostly used. However, surgical treatment is mostly used for patients with severe KOA [26]. In addition, although PRP combined with HA for KOA may be complicated and even more expensive, perhaps compared with the cost and risk of surgery, PRP combined with HA may be a better choice. However, a cost-effectiveness study of PRP combined with HA for KOA and PRP or HA alone is still lacking and needs further research. Moreover, surgical treatment has a long recovery period, unavoidable risks of surgery and complications [27]. Therefore, it is of great significance to study new treatments for KOA. In recent years, intra-articular injection of PRP or HA for the treatment of KOA has attracted strong interest from many clinicians, and in-depth research has been conducted [28, 29].
PRP is extracted by centrifugation from autologous blood, and the platelet concentration can be increased nearly 10-fold, which contains approximately 1500 proteins that can release macrophages and growth factors after activation, which is beneficial not only for removing necrotic tissue and reducing the inflammatory response but also for articular cartilage repair and regeneration [30,31,32]. HA is a high molecular weight polysaccharide that is an important part of synovial fluid and articular cartilage. Injecting HA into the knee joint cavity can physically lubricate the articular surface, reduce wear, and biologically nourish articular cartilage and promote the synthesis of endogenous HA, thereby delaying further joint disease [33,34,35]. A large number of RCTs and systematic reviews of the intra-articular injection of PRP or HA for KOA have been published [8, 36,37,38], and most studies have concluded that intra-articular injection of PRP, compared with HA, can relieve knee pain and improve the function of patients with KOA. Moreover, research has shown that the combined application of PRP and HA can repair the degeneration of cartilage and delay the progression of KOA [11, 14, 39]. This synergistic effect mainly changes the role of inflammatory cytokines in the process of chondrocyte degeneration through specific mediators (CD44, TGF-βRII), thereby promoting cartilage regeneration and inhibiting the inflammatory response [40].
In this study, a meta-analysis showed that there was no significant difference between PRP combined with HA and PRP alone for KOA at 1 month or 3 months after treatment. This outcome shows that the effects of the two intervention methods in relieving knee pain are similar at 1 month and 3 months after treatment. However, the VAS score at 6 months after treatment showed that intra-articular injection of PRP combined with HA, compared with PRP alone, could relieve pain in patients with KOA. Intra-articular injection of PRP combined with HA has a unique advantage in the long-term relief of pain in patients with KOA, but the results from longer follow-up periods are still needed for comparison. This meta-analysis found that PRP combined with HA at 6 months after treatment was superior to PRP alone, which may suggest that PRP combined with HA may be a better treatment for patients with long-term knee pain in the future. Previous studies have shown that PRP combined with HA for KOA can reduce WOMAC pain scores more than PRP alone can, which is consistent with the present findings [18]. In terms of the improved WOMAC Function Score and WOMAC Total Score, intra-articular injection of PRP combined with HA, compared with PRP alone, can improve patients’ knee joint function scores and overall WOMAC scores. Studies have reported that compared with PRP alone, HA combined with PRP in KOA treatment significantly improves physical function at 1 and 3 months after treatment [11]. From the analysis of Lequesne Index scores, it was found that PRP combined with HA reduced the Lequesne Index scores more than PRP alone did, which showed that PRP was more effective in relieving knee pain. This systematic review compared and analysed the adverse reactions of PRP combined with HA compared with PRP and HA alone for KOA. The results showed that no significant difference was found in the incidence of adverse reactions, whether PRP, HA, or both were applied, indicating that the safety of the three treatments was not different. The meta-analysis conducted Begg’s and Egger’s tests on VAS after 1 month and 6 months of treatment and AEs. The results suggest that there was no publication bias, indicating that the above results are reliable.
This study is the first systematic evaluation of the efficacy and safety of PRP combined with HA compared with that of PRP or HA alone for KOA. Additionally, this meta-analysis systematically summarizes the preparation process of the combined application of PRP and HA. Studies have shown that HA is not effective for patients with severe KOA disease, and the effect of HA decreases with time, especially in elderly patients [41, 42]. HA mainly provides nutrition and protection to joints and cannot regenerate damaged cartilage, while PRP contains a large number of growth factors that can promote chondrocyte proliferation and cartilage matrix synthesis [40]. Therefore, the combination of PRP and HA may have a better effect in patients who are elderly, have severe KOA, or exhibit a poor response to treatment with HA or PRP alone.
Nevertheless, several limitations were unavoidable. First, 2 articles were non-RCTs, which may have led to heterogeneity of the combined indicators. Second, the follow-up time was short, with the longest follow-up period being 1 year, and the long-term efficacy and safety of PRP combined with HA could not be evaluated. Third, from the indicators related to WOMAC, it was found that due to the inconsistent indicators reported in the literature, the combined results of the indicators related to WOMAC Pain Score and WOMAC Stiffness Score are lacking. In later clinical studies, attention should be paid to the comprehensiveness and consistency of the outcome indicators. Fourth, there are few studies that directly compare the efficacy and safety of the intra-articular injection of PRP combined with HA with those of the intra-articular injection of HA alone. Therefore, this meta-analysis failed to fully compare the efficacy of PRP combined with HA to that of HA alone. Fifth, the inclusion criteria and exclusion criteria of the included literature did not mention information about knee joint conditions, such as ligament instability, meniscus lesions, and alignment of the limb, which may affect the comparability of related data. Sixth, in terms of the KL Score evaluation, due to the limitation of the data included in the literature, the KL classification included grades I to IV, which may affect the credibility of the Lequesne Index evaluation.
