Abstract
Purpose
Cell migration plays an essential role in development, wound healing, and tissue regeneration. Plasma rich in growth factors (PRGF-Endoret) technology offers a potential source of growth factors involved in tissue regeneration. Here, we evaluate the potential of PRGF-Endoret over tendon cells and synovial fibroblasts migration and study whether the combination of this autologous technology with hyaluronic acid (HA) improves the effect and potential of the biomaterials over the motility of both types of fibroblasts.
Methods
Migration of primary tendon cells and synovial fibroblasts after culturing with either PRGF or PPGF (plasma poor in growth factors) at different doses was evaluated. Furthermore, the migratory capacity induced by the combination of PPGF and PRGF with HA was tested.
Results
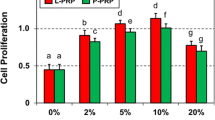
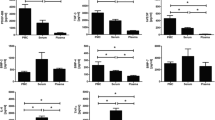
PPGF stimulated migration of both types of cells but this effect was significantly higher when PRGF was used. Tendon cells showed an increase of 212% in migratory ability when HA was combined with PPGF and of 335% in the case of HA + PRGF treatment compared with HA alone.
Conclusions
PRGF-Endoret stimulates migration of tendon cells and synovial fibroblasts and improves the biological properties of HA.






Similar content being viewed by others
References
Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT (2004) Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 91:4–15
Anitua E, Sánchez M, Orive G, Andia I (2008) Delivering growth factors for therapeutics. Trends Pharmacol Sci 29:37–41
Anitua E, Sánchez M, Orive G, Andia I (2007) The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 28:4551–4560
Anitua E, Sánchez M, Zalduendo MM, De la Fuente M, Prado R, Orive G, Andía I (2009) Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif 42:162–170
Biname G, Lassus P, Hibner U (2008) Transforming growth factor β controls the directional migration of hepatocyte cohorts by modulating their adhesion to fibronectin. Mol Biol Cell 19:945–956
Boilly B, Vercoutter-Edouart AS, Hondermarck H, Nurcombe V, Le Bourthis X (2000) FGF signals for cell proliferation and migration through different pathways. Cytokine Growth Factor Rev 11:295–302
Bourguignon LY, Singleton PA, Zhu H, Zhou B (2002) Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem 277:39703–39712
Caceres M, Hidalgo R, Sanz A, Martinez J, Riera P, Simth P (2008) Effect of platelet-rich plasma on cell adhesion, cell migration, and myofibroblastic differentiation in human gingival fibroblasts. J Periodontol 79:714–720
Celotti A, Colciago A, Negri-Cesi P, Pravettoni A, Zaninetti R, Sacchi MC (2006) Effect of platelet-rich plasma on migration and proliferation of SaOS-2 osteoblasts: role of platelet-derived growth factor and transforming growth factor-b. Wound Repair Regen 14:195–202
Chen WY, Abatangelo G (1999) Functions of hyaluronan in wound repair. Wound Repair Regen 7:79–89
Creeper F, Lichanska AM, Marshall RI, Seymour GJ, Ivanovski S (2009) The effect of platelet-rich plasma on osteoblast and periodontal ligament cell migration, proliferation and differentiation. J Periodontal Res 44:258–265
Englesbe MJ, Deou J, Bourns BD, Clowes AW, Daum G (2004) Interleukin-1beta inhibits PDGF-BB-induced migration by cooperating with PDGF-BB to induce cyclooxygenase-2 expression in baboon aortic smooth muscle cells. J Vasc Surg 39:1091–1096
Frey MR, Golovin A, Polk DB (2004) Epidermal growth factor-stimulated intestinal epithelial cell migration requires src family kinase-dependent p38 MAPK signaling. J Biol Chem 279:44513–44521
Gouëffic Y, Guilluy C, Guérin P, Patra P, Pacaud P, Loirand G (2006) Hyaluronan induces vascular smooth muscle migration through RHAMM-mediated PI3 K-dependent Rac activation. Cardiovasc Res 72:339–348
Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M (2006) The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 17:212–219
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 435:314–321