Conclusions
The results of this study indicate that PRP combined with HA may have promising clinical effects on KOA. Based on this meta-analysis, compared with intra-articular injection of PRP alone, PRP combined with HA can improve WOMAC Function Scores, WOMAC Total Scores, VAS ratings (after 6 months of treatment), and Lequesne Index scores. Additionally, in terms of the incidence of AEs, intra-articular injection of PRP combined with HA is not significantly different from intra-articular injection of PRP or HA alone, and the safety of the three treatment regimens is similar.
Availability of data and materials
All data and materials are contained within the manuscript.
Abbreviations
- PNAS:
-
Proceedings of the National Academy of Sciences
- PRP:
-
Platelet-rich plasma
- HA:
-
Hyaluronic Acid
- KOA:
-
Knee Osteoarthritis
- WOMAC:
-
Western Ontario and McMaster Universities Arthritis Index
- VAS:
-
Visual analog scale
- AEs:
-
Adverse events
- CNKI:
-
China National Knowledge Infrastructure
- RCT:
-
randomized controlled trail
- NOS:
-
Newcastle-Ottawa Scale
- RR:
-
Relative risk
- SMD:
-
Standard Mean Difference
References
Pereira D, Peleteiro B, Araújo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthr Cartilage. 2011;19(11):1270–85.
Williams QI, Gunn AH, Beaulieu JE, Benas BC, Buley B, Callahan LF, Cantrell J, Genova AP, Golightly YM, Goode AP, et al. Physical therapy vs. internet-based exercise training (PATH-IN) for patients with knee osteoarthritis: study protocol of a randomized controlled trial. BMC Musculoskel Dis. 2015;16(1):264.
Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, Woods RJ, Lieberman DE. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114(35):9332–6.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra S, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–89.
Guidelines AACO. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on osteoarthritis guidelines. Arthritis Rheum. 2000;43(9):1905–15.
Han Y, Huang H, Pan J, Lin J, Zeng L, Liang G, Yang W, Liu J. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20(7):1418–29.
Hepper CT, Halvorson JJ, Duncan ST, Gregory AJ, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17(10):638–46.
Dai W, Zhou A, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659–70.
Chen P, Huang L, Ma Y, Zhang D, Zhang X, Zhou J, Ruan A, Wang Q. Intra-articular platelet-rich plasma injection for knee osteoarthritis: a summary of meta-analyses. J Orthop Surg Res. 2019;14(1):16.
Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, Kon E, Filardo G. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47(2):347–54.
Lana JF, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, Ambach MA, Vincent H, Urban-Paffaro A, Onodera CM, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69–78.
Anitua E, Sanchez M, De la Fuente M, Zalduendo MM, Orive G. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1657–65.
Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, Maiello A, Realmuto C, Peretti GM. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2590–601.
Saturveithan C, Premganesh G, Fakhrizzaki S, Mahathir M, Karuna K, Rauf K, William H, Akmal H, Sivapathasundaram N, Jaspreet K. Intra-articular hyaluronic acid (HA) and platelet rich plasma (PRP) injection versus hyaluronic acid (HA) injection alone in patients with grade III and IV knee osteoarthritis (OA): a retrospective study on functional outcome. Malays Orthop J. 2016;10(2):35–40.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928.
Feng Q, Zhou A, Zou H, Ingle S, May MT, Cai W, Cheng C, Yang Z, Tang J. Quadruple versus triple combination antiretroviral therapies for treatment naive people with HIV: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;366:l4179.
Abate M, Verna S, Schiavone C, Di Gregorio P, Salini V. Efficacy and safety profile of a compound composed of platelet-rich plasma and hyaluronic acid in the treatment for knee osteoarthritis (preliminary results). Eur J Orthopaedic Surg Traumatol. 2015;25(8):1321–6.
Yu W, Xu P, Huang G, Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med. 2018;16(3):2119–25.
Guo Y, Yu H, Yuan L. Treatment of knee osteoarthritis with platelet-rich plasma plus hyaluronic acid in comparison with platelet-rich plasma only. Int J Clin Exp Med. 2016;9(6):12085–90.
Quanwei DING, Shuaijie LV, Xingchao SHEN, Peijian TONG. A prospective randomized controlled study on platelet-rich plasma (PRP) combined with sodium hyaluronate (HA) intra-articular injection in the treatment of knee osteoarthritis. Shanghai Med Pharmaceut J. 2017;38(05):25–8.
Xin-liang ZHAO. Clinical effect of sodium hyaluronate injection combined with autologous platelet rich plasma injection for knee osteoarthritis. Clin Res Pract. 2018;3(25):37–8.