Han J, Li L, Yu L, Zheng Y, Guo J, Zheng X, Ping Yi, Zhou Y (2010) Epidermal growth factor stimulates human trophoblast cell migration through Rho A and Rho C activation. Endocrinology 151:1732–1742
Han J, Meng HX, Tang JM, Li SL, Tang Y, Chen ZB (2007) The effect of different platelet-rich plasma concentrations on proliferation and differentiation of human periodontal ligament cells in vitro. Cell Prolif 40:241–252
Kim JS, Kim JG, Moon MY, Jeon CY, Won HY, Kim HJ, Jeon YJ, Seo JY, Kim JI, Kim J, Lee JY, Kim PH, Park JB (2006) Transforming growth factor-β1 regulates macrophage migration via RhoA. Blood 108:1821–1829
Lee HK, Lee JH, Kim M, Kariya Y, Miyazaki K, Kim EK (2006) Insulin-like growth factor-1 induces migration and expression of laminin-5 in cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci 47:873–882
Lopez-Ponte A, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J (2007) The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25:1737–1745
Maheshwari G, Wiley HS, Lauffenburger DA (2001) Autocrine epidermal growth factor signaling stimulates directionally persistent mammary epithelial cell migration. J Cell Biol 155:1123–1128
Maier KG, Sadowitz B, Cullen S, Han X, Gahtan V (2009) Thrombospondin-1-induced vascular smooth muscle cell migration is dependent on the hyaluronic acid receptor CD44. Am J Surg 198:664–669
Maniwa S, Ochi M, Motomura T, Nishikori T, Chen J, Naora H (2001) Effects of hyaluronic acid and basic fibroblast growth factor on motility of chondrocytes and synovial cells in culture. Acta Orthop Scand 72:299–303
Ranzato E, Balbo V, Boccafoschi F, Mazzuco L, Burlando B (2009) Scratch wound closure of C2C12 mouse myoblasts is enhanced by human platelet lysate. Cell Biol Int 33:911–917
Rughetti A, Giusti I, D’Ascenzo S, Leocata P, Carta G, Pavan A, Dell’Orso L, Dolo V (2008) Platelet gel-released supernatant modulates the angiogenic capability of human endothelial cells. Blood Transfus 6:12–17
Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I (2007) Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med 35:245–251
Sanchez M, Anitua E, Azofra J, Prado R, Muruzabal F, Andia I (2010) Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy 26:269–278
Sanchez M, Anitua E, Lopez-Vidriero E, Andia I (2010) The future: optimizing the healing enviroment in anterior cruciate ligament reconstruction. Sports Med Arthrosc Rev 18:48–53
Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, Satir P, Christensen S (2010) Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem 25:279–292
Senior RM, Griffin GL, Huang JS, Walz DA, Deuel TF (1983) Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol 96:382–385
Ware MF, Wells A, Lauffenburger DA (1998) Epidermal growth factor alters fibroblasts migration speed and directional persistence reciprocally and in a matrix-dependent manner. J Cell Sci 111:2423–2432
Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE (2004) Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 34:665–671
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83:835–870
Wobus M, Rangwala R, Sheyn I, Hennigan R, Coila B, Lower EE, Yassin RS, Sherman LS (2002) CD44 associates with EGFR and erbB2 in metastasizing mammary carcinoma cells. Appl Immunohistochem Mol Morphol 10:34–39
Yagi M, Sato N, Mitsui Y, Gotoh M, Hamada T, Nagata K (2010) Hyaluronan modulates proliferation and migration of rabbit fibroblasts derived from flexor tendon epitenon and endotenon. J Hand Surg 35A:791–796
Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD (2006) The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 24:928–935
Acknowledgments
We thank Basque Government (SAIOTEK project BIONE) for the partial funding of the present project.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Anitua, E., Sanchez, M., De la Fuente, M. et al. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg Sports Traumatol Arthrosc 20, 1657–1665 (2012). https://doi.org/10.1007/s00167-011-1697-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1697-4