Guo Y, Yu H, Yong M, Guandong L, Huili L, Lin Y, Zhentao M, Wei L, Shui S. Treatment of knee osteoarthritis with mixture of platelet-rich-plasma plus hyaluronic acid. Chinese J Joint Surg (Electronic Edition). 2018;12(06):874–8.
Chen-rong KE, Rui ZHANG, Ji-xin XUE. Clinical efficacy of autologous platelet-rich plasma combined with intra-articular hyaluronic acid injection for knee osteoarthritis. Chinese J General Pract. 2016;14(11):1810–2.
Oo WM, Liu X, Hunter DJ. Pharmacodynamics, efficacy, safety and administration of intra-articular therapies for knee osteoarthritis. Expert Opin Drug Met. 2019;15(12):1021–32.
Huétink K, Stoel BC, Watt I, Kloppenburg M, Bloem JL, Malm SH, van T Klooster R, Nelissen RGHH. Identification of factors associated with the development of knee osteoarthritis in a young to middle-aged cohort of patients with knee complaints. Clin Rheumatol. 2015;34(10):1769–79.
Charlesworth J, Fitzpatrick J, Perera NKP, Orchard J. Osteoarthritis- a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskel Dis. 2019;20(1):151.
Kanchanatawan W, Arirachakaran A, Chaijenkij K, Prasathaporn N, Boonard M, Piyapittayanun P, Kongtharvonskul J. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1665–77.
Laudy ABM, Bakker EWP, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Brit J Sport Med. 2015;49(10):657–72.
Smelter E, Hochberg MC. New treatments for osteoarthritis. Curr Opin Rheumatol. 2013;25(3):310–6.
Simental-Mendía MA, Vílchez-Cavazos JF, Martínez-Rodríguez HG. El plasma rico en plaquetas en osteoartrosis de rodilla: una alternativa de tratamiento. Artículo de revisión Cirugía y Cirujanos. 2015;83(4):352–358.
Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep. 2017;19(5):24.
Say F, Gurler D, Yener K, Bulbul M, Malkoc M. Platelet-rich plasma injection is more effective than hyaluronic acid in the treatment of knee osteoarthritis. Acta Chir Orthop Traumatol Cechoslov. 2013;80(4):278–83.
Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):485–92.
Richette P. Hyaluronic acid: Still useful in knee osteoarthritis? Joint Bone Spine. 2017;84(6):655–6.
Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: results of an extensive critical literature review. Semin Arthritis Rheu. 2019;48(4):563–72.
Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12(1):16.
Al-Moraissi EA, Wolford LM, Ellis E, Neff A. The hierarchy of different treatments for arthrogenous temporomandibular disorders: a network meta-analysis of randomized clinical trials. J Cranio Maxill Surg. 2019;48(1):9–23.
Wu Q, Luo X, Xiong Y, Liu G, Wang J, Chen X, Mi B. Platelet-rich plasma versus hyaluronic acid in knee osteoarthritis: a meta-analysis with the consistent ratio of injection. J Orthop Surg-Hong K. 2020;28(1):920548254.
Patel S, Dhillon MS, Bansal T. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee- Letter to the Editor & Author Response. J Stem Cells Regenerative Med. 2017;13(2):80.
Andia I, Abate M. Knee osteoarthritis: hyaluronic acid, platelet-rich plasma or both in association? Expert Opin Biol Th. 2014;14(5):635–49.
Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis--meta-analysis. Osteoarthr Cartil. 2011;19(6):611–9.
Uçar D, Dıraçoğlu D, Süleyman T, Capan N. Intra-articular hyaluronic acid as treatment in elderly and middle-aged patients with knee osteoarthritis. Open Rheumatol J. 2013;7:38–41.
Acknowledgements
We thank American Journal Experts for linguistic assistance during the preparation of this manuscript.
Funding
This work was funding by the National Natural Science Foundation of China (No.81873314, No.81974574, No. 81473698), the China Postdoctoral Science Foundation (No. 2018 M633036), the Medical Science Research Foundation of Guangdong Province (No. B2019091), Key scientific research platforms and research projects of universities in Guangdong Province (No. 2018KQNCX041), the Project of Guangdong Provincial Department of Finance (No.[2014]157, No.[2018]8), and the Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No.YN2019ML08, No. YK2013B2N19, YN2015MS15).
Author information
Authors and Affiliations
Contributions
JLZ, HTH and GHL had the original idea for the study and proposed the study design. LFZ, WYY and GHL conducted the literature search, screened and selected the studies initially identified. JLZ, HTH and GHL read and evaluated the quality of the studies included. JLZ and HTH conducted the meta-analysis. JL wrote the initial manuscript and serves as guarantor. All authors contributed to interpreting the study findings and to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since our study is a meta-analysis, an Ethical Review Committee Statement is not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, J., Huang, H., Liang, G. et al. Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord 21, 224 (2020). https://doi.org/10.1186/s12891-020-03262-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-020-03262-w